Abstract
Background:
Although breastfeeding confers significant benefits to infants, women with diabetes in pregnancy experience unique nutrition and health challenges, which may influence infant feeding practice. This study aimed to determine the association between nutrition and exercise behaviors of women with diabetes in pregnancy and breastfeeding at birth and 6 months.
Methods:
A secondary data analysis of a longitudinal study on maternal pregestational diabetes mellitus (DM) and gestational diabetes (GDM) and infant development was conducted. Women self-reported engaging in nutrition behaviors, such as using meal plans, and exercise health behaviors. Primary outcomes were exclusive breastfeeding at birth and any breastfeeding at 6 months. Logistic regression models adjusted for significant maternal-infant covariates.
Results:
Of n = 48 women with diabetes in pregnancy, 94% had GDM and 6% had pregestational type 1 or type 2 DM. Forty percent of women exclusively breastfed at birth and 68% partially or exclusively breastfed at 6 months (of n = 34 with complete 6-month data). Women who cooked their own meals had two times greater adjusted odds of exclusive breastfeeding at birth (adjusted odds ratio [AOR] = 1.94, 95% confidence interval [CI] = 1.12–5.11), and women who exercised during pregnancy had seven times greater adjusted odds of any breastfeeding at 6 months (AOR = 7.2, 95% CI = 1.10–42.8).
Conclusion:
Nutrition and exercise behaviors were associated with exclusive breastfeeding at birth and any breastfeeding at 6 months. Health behaviors to effectively manage diabetes during pregnancy may inform efforts to improve breastfeeding initiation and duration, and future studies in a larger sample are needed.
Keywords: breastfeeding, gestational diabetes, health behaviors, infant feeding, maternal diabetes, prenatal exercise
Introduction
Diabetes in pregnancy, defined as pregestational diabetes (diagnosis of type 1 [T1DM] or type 2 diabetes mellitus [T2DM] before pregnancy) and gestational diabetes (GDM) (diagnosis of diabetes mellitus [DM] during pregnancy), can confer significant health risks to women and infants, such as maternal preeclampsia and fetal macrosomia. Treatment of diabetes in pregnancy to achieve and maintain glycemic control generally includes lifestyle and dietary modification and potential pharmacotherapy with insulin and other medications. These management strategies introduce unique nutrition and health challenges that may burden women during pregnancy, especially women from low-income and racial and ethnic minoritized communities in the United States.
Diabetes in pregnancy disproportionately burdens U.S. women from low-income and racial and ethnic minoritized communities, and these communities also experience disparities in successful breastfeeding initiation and continuation.1 Extensive literature has demonstrated the relationship between diabetes in pregnancy and reduced infant breastfeeding; however, less is known of the influences of modifiable, diabetes-related perinatal nutrition and health behaviors on infant breastfeeding practice. Understanding the lifestyle behaviors of women with diabetes in pregnancy from predominantly historically underserved and socioeconomically disadvantaged backgrounds is critical to informing programs and interventions targeted at improving breastfeeding initiation and continuation in these communities. We aimed to determine the associations between nutrition and exercise health behaviors of women with diabetes in pregnancy and infant breastfeeding practice at birth and 6 months.
Materials and Methods
A secondary data analysis of an on-going longitudinal study on maternal diabetes and infant neurodevelopment at an academic medical center in New York City was conducted.2 The parent study enrolled postpartum women between 18 and 46 years of age with a history of gestational or pregestational diabetes as the only pregnancy complication and their full-term newborns; postpartum women with history of other pregnancy complications, such as hypertension, twin or multiple pregnancy, or a preterm newborn <37 weeks gestational age were excluded. Nondiabetic mother-infant dyads (controls) were matched to case dyads by infant gestational age at birth, sex, and ethnicity. The parent study obtained approval from the New York State Psychiatric Institute institutional review board (protocol no. 7050R) in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983.
The main predictors were diabetes-related self-reported nutrition and exercise health behaviors: using a meal plan, using nutrition labels when grocery shopping and cooking, cooking own meals at home, eating outside the home, and exercising before and during pregnancy.
The main outcomes were exclusive breastfeeding at birth and any breastfeeding (partial or exclusive breastfeeding) at 6 months. Covariates included sociodemographic characteristics, prepregnancy body mass index (BMI), delivery mode, and postpartum depression risk. Maternal pregnancy nutrition and activity behaviors and sociodemographic characteristics were obtained through self-reported questionnaires; the parent study abstracted all other covariates from electronic medical records.
SAS® (version 9.4; SAS Institute, Inc., Cary, NC) was used for statistical analyses (significance at α < 0.05). Bivariate analyses compared maternal diabetes status and outcomes and covariates. Logistic regression models estimated the association of diabetes-related nutrition and exercise behaviors with breastfeeding at birth and at 6 months. After identification of potential confounders significant on bivariate analyses and removing multicollinearity, final logistic regression models estimating exclusive breastfeeding at birth were each adjusted for prepregnancy BMI and infant sex, and models estimating any breastfeeding at 6 months were each adjusted for maternal Hispanic ethnicity and employment status.
Results
The parent study comprised N = 108 women; n = 48 women had diabetes in pregnancy and were included in this analytic sample. Six percent of women with diabetes in pregnancy had pregestational T1DM or T2DM and 94% had GDM. Women with diabetes in pregnancy had mean age 34.2 ± 5.9 years, and the majority self-identified as Hispanic (81.3%) and Other Race (67%) and reported a household income of <$50,000 (Table 1). Almost half of the sample were married (49%) and a third of the women were unemployed when their infant was born (31%). Nineteen women (40%) exclusively breastfed at birth and 23 (68%) reported any breastfeeding (partial or exclusive breastfeeding) at 6 months (out of N = 34 with complete data at 6 months).
Table 1.
Descriptive Statistics of Women with Pregestational and Gestational Diabetes in Pregnancy (N = 48)
| Variable | Maternal diabetes (n = 48) |
|---|---|
| Age, years | 34.2 ± 5.9 |
| Racea | |
| Black | 5 (12) |
| Asian | 1 (2) |
| White | 8 (19) |
| Native American | 2 (4) |
| Other | 29 (67) |
| Hispanic ethnicity | 39 (81.3) |
| Birth region of origin in the Caribbean | 20 (48.8) |
| Married | 19 (49) |
| Highest education level completed | |
| Less than high school | 5 (12) |
| High school degree or equivalent | 12 (29) |
| College degree or greater | 25 (59) |
| Unemployed | 12 (31) |
| Income, U.S. dollars | |
| <$50,000 | 25 (68) |
| $50,001–$100,000 | 6 (16) |
| >$100,000 | 6 (16) |
| Sometimes or often not having enough to eat | 5 (13) |
| Housing hardshipb | 5 (12) |
| Prepregnancy BMI, kg/m2 | 29.7 ± 6.0 |
| Parity | 1.4 ± 1.2 |
| Cesarean section delivery | 31 (65) |
| Number of other children in household | 1.7 (41) |
| Read nutrition food labels as dietary guides | 31 (76) |
| Cooks own home meals >5 days/week | 30 (63) |
| Eats out 3 or more times/week | 29 (67) |
| Used a meal plan | 34 (81) |
| Exercised before pregnancy | 17 (38) |
| Exercised during pregnancy | 14 (33) |
| Gestational age, weeks | 38.8 ± 0.8 |
| Infant birthweight, grams | 3,273.7 ± 663.6 |
| Infant female sex | 23 (48) |
| No childcare at 6 months | 11 (34) |
| EPDS depression score at 6 months | 3.9 ± 5.3 |
Due to missing responses, not all variables reflected the total sample size.
Housing hardship was defined as one or more of the following: ever homeless or lived in a homeless shelter, not being able to afford paying full amount of rent or mortgage, or having serious financial problems with inability to pay monthly bills at least once in the 12 months before the study.
BMI, body mass index; EPDS, Edinburgh Postnatal Depression Scale.
On bivariate analyses, prepregnancy BMI (p = 0.04) and infant sex (p = 0.04) were significantly associated with infant breastfeeding at birth, and maternal Hispanic ethnicity (p = 0.03) and employment (p = 0.02) were significantly associated with any infant breastfeeding at 6 months. Among the diabetes-related nutrition and exercise health behaviors, eating homecooked meals (p = 0.04) and using nutrition labels (p = 0.04) were significantly associated with breastfeeding at birth and exercise during pregnancy (p = 0.05) was significantly associated with breastfeeding at 6 months.
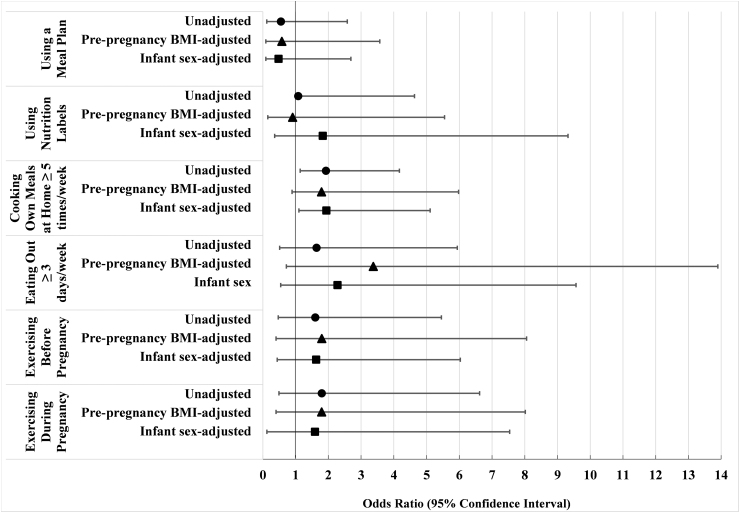
On adjusted analyses, women who reported cooking their own meals at home at least 5 days a week had almost two times greater adjusted odds of exclusive breastfeeding at birth compared to women who cooked their own meals 3 days or less a week (adjusted odds ratio [AOR] = 1.94, 95% confidence interval [CI] = 1.12–5.11) (Fig. 1). Use of nutrition labels conferred almost two times greater odds of exclusive breastfeeding at birth compared to women who did not use nutrition labels, although no longer statistically significant on the adjusted model (AOR = 1.83, 95% CI = 0.36–9.32).
FIG. 1.
Forest plot of unadjusted and adjusted ORs for the association between diabetes-related nutrition and exercise behaviors and exclusive breastfeeding at birth among women with a history of diabetes in pregnancy. Logistic regression models estimated associations between diabetes-related nutrition behaviors (using a meal plan, using nutrition labels when grocery shopping and cooking, eating out ≥3 times/week, and cooking own meals at home ≥5 times/week) and health behaviors (exercise before pregnancy and exercise during pregnancy) and exclusive breastfeeding practice at birth among women with a history of diabetes in pregnancy (N = 48 women with pregestational and gestational diabetes). Unadjusted ORs shown with filled circle markers. Adjusted models were each adjusted for prepregnancy BMI (triangle markers) and infant sex (square markers). Using nutrition labels (unadjusted OR = 1.08, 95% CI = 1.05–4.63) and cooking own meals (OR = 1.925, 95% CI = 1.14–3.387) were significantly associated with exclusive breastfeeding at birth. Cooking own meals remained statistically significant after adjusting for infant sex (AOR = 1.94, 95% CI = 1.1–5.11). AOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; OR, odds ratio.
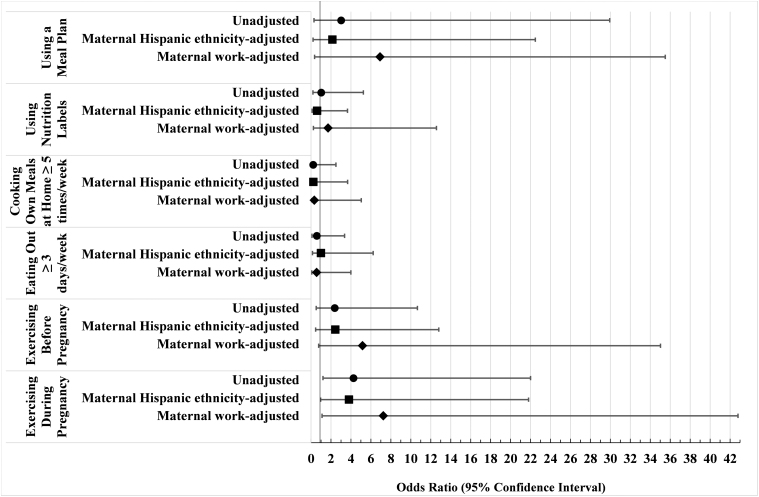
Women who reported exercising during pregnancy had seven times greater adjusted odds of any breastfeeding, partial or exclusive, at 6 months postpartum (AOR = 7.2, 95% CI = 1.10–42.8) compared to women who did not exercise during pregnancy (Fig. 2).
FIG. 2.
Forest plot of unadjusted and adjusted ORs for the association between diabetes-related nutrition and exercise behaviors and any breastfeeding practice at 6 months among women with a history of diabetes in pregnancy. Logistic regression models estimated associations between diabetes-related nutrition behaviors (using a meal plan, using nutrition labels when grocery shopping and cooking, eating out ≥3 times/week, and cooking own meals at home ≥5 times/week) and health behaviors (exercise before pregnancy and exercise during pregnancy) and any breastfeeding practice (partial or exclusive) at 6 months among women with a history of diabetes in pregnancy (N = 34 women with pregestational and gestational diabetes with complete infant feeding data at 6 months). Unadjusted ORs shown with filled circle markers. Adjusted models were each adjusted for maternal Hispanic ethnicity (square markers) and maternal employment (diamond markers). Exercising during pregnancy remained statistically significant after adjusting for maternal employment (AOR = 7.25, 95% CI = 1.1–42.8). AOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
Discussion
In an urban, racially/ethnically and economically diverse sample of women with a history of pregestational diabetes and GDM, cooking own meals almost every day, using nutrition labels when shopping and cooking, and exercise behaviors during pregnancy were associated with infant breastfeeding practice on unadjusted models. These nutritional and behavioral health factors correlate with successful management of diabetes, as well as an overall healthy lifestyle. After adjusting for infant sex and maternal demographic characteristics, cooking own meals and exercise during pregnancy maintained a positively significant association with breastfeeding at birth and any breastfeeding at 6 months of age, respectively. Less is known of influences of nutrition and exercise factors utilized by women with diabetes in pregnancy on infant feeding, and our findings provide novel insights on factors that may impact breastfeeding practice among this medically vulnerable population.
The positive association between cooking own meals and exclusive breastfeeding at birth found in our study suggests that maternal prenatal diet quality may influence breastfeeding exclusivity. Pregnant women cooking their own meals at home are potentially more knowledgeable about the nutritional components of their diets and are more likely to appreciate the benefits of exclusive breastfeeding as the modality to supply all the necessary nutrients to infants in their 1st month of life. While no longer statistically significant on adjusted models in our study, using nutrition labels has been associated with decreased risk for poorer dietary quality,3 elements of healthful dietary behaviors,4 and increased health literacy.5 Health literacy is suggested as a protective factor against breastfeeding cessation.6 Previous studies have shown that mother's dietary quality and nutritional knowledge may influence their breastfeeding initiation and continuation decisions.7
Exercise during pregnancy was significantly associated with any breastfeeding at 6 months, although the wide CI was a likely consequence of the smaller sample size with infant feeding data at that follow-up time point. The World Health Organization and the American College of Obstetricians and Gynecologists recommend at least 150 minutes of moderate-intensity aerobic exercise throughout the week for a healthy pregnancy,8 and exercise has been prescriptive for glucose control in women with pregestational diabetes and GDM.9
Only 17% of women in our sample reported any exercising during pregnancy, less than the estimated national prevalence of 40% of U.S. women with healthy pregnancies.10 Exercise has also been associated with positive emotional and mental health, and women with diabetes who exercise in pregnancy may have improved emotional wellbeing that supports breastfeeding practices postpartum. A qualitative study of prenatal physical activity and breastfeeding decisions reported that mothers believed prenatal exercise behavior, as a continuation of exercise habits before pregnancy, and breastfeeding practice were indirectly connected around themes of healthy lifestyle and health benefits for both mother and baby.11 A study of Vietnamese mothers found a significant association between prenatal physical activity and breastfeeding initiation and duration, possibly due to physical activity behaviors correlating with other healthy lifestyle knowledge and choices.12
Other nutrition-related diabetes management behaviors and reports of exercising before pregnancy were not significantly associated with infant breastfeeding at birth or 6 months in our study. There may be other social support, economic, familial, and maternal health experiences among women with pregestational DM or GDM, which were stronger contributors to infant breastfeeding practice that should be investigated with a larger sample. In previous literature, modifiable factors correlated with breastfeeding to at least 6 months postpartum include breastfeeding intention, self-efficacy, social support, especially from a close family member and/or partner, continuity of breastfeeding education and support, limited pacifier use, and workplace reintegration supports.13–16
Our study was limited by small sample size, which likely inhibited the ability to identify small effects from the other potential predictors and contributed to the wide CI for the estimate of the effect of exercising during pregnancy on any breastfeeding practice at 6 months. There was an absence of information about expressed breast milk feeding, and mothers who reported no breastfeeding may have given their infants expressed breast milk rather than latched the infant. The small sample size and data from a single hospital center limited generalizability of findings. In addition, maternal nutrition and exercise behaviors were self-reported and potentially susceptible to recall and response biases.
Despite the limitations, these preliminary results contribute valuable insights into the role of nutritional and exercise behaviors in shaping breastfeeding practices of women with a history of pregestational diabetes and GDM during pregnancy. Further research on perinatal nutrition and lifestyle health behaviors in larger samples of women with a history of diabetes in pregnancy is necessary to confirm these associations and to inform nutrition- and exercise-related components of future pregnancy and postpartum interventions that support breastfeeding initiation and duration among high-risk women and infants.
Acknowledgments
We thank the families and hospital staff whose participation and support contributed to the parent study efforts.
Authors' Contributions
C.R.F.: Conceptualization, funding, methodology, project administration, formal analysis, and writing–original and revised draft preparation. L.G.: Formal analysis, visualization, and writing–review and editing. C.R.: Investigation and resources. N.Z.: Writing–review and editing. J.B.: Data curation, investigation, and resources. L.C.S.: Funding acquisition, resources, supervision, and writing–review and editing.
Disclaimer
The content is solely the responsibility of authors and does not necessarily represent the official views of the NIH.
Data Accessibility
Given the data utilized for this analysis are part of an ongoing longitudinal study, data may be shared upon reasonable request by contacting senior author Dr. Shuffrey at lcg2129@cumc.columbia.edu.
Disclosure Statement
All authors declare no conflicts of interest.
Funding Information
This research was supported by the National Center for Advancing Translational Sciences (Grant No. KL2TR001874) and L.C.S. is supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant No. K99HD103910), U.S. National Institutes of Health.
References
- 1. De Bortoli J, Amir LH. Is onset of lactation delayed in women with diabetes in pregnancy? A systematic review. Diabet Med 2016;33(1):17–24; doi: 10.1111/dme.12846. [DOI] [PubMed] [Google Scholar]
- 2. Shuffrey LC, Rodriguez C, Rodriguez DJ, et al. Delayed maturation of P2 flash visual evoked potential (VEP) latency in newborns of gestational diabetic mothers. Early Hum Dev 2021;163:105503; doi: 10.1016/j.earlhumdev.2021.105503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilson MD, Ramírez AS, Arsenault JE, Miller LMS. Nutrition label use and its association with dietary quality among Latinos: The roles of poverty and acculturation. J Nutr Educ Behav 2018;50(9):876–887; doi: 10.1016/j.jneb.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Persoskie A, Hennessy E, Nelson WL. US consumers' understanding of nutrition labels in 2013: The importance of health literacy. Prev Chronic Dis 2017;14:E86; doi: 10.5888/pcd14.170066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rothman RL, Housam R, Weiss H, et al. Patient understanding of food labels: The role of literacy and numeracy. Am J Prev Med 2006;31(5):391–398; doi: 10.1016/j.amepre.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 6. Valero-Chillerón MJ, Mena-Tudela D, Cervera-Gasch Á, et al. Influence of health literacy on maintenance of exclusive breastfeeding at 6 months postpartum: A multicentre study. Int J Environ Res Public Health 2022;19(9):5411; doi: 10.3390/ijerph19095411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amir LH, Donath SM. Maternal diet and breastfeeding: A case for rethinking physiological explanations for breastfeeding determinants. Early Hum Dev 2012;88(7):467–471; doi: 10.1016/j.earlhumdev.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 8. ACOG Committee Opinion No. 650: Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet Gynecol 2015;126(6):e135–e142; doi: 10.1097/AOG.0000000000001214. [DOI] [PubMed] [Google Scholar]
- 9. Dipla K, Zafeiridis A, Mintziori G, et al. Exercise as a therapeutic intervention in gestational diabetes mellitus. Endocrines 2021;2(2):65–78; doi: 10.3390/endocrines2020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hesketh KR, Evenson KR. Prevalence of U.S. Pregnant Women Meeting 2015 ACOG Physical Activity Guidelines. Am J Prev Med 2016;51(3):e87–e89; doi: 10.1016/j.amepre.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tucker EA, Fouts HN. Connections between prenatal physical activity and breastfeeding decisions. Qual Health Res 2017;27(5):700–713; doi: 10.1177/1049732316628514. [DOI] [PubMed] [Google Scholar]
- 12. Nguyen PTH, Binns CW, Nguyen CL, et al. Physical activity during pregnancy is associated with improved breastfeeding outcomes: A prospective cohort study. Int J Environ Res Public Health 2019;16(10):1740; doi: 10.3390/ijerph16101740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meedya S, Fahy K, Kable A. Factors that positively influence breastfeeding duration to 6 months: A literature review. Women Birth J Aust Coll Midwives 2010;23(4):135–145; doi: 10.1016/j.wombi.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 14. Muelbert M, Giugliani ERJ. Factors associated with the maintenance of breastfeeding for 6, 12, and 24 months in adolescent mothers. BMC Public Health 2018;18(1):675; doi: 10.1186/s12889-018-5585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernández-Cañadas Morillo A, Durán Duque M, Hernández López AB, et al. A comparison of factors associated with cessation of exclusive breastfeeding at 3 and 6 months. Breastfeed Med 2017;12(7):430–435; doi: 10.1089/bfm.2017.0045. [DOI] [PubMed] [Google Scholar]
- 16. Cummins L, Meedya S, Wilson V. Factors that positively influence in-hospital exclusive breastfeeding among women with gestational diabetes: An integrative review. Women Birth 2022;35(1):3–10; doi: 10.1016/j.wombi.2021.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Given the data utilized for this analysis are part of an ongoing longitudinal study, data may be shared upon reasonable request by contacting senior author Dr. Shuffrey at lcg2129@cumc.columbia.edu.