Abstract
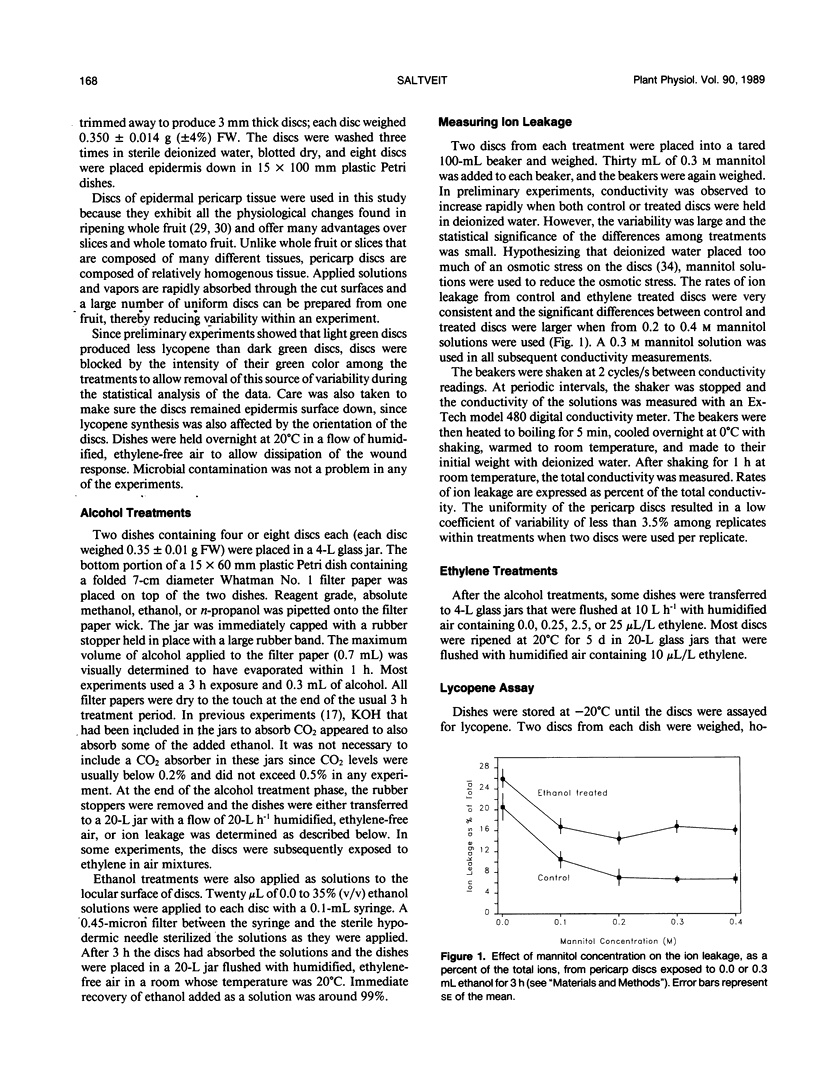
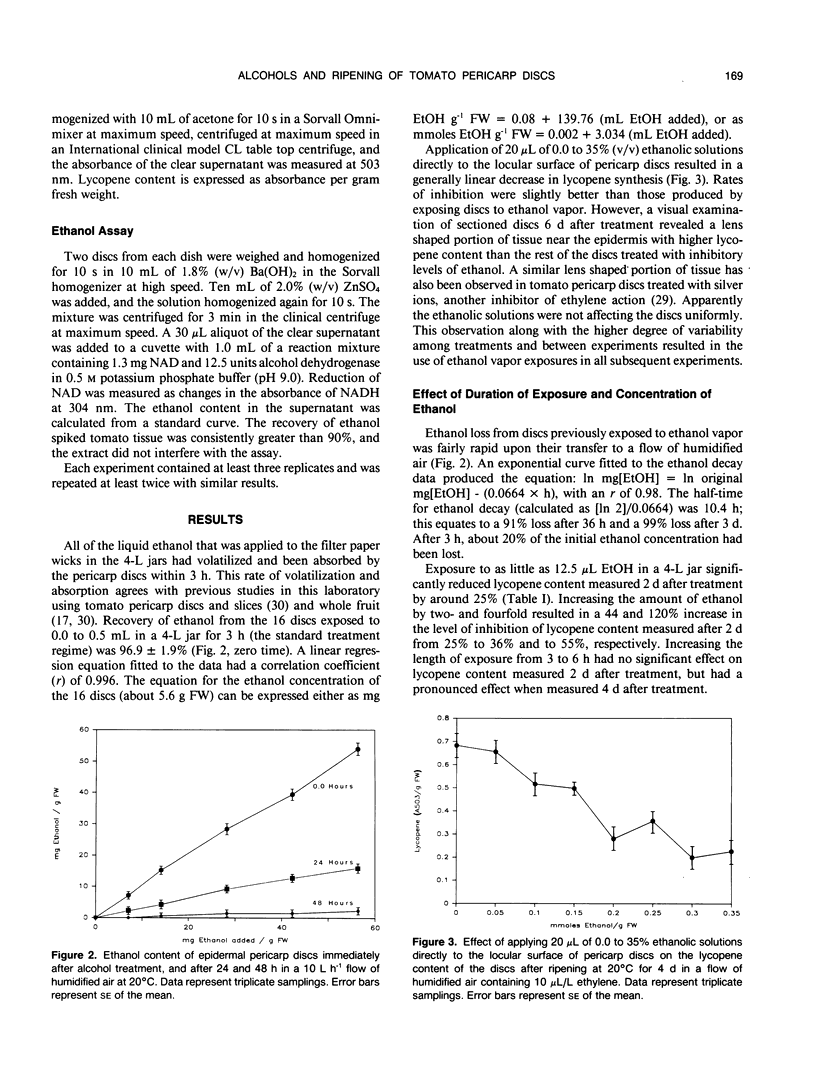
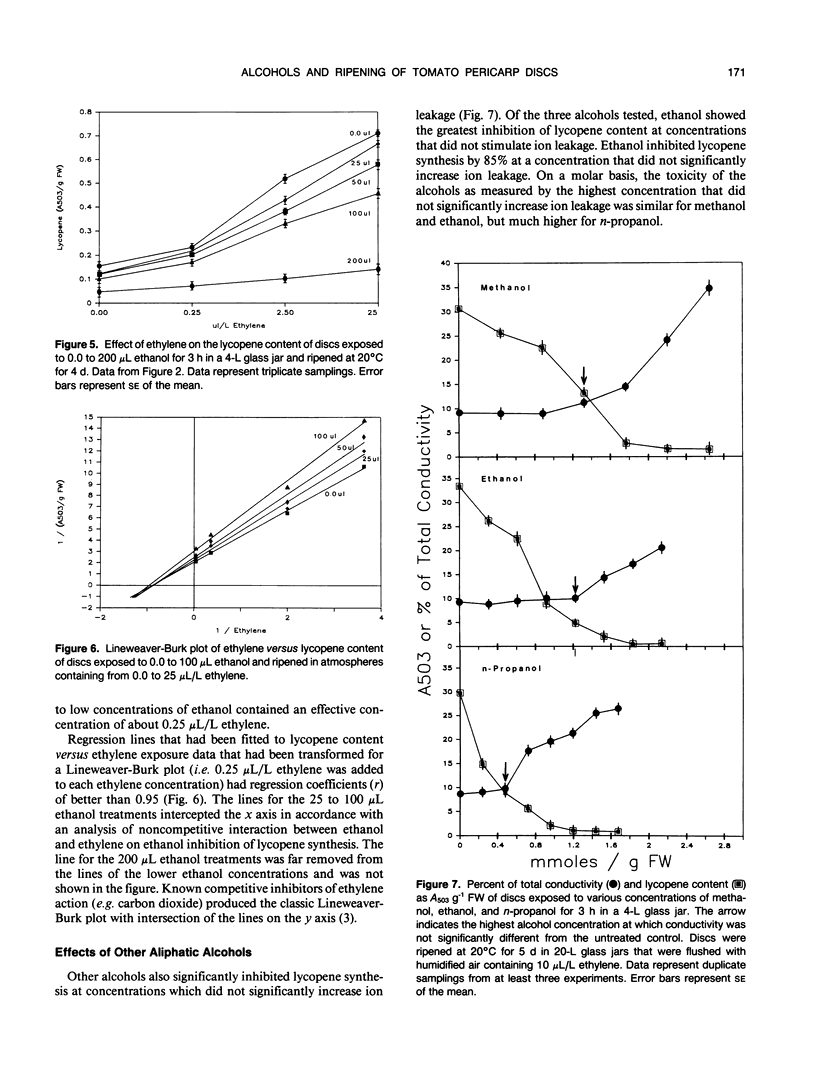
Ethanol concentrations that were induced in pericarp discs of mature-green tomato fruit (Lycopersicon esculentum Mill, cv Castlemart) either by anaerobic metabolism or by exposure to ethanol vapor inhibited ripening without increasing the rate of ion leakage. Inhibition of ripening (i.e. lycopene synthesis) of excised tomato pericarp tissue by ethanol vapor was reversed by increasing concentrations of the plant hormone ethylene. A Lineweaver-Burk plot indicated noncompetitive interaction between ethanol and ethylene. Methanol and n-propanol also inhibited lycopene synthesis without significantly increasing ion leakage. The similar inhibitory effects of methanol, ethanol, and n-propanol at concentrations which did not stimulate ion leakage, and the relationship between activity and lipophilia of the alcohols suggest that their mode of action was through disruption of membranes associated with ethylene action.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews C. J., Pomeroy M. K. Toxicity of Anaerobic Metabolites Accumulating in Winter Wheat Seedlings during Ice Encasement. Plant Physiol. 1979 Jul;64(1):120–125. doi: 10.1104/pp.64.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. A., Hammett L. K., Pharr D. M. Carbon dioxide effects on ethanol production, pyruvate decarboxylase, and alcohol dehydrogenase activities in anaerobic sweet potato roots. Plant Physiol. 1983 Jan;71(1):59–62. doi: 10.1104/pp.71.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. H., Goldstein D. B. Effects of low concentrations of ethanol on the fluidity of spin-labeled erythrocyte and brain membranes. Mol Pharmacol. 1977 May;13(3):435–441. [PubMed] [Google Scholar]
- Cockburn A., Barraco R. A., Reyman T. A., Peck W. H. Autopsy of an Egyptian mummy. Science. 1975 Mar 28;187(4182):1155–1160. [PubMed] [Google Scholar]
- Goodenough-Trepagnier C., Tarry E., Prather P. Derivation of an efficient nonvocal communication system. Hum Factors. 1982 Apr;24(2):163–172. doi: 10.1177/001872088202400202. [DOI] [PubMed] [Google Scholar]
- Grisham C. M., Barnett R. E. The interrelationship of membrane and protein structure in the functioning of the (Na + = K + )-activated ATPase. Biochim Biophys Acta. 1972 Jun 20;266(3):613–624. doi: 10.1016/0006-3002(72)90005-4. [DOI] [PubMed] [Google Scholar]
- Gustafson F. G. PRODUCTION OF ALCOHOL AND ACETALDEHYDE BY TOMATOES. Plant Physiol. 1934 Apr;9(2):359–367. doi: 10.1104/pp.9.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. P., Greenfield P. F. Ethanol and the fluidity of the yeast plasma membrane. Yeast. 1987 Dec;3(4):223–232. doi: 10.1002/yea.320030403. [DOI] [PubMed] [Google Scholar]
- Kelly M. O., Saltveit M. E. Effect of endogenously synthesized and exogenously applied ethanol on tomato fruit ripening. Plant Physiol. 1988 Sep;88(1):143–147. doi: 10.1104/pp.88.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerer T. W., Kozlowski T. T. Ethylene, Ethane, Acetaldehyde, and Ethanol Production By Plants under Stress. Plant Physiol. 1982 Apr;69(4):840–847. doi: 10.1104/pp.69.4.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerer T. W., Macdonald R. C. Acetaldehyde and ethanol biosynthesis in leaves of plants. Plant Physiol. 1987 Aug;84(4):1204–1209. doi: 10.1104/pp.84.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerer T. W., Stringer M. A. Alcohol dehydrogenase and ethanol in the stems of trees : evidence for anaerobic metabolism in the vascular cambium. Plant Physiol. 1988 Jul;87(3):693–697. doi: 10.1104/pp.87.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury A. C., Frost F., Cookson W. O. Dysgerminoma, gonadoblastoma, and testicular germ cell neoplasia in phenotypically female and male siblings with 46 XY genotype. Cancer. 1987 Jan 15;59(2):288–291. doi: 10.1002/1097-0142(19870115)59:2<288::aid-cncr2820590219>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Kondo M., Kasai M. The effects of n-alcohols on sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1973 Jul 6;311(3):391–399. doi: 10.1016/0005-2736(73)90319-2. [DOI] [PubMed] [Google Scholar]
- Lenaz G., Parenti-Castelli G., Monsigni N., Silvestrini M. G. Effect of alcohols on the functional organization of the inner mitochondrial membrane. J Bioenerg. 1971 May;2(2):119–127. doi: 10.1007/BF01648927. [DOI] [PubMed] [Google Scholar]
- Rumpho M. E., Kennedy R. A. Anaerobiosis in Echinochloa crus-galli (Barnyard Grass) Seedlings : Intermediary Metabolism and Ethanol Tolerance. Plant Physiol. 1983 May;72(1):44–49. doi: 10.1104/pp.72.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satler S. O., Thimann K. V. The influence of aliphatic alcohols on leaf senescence. Plant Physiol. 1980 Sep;66(3):395–399. doi: 10.1104/pp.66.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thore A., Baltscheffsky H. Inhibitory effects of lower aliphatic alcohols on electron transport phosphorylation systems. I. Straight-chain, primary alcohols. Acta Chem Scand. 1965;19(7):1591–1599. doi: 10.3891/acta.chem.scand.19-1591. [DOI] [PubMed] [Google Scholar]


