Abstract
The neurotransmitter acetylcholine (ACh) plays a central role in the regulation of multiple cognitive and behavioral processes, including attention, learning, memory, motivation, anxiety, mood, appetite and reward. As a result, understanding ACh dynamics in the brain is essential for elucidating the neural mechanisms underlying these processes. In vivo measurements of ACh in the brain have been challenging due to low concentrations and rapid turnover of this neurotransmitter. Here we review a number of techniques that have been developed to measure ACh levels in the brain in vivo. We follow this with a deeper focus on use of genetically-encoded fluorescent sensors coupled with fiber photometry, an accessible technique that can be used to monitor neurotransmitter release with high temporal resolution and specificity. We conclude with a discussion of methods for analyzing fiber photometry data and their respective advantages and disadvantages. The development of genetically-encoded fluorescent ACh sensors is revolutionizing the field of cholinergic signaling, allowing temporally-precise measurement of ACh release in awake, behaving animals. Use of these sensors has already begun to contribute to a mechanistic understanding of cholinergic modulation of complex behaviors.
Graphical Abstract
Measurement of acetylcholine (ACh) dynamics in the brain is essential for understanding a wide range of behavioral processes, including attention, learning, memory, motivation, anxiety, mood, appetite and reward. This review outlines a number of techniques that have been developed to measure ACh levels in the brain in vivo with a deeper focus on use of genetically-encoded fluorescent sensors coupled with fiber photometry, an accessible technique that can be used to monitor neurotransmitter release with high temporal resolution and specificity.
INTRODUCTION
Acetylcholine (ACh) was first discovered by Sir Henry Dale and Otto Loewi in the early 1900s (Tansey 2006). In several landmark experiments, Dale discovered that a substance was released from nerve endings and Loewi later showed that chemical stimulation of the vagus nerve caused a decrease in heart rate, which they attributed to the release of an unknown substance they called “Vagusstoff.” Dale later identified this substance as acetylcholine (ACh), a choline ester that acts as a neurotransmitter in the central and peripheral nervous systems. The discovery of ACh was significant for several reasons. First, it was the first neurotransmitter to be identified, paving the way for further research into the chemical basis of nervous system function. Second, ACh was found to be involved in a wide range of physiological processes, including muscle contraction, autonomic regulation, and cognitive function.
ACh in the periphery
ACh is widely distributed in the peripheral nervous system (PNS) and acts as the primary neurotransmitter at the neuromuscular junction and autonomic ganglia (Burnstock 1978; Wessler et al. 1987). At the neuromuscular junction, ACh binds to the family of nicotinic acetylcholine receptor (nAChRs) ligand-gated ion channels on muscle fibers, initiating muscle contraction (Kuffler, 1949). Inhibiting ACh release or blocking nAChRs can lead to muscle weakness and paralysis, as is seen in myasthenia gravis and botulism (Nicolle 2002). In the autonomic nervous system, ACh activates nAChRs on ganglionic neurons, thereby regulating autonomic responses (Burnstock 1978).In addition to its role as a neurotransmitter, ACh also modulates immune responses and inflammation in the PNS. It inhibits the release of pro-inflammatory cytokines and promotes the differentiation of anti-inflammatory regulatory T cells (Tracey 2007).
ACh in the brain
In the brain, ACh is synthesized by cholinergic neurons and is implicated in attention, learning, memory, motivation, mood, anxiety, appetite, and reward (Mineur & Picciotto 2021). Altered ACh signaling is associated with cognitive dysfunction and mood disorders, including depression and anxiety. ACh signaling also modulates the hypothalamic-pituitary-adrenal (HPA) axis, which plays a role in the stress response (Balkan & Pogun 2018) and alters activity of multiple cells types in brain regions involved in emotion processing, such as the amygdala and prefrontal cortex through various receptors, including both the nAChR and muscarinic ACh receptor (mAChR) families (Likhtik & Johansen 2019; Mineur et al. 2022a). As a result, the cholinergic system has been targeted for drugs to treat several brain disorders (Yu et al. 2014). For example, cholinesterase inhibitors increase ACh levels and are used to treat for cognitive deficits in patients with Alzheimer’s disease (Cummings et al. 2019), whereas both nAChR antagonists and partial agonists have been tested as potential therapeutics for depression and anxiety disorders (Mineur et al. 2009; Rollema et al. 2009; Mineur et al. 2011).
ACh receptors
ACh signals via both nAChRs and G-coupled mAChRs to regulate cognitive and behavioral processes (Hasselmo & Sarter 2011; Picciotto et al. 2012). nAChRS are ligand-gated ion channels composed of five subunits arranged symmetrically around a central pore. α4β2 nAChRs are the most abundant subtype in the brain and modulates both cognitive and emotional processes. This subtype is also essential for the reinforcing properties of nicotine, highlighting their relevance in addiction (Picciotto et al. 1998). α7 nAChRs are involved in learning, memory, and synaptic plasticity. Activation of α7 receptors enhances cognitive functions and has been suggested to be a therapeutic target for neurological and psychiatric disorders, including Alzheimer’s disease and schizophrenia (Ma & Qian 2019; Tregellas & Wylie 2019). mAChRs (M1 through 5) belong to the metabotropic G protein-coupled receptor (GPCR) family (Enz 2007). M1, M3, M5 are Gq-coupled and are therefore excitatory while M2 and M4 are Gi/o-coupled and are inhibitory. mAChRs regulate cognitive processes (Moran et al. 2019), motor function (Valuskova et al. 2018) and behaviors relevant to anxiety and depression (Wohleb et al. 2016; Fogaca et al. 2023).
ACh measurement
Traditional post-mortem approaches have provided valuable insights into ACh distribution and function. However, in vivo methods offer the advantage of allowing real-time evaluation of ACh turn-over, enabling researchers to monitor its release and reuptake under various physiological or pharmacological conditions (Wightman 2006).Thus, quantifying ACh levels in vivo with temporal precision is essential for assessing its role in both fundamental behaviors and pathological conditions.
Despite its importance, measurement of ACh in vivo has been challenging. One reason is that ACh is rapidly metabolized by the enzyme acetylcholinesterase (AChE) (Soreq & Seidman 2001). ACh levels in the brain therefore change rapidly and accurate measurement requires precise timing of sampling. Another challenge is the low concentrations of ACh in the CNS. ACh is present in the brain in the nanomolar range (Chang et al. 2006), making detection difficult. Furthermore, the distribution of ACh and ACh neurons in the CNS is not uniform, and the levels of ACh in different brain regions can vary widely (Li et al. 2018; Picciotto et al. 2012). This heterogeneity makes it difficult to compare ACh levels across brain regions and to draw conclusions about the function of ACh in specific brain circuits. Moreover, ACh is released and modulates neuronal function via both tonic and phasic release. While electrophysiological recording is suitable for evaluating both tonic and phasic firing of cholinergic neurons, most available methods for ACh detection primarily assess tonic levels (ie, slow methods) and are not suitable for detecting phasic events.
Finally, the complex interactions between ACh and other neurotransmitters, such as dopamine, norepinephrine, and serotonin, (Perry et al. 2002; Mineur et al. 2018), further complicate the measurement of ACh. These interactions can make it difficult to attribute specific cognitive or behavioral processes to changes in ACh levels.
Several in vivo measurement techniques have emerged over the years, offering unique advantages and limitations, including, but not limited to, microdialysis, biosensors, electrophysiological recordings, electrochemical recordings, advanced imaging modalities such as magnetic resonance spectroscopy (MRS) and positron emission tomography (PET) (Wightman 2006; Hyder & Rothman 2017) and more recently, fluorescent sensor-based fiber photometry. Each technique offers different spatial and temporal resolution, invasiveness, and sensitivity, making the choice of an appropriate method contingent on the specific research question and experimental design.
This review will provide an overview of techniques used to measure ACh levels, comparing and contrasting their strengths and weaknesses in vitro and in vivo (see Table 1 for an overview and comparisons). By gaining a thorough understanding of available methods for ACh measurement, researchers can make informed decisions on the most suitable approach for their experimental needs, ultimately contributing to the advancement of our understanding of the role of ACh in circuit-level and behavioral processes. We will focus in most depth on recently developed genetically-encoded fluorescent ACh sensors combined with fiber photometry, and provide a basic walkthrough for researcher interested in using this technique.
Table 1:
Techniques used to measure ACh fluctuations in vivo
| Technique | Analyte detected | Spatial Resolution | Range of sensitivity | Application |
|---|---|---|---|---|
| Positron Emission Tomography (PET) | Can target AChE, VAChT or mAChRs (M1) and nAChRs (α4β2) (indirect estimate of ACh levels or cholinergic activity) | Several mm | Relative to a baseline and dependent on KD of probe targets (Smart et al. 2021) | Mainly clinical but can be used in animal models |
| Magnetic resonance spectroscopy (MRS) | Choline (indirect estimate of ACh levels or cholinergic activity assuming a linear ratio between changes in choline levels and ACh release) | Several mm | Relative to baseline (Lindner et al. 2017) empirically quantified in animal models (Westman et al. 2009) | Mainly clinical but can be used in animal models |
| Microdialysis | Acetylcholine (direct) | Depends on sampling rate; within mm | Depends on the method of analysis of ACh concentration in the sample; subnanomolar range by mass spectroscopy (Keski-Rahkonen et al. 2007) | Clinical (from blood samples or CSF) and animal models in vivo |
| Amperometry/Voltammetry | Choline or acetylcholine (after derivatization) | Sub-mm | Low nanomolar range (Bruno et al. 2006) | Animal models |
| Fiber photometry combined with fluorescent sensors | Acetylcholine (direct via ACh sensors) or activity of cholinergic neurons (indirect via GCaMP sensors) | Sub-mm | Low nanomolar range (Borden et al. 2020; Jing et al. 2020) | Animal models |
TECHNIQUES FOR MEASURING ACETYCHOLINE LEVELS IN BRAIN
Positron Emission Tomography (PET) for In Vivo Measurement of Acetylcholine in human Brain
Positron emission tomography (PET) is a non-invasive imaging technique widely used for in vivo quantification of neurotransmitter, receptor, and enzyme levels in the brain of human subjects and model organisms, including those involved in ACh transmission. PET relies on the administration of radiolabeled tracers that selectively bind to the target molecule, allowing visualization and quantification of its distribution and dynamics in the brain (Phelps 2000). In addition to use in research, PET is used widely in clinical settings.
The development of sensitive and selective PET tracers for ACh imaging has been essential for accurate and reliable measurements of brain cholinergic signaling. ACh is rapidly degraded by acetylcholinesterase (AChE) in the synaptic cleft, and most PET tracers target AChE rather than ACh itself (Kikuchi et al. 2013; Kadir et al. 2008). By quantifying AChE distribution and activity, PET has been used to estimate ACh levels and function in the brain indirectly.
A commonly used AChE-targeting PET tracer is [δ11C]N-methyl-4-piperidyl acetate ([δ 11C]MP4A), which has demonstrated high selectivity and sensitivity for AChE imaging in the brain (Kuhl et al. 1999). Other PET tracers for AChE include [δ δ11C]PMP (N-[ δ 11C]methylpiperidin-4-yl propionate) (Kilbourn et al. 1996;). These tracers have been used to investigate changes in AChE activity and distribution in the brains of individuals with various brain disorders, such as Alzheimer’s disease, Parkinson’s disease, and schizophrenia.
In addition to AChE, PET tracers targeting other components of the cholinergic system, such as the vesicular acetycholine transporter (VAChT), or nicotinic and muscarinic ACh receptors, have also been developed. For example, [δ 18F]FEOBV (fluoroethoxybenzovesamicol ) binds to VAChT (Mulholland et al. 1998) while [δ 18F]FP-TZTP (3-(3-[^18F]fluoropropylthio)-1,2,5-thiadiazol-4-yl-1,2,5,6-tetrahydro-1-methylpyridine) and [δ 11C]AF150(S) are selective tracers for muscarinic M2 and M1 receptors, respectively (Ravasi et al. 2012; Buiter et al. 2013). Similarly, [δ 18F]AZAN and [δ 18F]Nifene or [123I]5-IA-A85380 are tracers for the α4β2 nicotinic ACh receptor subtype (Hillmer et al. 2011; Wong et al. 2013; Mukhin et al. 2000) that have been used to investigate cholinergic risk factors in schizophrenia and depression (Saricicek et al. 2012; Hannestad et al. 2013; Esterlis et al. 2014) or in response to manipulations of the cholinergic system (Esterlis et al. 2013) in human subjects. [123I]5-IA-A85380 is of particular interest since it is a competitive antagonist of the ACh binding site on α4β2 nicotinic ACh receptors, so that receptor occupancy by endogenous ACh can be inferred from binding availability for the tracer (Esterlis et al. 2013). This tracer has therefore been used to estimate levels of ACh in the brains of individuals with mood disorders, based on comparison with binding to postmortem brain tissue (Saricicek et al. 2012; Hannestad et al. 2013; Esterlis et al. 2014).
PET offers several advantages for in vivo assessment of ACh function in the brain. This non-invasive technique allows for longitudinal studies, making it possible to monitor changes in the cholinergic system over time and in response to therapeutic interventions. PET can also provide whole-brain coverage, enabling the assessment of ACh-related targets in different brain regions and across various neural networks. However, PET has relatively low temporal resolution compared to methods such as electrochemical techniques that can be used in model organisms. In addition, specialized radiochemistry facilities are necessary to generate PET tracers and most tracers have a short half-life (δ11C (T_1/2 ≈ 20.4 min) or 123I (T_1/2 ≈13.2 hours)) for safe use in human subjects. Even so, PET imaging involves the use of ionizing radiation which limits the frequency and duration of longitudinal studies, particularly in vulnerable populations. To address the limitations of PET for in vivo ACh measurement, researchers are focusing on the development of novel PET tracers with improved selectivity, sensitivity, and pharmacokinetics. For example, efforts are being made to develop AChE-targeting tracers labeled with longer-lived radioisotopes, such as δ18F (T_1/2 ≈ 110 min), to facilitate more flexible imaging protocols (Ishibashi et al. 2018).
Another area of interest is the development of PET tracers targeting vesicular ACh transporters (VAChT), which play a critical role in ACh storage and release. The development of VAChT-targeting tracers, such as [δ18F]VAT, may provide a more direct measure of ACh release and function in the brain including in clinical settings (Yue et al. 2016; Karimi et al. 2015)
Use of Magnetic resonance spectroscopy (MRS) to Measure ACh in Human Brain
Magnetic resonance spectroscopy (MRS) is used to measure ACh levels in vivo by detecting the characteristic magnetic resonance signals of choline in the brain (MRS) (Govindaraju et al. 2000). Choline and ACh levels are highly interdependent in the brain, with changes in the levels of the precursor/metabolite choline linearly related to levels of ACh. Accordingly, quantifying choline levels can serve as a reliable indicator to assess cholinergic turnover in the central nervous system (Loffelholz et al. 1993). MRS offers several advantages, such as non-invasiveness, high spatial resolution, and the ability to detect choline in specific brain regions. MRS can also be used to measure other neurotransmitters and metabolites simultaneously, providing a comprehensive understanding of biochemical changes in the brain (Henry et al. 2001). MRS relies on the same principles as magnetic resonance imaging (MRI), but instead of detecting signals from water molecules, MRS measures signals from specific molecules of interest, such as choline. Choline signals can be estimated using proton MRS (1H-MRS) or phosphorus MRS (δ31P-MRS). δ1H-MRS detects the magnetic resonance signals of the protons in choline, while δ31P-MRS detects the signals from the phosphorus atom in choline.
δ1H-MRS is the more commonly used technique for choline detection, as it offers higher sensitivity and better spatial resolution, however, it is limited by the presence of overlapping signals from other molecules in the brain. To overcome this limitation, researchers have developed advanced techniques such as two-dimensional (2D) δ1H-MRS and δ1H-difference spectroscopy (Bogner et al. 2017). These techniques allow for the separation and quantification of choline signals from other overlapping signals, providing a more accurate measurement of choline levels in the brain.
δ31P-MRS is less commonly used for choline detection due to its lower sensitivity and spatial resolution. However, like other MRS techniques, it offers the advantage of measuring ACh metabolism, which can provide insights into the underlying biochemical mechanisms of ACh dysregulation in neurological disorders (Lindner et al. 2017). One limitation of MRS is that it requires specialized equipment and expertise to perform, making it less accessible than other techniques. In addition, MRS is limited by the signal-to-noise ratio, which can be affected by factors such as magnetic field strength and tissue composition. Despite these limitations, MRS is a valuable technique for investigating the role of ACh in human brain function and pathology (Kintner et al. 2000; Eliassen et al. 2008).
Future advances in MRS technology and data analysis techniques could enhance the sensitivity and specificity of ACh detection itself, allowing for a more comprehensive understanding of ACh dynamics in the brain. In addition, combining MRS with other imaging techniques, such as functional MRI (fMRI), could provide insights into the relationship between ACh levels and brain activity (Logothetis 2008).
Microdialysis
One of the most widely used techniques to measure ACh in vivo has been microdialysis, which involves the insertion of a small probe into the brain to collect extracellular fluid, allowing continuous monitoring of ACh levels. Although microdialysis has poor temporal resolution (several minutes) compared to the rapid changes in behavior mediated by the neurotransmitter (Giovannini et al. 2001), it is particularly valuable for measuring tonic ACh levels in vivo. After fluid collection, techniques such as high-performance liquid chromatography (HPLC) coupled with electrochemical detection (ECD) allow for the identification and quantification of ACh (Beley et al. 1987). The sensitivity of HPLC-ECD has been improved significantly with the use of advanced electrode materials and derivatization techniques (David et al. 2021).
In vivo microdialysis involves implantation of a semi-permeable membrane, into the brain region of interest. A perfusion fluid is pumped through the probe continuously, allowing ACh to diffuse across the membrane while minimizing passage of other molecules (Giovannini et al. 2001). Commonly used membrane materials for ACh microdialysis include polyacrylonitrile (PAN), cellulose ester, and polycarbonate (Chefer et al. 2009) all of which have suitable molecular weight cut-offs, allowing diffusion of ACh while restricting passage of larger molecules.
Membrane length and diameter play essential roles in recovery efficiency of ACh. Longer membranes provide a larger surface area for diffusion, potentially increasing recovery efficiency. However, this also increases the risk of tissue damage during probe insertion (Chefer et al. 2009). In contrast, shorter membranes minimize tissue damage but may result in lower recovery efficiency. Therefore, membrane length/recovery must be balanced against risk of tissue damage. Typically, membrane lengths of 1–2 mm and diameters of 200–500 μm are used for ACh microdialysis.
To prevent the rapid degradation of ACh by AChE in the dialysate, cholinesterase inhibitors, such as neostigmine or physostigmine, are often added to the perfusion fluid (Giovannini et al. 2001). A significant concern is that excessive inhibition of AChE could lead to artificially elevated ACh levels in the dialysate. Once again, a balance between enhanced signal-to-noise by addition of AChE inhibitors has to be balanced against perturbation of ACh levels and signaling by these enzymes.
Microdialysis is often used to measure relative levels of brain ACh following some perturbation or behavioral process, however, when differences in basal extracellular ACh levels across individuals may differ, no-net-flux microdialysis can be employed (Laplante et al. 2004). This technique involves inclusion of known concentrations of the analyte (in this case ACh) in the perfusate coupled with online detection, allowing determination of the concentration at which ACh influx is balanced with efflux across the dialysis membrane. The precise quantification of baseline ACh concentration is an advantage of no-net-flux microdialysis over other methods of in vivo ACh detection, which is particularly relevant for tonic ACh levels.
Following recovery, the collected dialysate must be analyzed to determine the concentration of ACh. Several analytical techniques have been used for evaluation of ACh concentration, each with advantages and limitations. Some common techniques include high-performance liquid chromatography (HPLC), capillary electrophoresis (CE) and microbiosensor-based detection. HPLC coupled with electrochemical detection (ECD) is widely used for sensitive and selective detection of ACh in microdialysate samples (Zapata et al. 2009) because it allows ACh to be separated from other molecules in the dialysate, and the detector selectively measures ACh based on its electrochemical properties (Zackheim & Abercrombie 2003). This method typically requires a derivatization step to convert ACh into an electroactive compound, such as acetylthiocholine, due to resistance of the ACh molecule to electrochemical oxidation (Perry et al. 2009). Capillary electrophoresis with laser-induced fluorescence (CE-LIF) detection involves separation of ACh from other molecules in the dialysate based on electrophoretic mobility (Bergquist et al. 1996). ACh is detected using LIF after derivatization with a fluorescent label. CE-LIF provides high separation efficiency and sensitivity, but can require more complex sample preparation and optimization of the derivatization procedure (Nguyen & Kang 2019). Finally, microbiosensors are miniature electrochemical devices that can be used for the direct measurement of ACh in microdialysate samples (Yang et al. 2005). These sensors consist of an enzyme, such as AChE or choline oxidase, immobilized on an electrode surface, which catalyzes the conversion of ACh into electroactive products. The resulting current is proportional to ACh concentration in the sample. Microbiosensors offer advantages such as rapid detection, high sensitivity, and minimal sample preparation. However, they may be subject to interference from other electroactive species present in the dialysate (Karube et al. 1990). Strategies that reduce this confound have been developed (Schuvailo et al. 2005), but this method is less selective than HPLC-ECD and CE-LIF.
While microdialysis remains a powerful technique for measuring ACh levels in vivo (Damsma et al. 1987), several challenges still remain. The spatial resolution of microdialysis is limited by the probe size and is often in the millimeter range, which may not accurately represent the local ACh concentration in small brain regions or across different cell populations. In addition, the temporal resolution of microdialysis is limited by factors such as probe recovery efficiency, perfusion rate, and the time required for sample analysis (Konig et al. 2018). These factors may prevent detection of rapid changes in ACh concentration, since even the most efficient ACh detection methods still require a few minutes of sampling which are not suitable to detect rapid ACh concentration changes that are behaviorally relevant.
Recent developments in microdialysis probe design, such as the use of smaller, more biocompatible materials, more sensitive and selective detectors and the integration of biosensors into the probe, may help address some of these challenges (Stangler et al. 2021). For example, recent developments in mass spectrometry, such as liquid chromatography-tandem mass spectrometry (LC-MS/MS), have allowed for the simultaneous quantification of multiple neurotransmitters, including ACh, in a single analysis (Becker et al. 2023).
Electrochemical techniques: Amperometry/Voltammetry
As noted above, one challenge for the detection of ACh in vivo with high temporal resolution is its low concentration in the brain. ACh levels in the CNS are typically in the range of 0.1–1 μM, while ACh concentrations are higher in the PNS, around 10–100 μM (Meriney & Fanselow 2019). Moreover, fast metabolism by AChE limits the time window for detection of ACh after release (Picciotto et al. 2012). To overcome these challenges, several electrochemical methods have been developed, including several versions of voltammetry (Armstrong-James & Millar 1979; Baur et al. 1988; Schuvailo et al. 2005; Asri et al. 2016), and amperometry (Parikh et al. 2004; Parikh et al. 2007), to measure ACh in the brain with greater spatial and temporal (seconds) resolution. These techniques involve the use of enzyme-coated electrodes to measure ACh release directly in specific brain regions (Bruno et al. 2006).
Fast Scan Voltammetry (FSCV) can detect rapid changes in electroactive analytes with high temporal resolution. FSCV involves application of a waveform potential (constant or varying intensity) to the working electrode at a high scan rate revealing the reduction potential of the molecules in contact with the electrode. When the lysate is oxidized at the electrode surface, it produces a characteristic oxidation peak proportional to the concentration of the analyte in the sample. One advantage of FSCV is its ability to distinguish different electroactive species, such as dopamine and serotonin (Rodeberg et al. 2017; Espin et al. 2021). By using different waveform potentials, the oxidation potentials of these species can be separated. ACh, however, is not electroactive and its direct detection is generally not possible; thus, ACh generally requires enzymatic conversion at the electrode to be detected, although recent methodological developments may overcome this hurdle via non-enzymatic processes (Mohammadi et al. 2018).
Amperometry has also been used to measure ACh levels in vivo with high sensitivity, selectivity, and rapid response times. Amperometry also relies on oxidation of the target analyte (ACh or its derivatives), using a fixed current to generate an electrical signal that can be correlated directly with the concentration of the analyte in the sample (Adeloju 2005). Amperometric detection of ACh typically involves conversion of ACh to choline and acetate, which occurs at a potential of approximately +0.7 V vs. a reference electrode (Bruno et al. 2006). As is the case for FSCV, ACh requires enzymatic conversion at the electrode to be detected.
Carbon fiber microelectrodes (CFMEs) are now generally used for in vivo amperometric and voltametric detection due to their small size, electrochemical properties, and biocompatibility. In comparison to traditional macroelectrodes that were originally used for rapid detection of ACh in vivo, (Bruno et al. 2006; Parikh et al. 2007; Howe et al. 2010), CFMEs exhibit faster response times and lower background currents. CFMEs can be fabricated with tip diameters of less than 10 μm (Espin et al. 2021), which minimizes tissue damage upon implantation and allows detection of ACh in smaller brain regions than microdialysis (~1 mm) or macroelectrodes (~1–3 mm).
To develop electrochemical methods suitable for ACh detection, enzyme-modified electrodes have been developed. These electrodes are coated with both AChE, which hydrolyzes ACh to choline and acetic acid, and choline oxidase (ChOx), which subsequently converts choline to betaine and hydrogen peroxide (H2O2). The H2O2 generated can then be detected at the CFME, providing a highly selective and quantifiable signal (Sanford et al. 2010). Enzyme-modified electrodes exhibit excellent sensitivity and selectivity for ACh detection in vivo, with detection limits as low as 10 nM (Bruno et al. 2006)
Recent advances in the fabrication of enzyme-modified CFMEs include development of multi-enzyme electrodes that incorporate additional enzymes, such as catalase, to improve the stability and sensitivity of the sensor by removing endogenous H2O2. Nanoparticle-based enzyme immobilization strategies have also been employed to increase enzyme loading on the electrode surface and improve sensor response time (Tyagi et al. 2019). These improvements could enable real-time monitoring of ACh during various physiological and pharmacological conditions (Ahlawat et al. 2023).
Another critical aspect of in vivo amperometric detection of ACh is the development of implantable sensors (Reid & Finnerty 2017) that can continuously monitor ACh levels for extended periods of time. These sensors must be biocompatible, stable, and able to provide reliable measurements even after gliosis, biofouling and other biological challenges. Several approaches have been investigated to address these issues, including the use of biocompatible polymers to encapsulate enzyme-modified CFMEs (Hanssen et al. 2016) as well as stabilization strategies than could be used to keep electrodes potent (Hou et al. 2012).(Kucherenko et al. 2019).
Despite significant progress in development of amperometric sensors for in vivo ACh detection, several challenges remain. These include further improvement of selectivity in the presence of interfering species and long-term stability of the enzyme coatings.
Detection of Genetically-Encoded Fluorescent Sensors with Fiber photometry
More recently, development of genetically-encoded fluorescent sensors, coupled with in vivo fiber photometry (FP), has allowed measurement of ACh release in the brain with high temporal resolution and with relatively easy implementation (Jing et al. 2018). One approach consists of genetically modifying cholinergic neurons to express a calcium-sensitive protein (GCaMP)(Chen et al. 2013) that fluoresces in response to changes in intracellular calcium levels. The modified neurons are then targeted with an optical fiber, which is used to measure changes in fluorescence in response to activity changes leading to ACh release (Crouse et al. 2020). In this context, levels of GCaMP fluorescence are interpreted as activation of cholinergic neurons and can be used as a proxy for ACh release. More directly, genetically encoded ACh sensors have been developed that allow for direct measurement of ACh release in the brain with high sensitivity and specificity (Jing et al. 2018; Jing et al. 2020). One of these biosensors, GPCR-activation-based acetylcholine sensor (GRAB-ACh), is a modified muscarinic acetylcholine receptor M3 (M3R) with the insertion of a circularly permuted green fluorescent protein (cpGFP). Upon ACh binding to the M3R, conformational changes are induced that alter the fluorescence properties of cpGFP, allowing for the real-time detection of ACh levels in vivo. An alternative approach involves the development of a sensor based on periplasmic binding proteins (PBP), combined with an intensity-based fluorescent reporter for acetylcholine sensing known as iAChSnFR (Borden et al. 2020). This PBP-sensor exhibits rapid activation and decay kinetics at the millisecond level and is independent of most cholinergic drugs.
Fiber photometry (FP) is used to measure in vivo fluorescence from dyes or genetically-encoded fluorescent sensors (Gunaydin & Deisseroth 2014) and is increasingly used for continuous recording of neural activity in freely moving animals. FP is particularly advantageous for investigating ACh signaling in the brain, as it allows subsecond measurement of ACh dynamics in small brain regions. Use of FP to detect ACh fluctuations via fluorescent sensors involves implantation of an optical fiber into the brain region of interest, and delivery of light through the fiber to excite the fluorescent indicator(s) expressed in target neurons. The resulting fluorescence emission is then collected through the same fiber and detected by a sensitive photodetector. The intensity of the fluorescence signal is directly proportional to the concentration of the analyte (ACh, in this case) in the vicinity of the indicator (Jing et al. 2018; Jing et al. 2020; Crouse et al. 2020; Mineur et al. 2022b; Mineur et al. 2022a).
The development of genetically encoded ACh sensors has greatly improved the selectivity and sensitivity of fiber photometry for ACh detection (Jing et al. 2018; Jing et al. 2020). GRAB_ACh sensors have been optimized for various properties, including affinity, dynamic range, and brightness, enabling their application in a wide range of experimental settings. Other sensor such as iAChRSnFR were also developed with millisecond-level activation and decay kinetics, and showed to be orthogonal to most cholinergic drugs (Borden et al. 2020).
The combination of fiber photometry with fluorescent ACh sensors has enabled real-time monitoring of ACh release and uptake in the brain during various physiological and pharmacological conditions. For example, fiber photometry with GRAB_ACh sensors has been used to investigate ACh dynamics in the striatum, hippocampus, cortex and amygdala during stressful challenges and memory tasks, revealing the role and pattern of ACh signaling in these processes (Crouse et al. 2020; Lohani et al. 2022; Mineur et al. 2022b; Mineur et al. 2022a; Skirzewski et al. 2022). Furthermore, this approach has been applied to study the effects of pharmacological manipulation of ACh signaling on neuronal activity and behavior (Jing et al. 2020).
Despite the advantages of fiber photometry and fluorescent sensors for in vivo ACh detection, several challenges remain. One key challenge is that ACh levels measured at the fiber location is the result of release from a large number of neuronal terminals adjacent to the fiber, likely from multiple neurons. This method also does not indicate whether the ACh released is sufficient to alter activity of local neurons, since the sensors are generally expressed broadly in the target brain area and may not reflect the site of endogenous ACh receptors. Placement of the fiber also requires surgery and the diameter of the fiber required to detect sufficient fluorescent signal is significant (50 to 600 μm), which may result in tissue damage. Further, there is still room to improve ACh sensor kinetics and dynamic range for more accurate measurements of ACh levels in vivo. Finally, development of sensors with different spectral properties has recently enabled simultaneous monitoring levels of multiple neurotransmitters or signaling molecules simultaneously (Lohani et al. 2022). Furthermore, recent ex vivo studies have demonstrated the feasibility of recording from sensors in conjunction with cell-specific electrophysiological measurements. This technique allows for the detection of single-cell events and their correlation with ACh release. Moreover, electrophysiological recordings enable the detection of post-synaptic events, while ACh sensors assess presynaptic release from a pool of ACh neurons. By employing this multianalyte approach, a more comprehensive understanding of cholinergic turnover and its downstream neuronal effects can be achieved (Zhang et al. 2023).
For new users unfamiliar with FP, there may be several challenges in measuring ACh using this technique related to experimental design, equipment setup, data acquisition, and data analysis. FP experiments require fiber-coupled light sources, detectors, and optical fibers. In addition, FP signals are influenced by parameters such as light intensity, excitation wavelength, and integration time that must be optimized to obtain high-quality signals while minimizing background noise and photobleaching. Motion, heartbeat/hemoglobin artifacts, and autofluorescence can result in artefactual signals, and appropriate strategies to minimize their impact, such as filtering or artifact removal algorithms, must be used. Similarly, changes in temperature, pH, or other physiological parameters can affect the properties of the sensors (Creamer et al. 2022; Lerner et al. 2015; Zhang et al. 2022). Finally, FP data require specialized analysis techniques, such as deconvolution and baseline correction, and the choice of analysis software and parameters can affect data interpretation; however, a number of recent publications have described pipelines for data processing that can be a good starting point for design of analysis strategies (Lerner et al. 2015; Sherathiya et al. 2021; Bruno et al. 2021; Murphy et al. 2023).
FP hardware
Hardware components of a fiber photometry system include a light source, fiber optic cannula, and photodetector and must be optimized for high signal-to-noise ratios and minimal interference. Light sources such as LEDs, lasers, and arc lamps can be used, with LEDs preferred for their stability and low cost. The choice of excitation wavelength is crucial to optimize sensitivity and selectivity. The fiber optic cannula delivers the excitation light and collects the emitted fluorescence, and the material choice is important to prevent adsorption of the target molecule. Photodetectors like photomultiplier tubes (PMTs) and silicon photomultipliers (SiPMs) detect and measure the emitted fluorescence, with SiPMs being suitable for miniaturization and implantable devices.
FP signal analysis
Signal analysis in fiber photometry involves processing the raw fluorescence data. The data reflect concentration changes of the target molecule over time and provide valuable information on temporal dynamics. The noise level and signal-to-noise ratio determine sensitivity and precision and can be influenced by various factors. Processing steps include filtering, baseline correction, and normalization. Filtering removes noise and drifts, baseline correction corrects for gradual changes, and normalization allows for comparison across conditions. The ΔF/F method is commonly used for normalization. The choice of reference signal for normalization depends on the experimental design, and the isosbestic signal is a good starting point.
Pre-processing steps in fiber photometry data analysis involve background subtraction and artifact removal. Background subtraction accounts for baseline fluorescence unrelated to the target molecule, and artifact removal removes noise and interference. Techniques like filtering and regression can be used for artifact removal, whereas manual artifact removal may be necessary for specific cases. Baseline correction is a crucial step in analyzing fiber photometry data to remove slow changes unrelated to neural activity. Polynomial functions like linear, quadratic, and cubic fits can be used, but linear regression is generally preferred. The choice of baseline correction method depends on the experimental conditions and baseline fluctuations. It is important to evaluate the need for baseline correction as it may remove important information.
Software packages like PhAT(Murphy et al. 2023), pMAT (Bruno et al. 2021), and Guppy (Sherathiya et al. 2021) are available for signal analysis in fiber photometry studies. They provide tools for signal processing, baseline correction, and statistical analysis. Each software package has its own advantages and features for data analysis and visualization.
Conclusions and future directions
Levels of ACh have been measured in vitro and in vivo using multiple methods for many decades. Techniques such as PET, MRS, microdialysis, voltammetry/amperometry and FP/genetically-encoded fluorescent sensors provide different levels of invasiveness, molecular specificity, spatial resolution and temporal precision. Each technique has strengths and weaknesses, as well as uses in human subjects or animal models. Recent advances in development of genetically-encoded fluorescent ACh sensors provide a method to measure behaviorally-relevant changes in ACh signaling with high molecular precision in defined brain areas.
Despite their advantages, there are some situations where use of genetically-encoded fluorescent ACh sensors may not be appropriate. One caveat is that virally-expressed genetically-encoded ACh sensors are expressed on cell types that may represent only a subset of targets of endogenous ACh signaling or, alternatively, cells that generally do not express ACh receptors and do not normally respond to ACh release. In addition, expression levels of the ACh sensor may vary between animals infused with viral constructs. With proper normalization and Z-scoring, data can be combined across animals, but this may not be suitable for detection of low concentrations of ACh. This could also be overcome by using transgenic or knockin mice expressing an ACh sensor.
Perhaps most important at this time, currently-available fluorescent ACh sensors have limited temporal resolution compared to electrophysiological recording or FSCV/Amperometry. This means that current genetically-encoded fluorescent ACh sensors may not be suitable for studying rapid (millisecond) changes in ACh release, such as those that occur during phasic firing of ACh neurons or during attentional tasks that require rapid changes in ACh levels (Parikh et al., 2013; Jennings et al., 2015). Ongoing progress in engineering novel sensors and methods of detection have resulted in very rapid advances in development of novel sensors which is likely to continue to advance the field of in vivo ACh detection. These techniques are poised to revolutionize our understanding of the function of ACh signaling in health and disease.
Figure 1: Schematic representation of an ACh release site and the targets for in vivo recording of ACh levels and cholinergic activity.
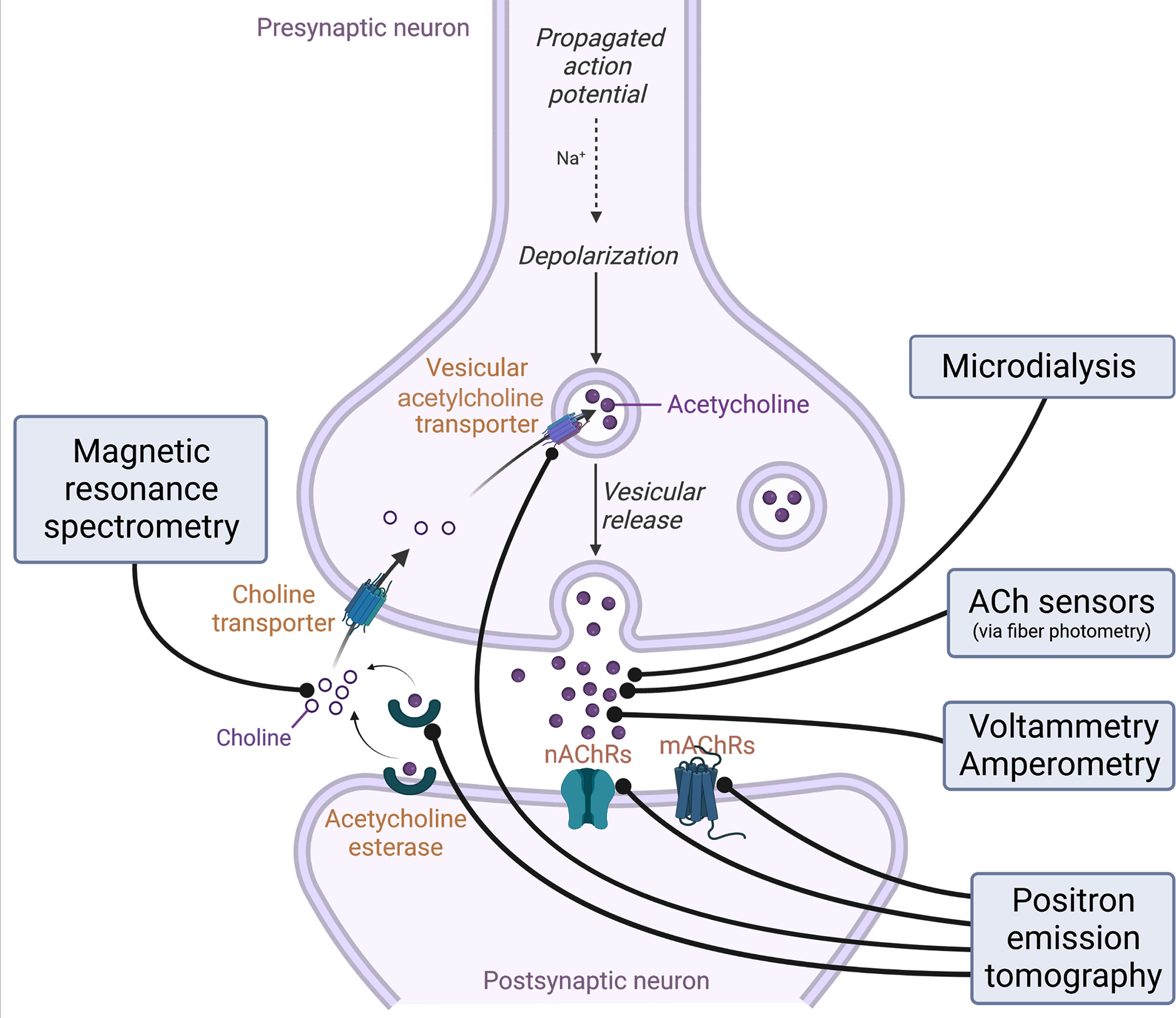
An example presynaptic cholinergic terminal is diagrammed, along with a generic postsynaptic cholinoceptive cell, with labeling of targets for several available methods of measuring ACh levels and activity. Positron emission tomography (PET) involves infusion of radioalabeled ligands that tag ACh binding sites followed by scanning, allowing estimation of various proxies for ACh levels and activity. Of note, some of these probes can compete for binding of ACh, and thus binding availability may represent either changes in levels of the binding site or changes in levels of endogenous ACh. Magnetic resonance imaging (MRS) can be used to detect the specific resonance spectrum of choline, a precursor of ACh whose levels are linearly related to ACh concentration and are altered in response to ACh release. Microdialysis samples fluid in the extracellular space and ACh levels are subsequently quantified using biochemical methods. Fiber photometry (FP) can be used to detect fluorescence changes due to calcium in the presynaptic terminal, as a proxy for activity of the cholinergic neurons, or as a result of ACh binding to fluorescence-based ACh sensors. Voltammetry and amperometry are used to detect specific electrochemical signatures from choline or ACh in the extracellular space.
Acknowledgements
This study was supported by grants DA050986, DA014241, MH077681, AA027989 and DA036151. This work was funded in part by the State of Connecticut, Department of Mental Health and Addiction Services, but this publication does not express the views of the Department of Mental Health and Addiction Services or the State of Connecticut.
Abbreviation list:
- [123I]5-IA-A85380
[123I]-5-iodo-3-[2(S)-azetidinylmethoxy]pyridine
- [δ 11C]AF150(S)
(2,8-dimethyl-1-thia-3,8-diaza-spiro[4.5]dec-2-ene
- [δ 11C]MP4A
[δ11C]N-methyl-4-piperidyl acetate
- [δ 18F]AZAN
(−)-7-Methyl-2-exo-[3’-(6-[18F]fluoropyridin-2-yl)-5’-pyridinyl]-7-azabicyclo[2.2.1]heptane[δ 18F]
- FEOBV
fluoroethoxybenzovesamicol
- [δ 18F]FP-TZTP
3-(3-[^18F]fluoropropylthio)-1,2,5-thiadiazol-4-yl-1,2,5,6-tetrahydro-1-methylpyridine
- [δ 18F]Nifene
3-[[(2S)-2,5-dihydro-1H-pyrrol-2-yl]methoxy]-2-(18F)fluoranylpyridine
- [δ δ11C]PMP
N-[ δ 11C]methylpiperidin-4-yl propionate
- [δ18F]VAT
(2R,3R)-3-[4-(4-fluorobenzoyl)piperidin-1-yl]-5-[2-(18F)fluoroethoxy]-1,2,3,4-tetrahydronaphthalen-2-ol
- ACh
acetylcholine
- AChE
acetylcholinesterase
- CE
capillary electrophoresis
- CE-LIF
Capillary electrophoresis with laser-induced fluorescence
- CFME
Carbon fiber microelectrodes
- ChOx
choline oxidase
- cpGFP
circularly permuted green fluorescent protein
- ECD
electrochemical detection
- fMRI
functional MRI
- FP
fiber photometry
- FP
fiber photometry
- FSCV
Fast Scan Voltammetry
- GCaMP
calcium-sensitive protein
- GRAB-ACH
GPCR-activation-based ACh
- H2O2
hydrogen peroxide
- HPLC
high-performance liquid chromatography
- iACh SnFR
ACh sensing fluorescent reporter
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- mAChR
muscarinic acetylcholine receptor
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- nAChR
nicotinic acetylcholine receptor
- PAN
polyacrylonitrile
- PBP
periplasmic binding protein
- PET
positron emission tomography
- PMT
photomultiplier tubes
- PNS
peripheral nervous system
- SiPMs
silicon photomultipliers
- vAChT
vesicular ACh transporter
Footnotes
Conflict of Interest Statement
Marina Picciotto is a former Editor with the Journal of Neurochemistry. The authors have no conflicts of interest to report.
Conflict of interest disclosure:
All experiments were conducted in compliance with the ARRIVE guidelines.
Involves human subjects:
Informed consent was achieved for all subjects, and the experiments were approved by the local ethics committee.
REFERENCES
- Adeloju SB (2005) AMPEROMETRY. In: Encyclopedia of Analytical Science (Second Edition), (Worsfold P, Townshend A and Poole C eds.), pp. 70–79. Elsevier, Oxford. [Google Scholar]
- Ahlawat J, Sharma M and Pundir CS (2023) An Amperometric Acetylcholine Biosensor Based on Co-Immobilization of Enzyme Nanoparticles onto Nanocomposite. Biosensors (Basel) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-James M and Millar J (1979) Carbon fibre microelectrodes. J Neurosci Methods 1, 279–287. [DOI] [PubMed] [Google Scholar]
- Asri R, O’Neill B, Patel JC, Siletti KA and Rice ME (2016) Detection of evoked acetylcholine release in mouse brain slices. Analyst 141, 6416–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkan B and Pogun S (2018) Nicotinic Cholinergic System in the Hypothalamus Modulates the Activity of the Hypothalamic Neuropeptides During the Stress Response. Curr Neuropharmacol 16, 371–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JE, Kristensen EW, May LJ, Wiedemann DJ and Wightman RM (1988) Fast-scan voltammetry of biogenic amines. Anal Chem 60, 1268–1272. [DOI] [PubMed] [Google Scholar]
- Becker S, Schulz A, Kreyer S, Dressler J, Richter A and Helmschrodt C (2023) Sensitive and simultaneous quantification of 16 neurotransmitters and metabolites in murine microdialysate by fast liquid chromatography-tandem mass spectrometry. Talanta 253, 123965. [DOI] [PubMed] [Google Scholar]
- Beley A, Zekhnini A, Lartillot S, Fage D and Bralet J (1987) Improved Method for Determination of Acetylcholine, Choline, and Other Biogenic Amines in a Single Brain Tissue Sample Using High Performance Liquid Chromatography and Electrochemical Detection. Journal of Liquid Chromatography 10, 2977–2992. [Google Scholar]
- Bergquist J, Vona MJ, Stiller CO, O’Connor WT, Falkenberg T and Ekman R (1996) Capillary electrophoresis with laser-induced fluorescence detection: a sensitive method for monitoring extracellular concentrations of amino acids in the periaqueductal grey matter. J Neurosci Methods 65, 33–42. [DOI] [PubMed] [Google Scholar]
- Bogner W, Hangel G, Esmaeili M and Andronesi OC (2017) 1D-spectral editing and 2D multispectral in vivo(1)H-MRS and (1)H-MRSI - Methods and applications. Anal Biochem 529, 48–64. [DOI] [PubMed] [Google Scholar]
- Borden PM, Zhang P, Shivange AV et al. (2020) A fast genetically encoded fluorescent sensor for faithful in vivo acetylcholine detection in mice, fish, worms and flies. bioRxiv, 2020.2002.2007.939504. [Google Scholar]
- Bruno CA, O’Brien C, Bryant S, Mejaes JI, Estrin DJ, Pizzano C and Barker DJ (2021) pMAT: An open-source software suite for the analysis of fiber photometry data. Pharmacol Biochem Behav 201, 173093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JP, Gash C, Martin B, Zmarowski A, Pomerleau F, Burmeister J, Huettl P and Gerhardt GA (2006) Second-by-second measurement of acetylcholine release in prefrontal cortex. Eur J Neurosci 24, 2749–2757. [DOI] [PubMed] [Google Scholar]
- Buiter HJ, Windhorst AD, Huisman MC, Yaqub M, Knol DL, Fisher A, Lammertsma AA and Leysen JE (2013) [11C]AF150(S), an agonist PET ligand for M1 muscarinic acetylcholine receptors. EJNMMI Res 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G (1978) Do some sympathetic neurones synthesize and release both noradrenaline and acetylcholine? Prog Neurobiol 11, 205–222. [DOI] [PubMed] [Google Scholar]
- Chang Q, Savage LM and Gold PE (2006) Microdialysis measures of functional increases in ACh release in the hippocampus with and without inclusion of acetylcholinesterase inhibitors in the perfusate. J Neurochem 97, 697–706. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Thompson AC, Zapata A and Shippenberg TS (2009) Overview of brain microdialysis. Curr Protoc Neurosci Chapter 7, Unit7 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y et al. (2013) Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer MS, Chen KS, Leifer AM and Pillow JW (2022) Correcting motion induced fluorescence artifacts in two-channel neural imaging. PLoS Comput Biol 18, e1010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse RB, Kim K, Batchelor HM et al. (2020) Acetylcholine is released in the basolateral amygdala in response to predictors of reward and enhances the learning of cue-reward contingency. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Tong G and Ballard C (2019) Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. J Alzheimers Dis 67, 779–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsma G, Westerink BH, Imperato A, Rollema H, de Vries JB and Horn AS (1987) Automated brain dialysis of acetylcholine in freely moving rats: detection of basal acetylcholine. Life Sci 41, 873–876. [DOI] [PubMed] [Google Scholar]
- David V, Moldoveanu SC and Galaon T (2021) Derivatization procedures and their analytical performances for HPLC determination in bioanalysis. Biomed Chromatogr 35, e5008. [DOI] [PubMed] [Google Scholar]
- Eliassen JC, Boespflug EL, Lamy M, Allendorfer J, Chu WJ and Szaflarski JP (2008) Brain-mapping techniques for evaluating poststroke recovery and rehabilitation: a review. Top Stroke Rehabil 15, 427–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz A (2007) Muscarinic Acetylcholine Receptors. In: xPharm: The Comprehensive Pharmacology Reference, (Enna SJ and Bylund DB eds.), pp. 1–6. Elsevier, New York. [Google Scholar]
- Espin LX, Asp AJ, Trevathan JK, Ludwig KA and Lujan JL (2021) Integral methods for automatic quantification of fast-scan-cyclic-voltammetry detected neurotransmitters. PLoS One 16, e0254594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterlis I, Hannestad JO, Bois F et al. (2013) Imaging changes in synaptic acetylcholine availability in living human subjects. J Nucl Med 54, 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterlis I, Ranganathan M, Bois F et al. (2014) In vivo evidence for beta2 nicotinic acetylcholine receptor subunit upregulation in smokers as compared with nonsmokers with schizophrenia. Biol Psychiatry 76, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogaca MV, Wu M, Li C, Li XY, Duman RS and Picciotto MR (2023) M1 acetylcholine receptors in somatostatin interneurons contribute to GABAergic and glutamatergic plasticity in the mPFC and antidepressant-like responses. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini MG, Rakovska A, Benton RS, Pazzagli M, Bianchi L and Pepeu G (2001) Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience 106, 43–53. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K and Maudsley AA (2000) Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed 13, 129–153. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA and Deisseroth K (2014) Dopaminergic Dynamics Contributing to Social Behavior. Cold Spring Harb Symp Quant Biol 79, 221–227. [DOI] [PubMed] [Google Scholar]
- Hannestad JO, Cosgrove KP, DellaGioia NF et al. (2013) Changes in the cholinergic system between bipolar depression and euthymia as measured with [123I]5IA single photon emission computed tomography. Biol Psychiatry 74, 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen BL, Siraj S and Wong DKY (2016) Recent strategies to minimise fouling in electrochemical detection systems. Reviews in Analytical Chemistry 35, 1–28. [Google Scholar]
- Hasselmo ME and Sarter M (2011) Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology 36, 52–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry PG, Dautry C, Hantraye P and Bloch G (2001) Brain GABA editing without macromolecule contamination. Magn Reson Med 45, 517–520. [DOI] [PubMed] [Google Scholar]
- Hillmer AT, Wooten DW, Moirano JM et al. (2011) Specific alpha4beta2 nicotinic acetylcholine receptor binding of [F-18]nifene in the rhesus monkey. Synapse 65, 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Ou Z, Chen Q and Wu B (2012) Amperometric acetylcholine biosensor based on self-assembly of gold nanoparticles and acetylcholinesterase on the sol-gel/multi-walled carbon nanotubes/choline oxidase composite-modified platinum electrode. Biosens Bioelectron 33, 44–49. [DOI] [PubMed] [Google Scholar]
- Howe WM, Ji J, Parikh V, Williams S, Mocaer E, Trocme-Thibierge C and Sarter M (2010) Enhancement of attentional performance by selective stimulation of alpha4beta2(*) nAChRs: underlying cholinergic mechanisms. Neuropsychopharmacology 35, 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F and Rothman DL (2017) Advances in Imaging Brain Metabolism. Annu Rev Biomed Eng 19, 485–515. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Onishi A, Fujiwara Y, Oda K, Ishiwata K and Ishii K (2018) Longitudinal effects of aging on (18)F-FDG distribution in cognitively normal elderly individuals. Sci Rep 8, 11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing M, Li Y, Zeng J et al. (2020) An optimized acetylcholine sensor for monitoring in vivo cholinergic activity. Nat Methods 17, 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing M, Zhang P, Wang G et al. (2018) A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies. Nat Biotechnol 36, 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadir A, Darreh-Shori T, Almkvist O et al. (2008) PET imaging of the in vivo brain acetylcholinesterase activity and nicotine binding in galantamine-treated patients with AD. Neurobiol Aging 29, 1204–1217. [DOI] [PubMed] [Google Scholar]
- Karimi M, Tu Z, Yue X, Zhang X, Jin H, Perlmutter JS and Laforest R (2015) Radiation dosimetry of [(18)F]VAT in nonhuman primates. EJNMMI Res 5, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karube I, Sode K and Tamiya E (1990) Microbiosensors. J Biotechnol 15, 267–281. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Okamura T, Zhang MR and Irie T (2013) PET probes for imaging brain acetylcholinesterase. J Labelled Comp Radiopharm 56, 172–179. [DOI] [PubMed] [Google Scholar]
- Kilbourn MR, Snyder SE, Sherman PS and Kuhl DE (1996) In vivo studies of acetylcholinesterase activity using a labeled substrate, N-[11C]methylpiperdin-4-yl propionate ([11C]PMP). Synapse 22, 123–131. [DOI] [PubMed] [Google Scholar]
- Kintner DB, Anderson MK, Fitzpatrick JH Jr., Sailor KA and Gilboe DD (2000) 31P-MRS-based determination of brain intracellular and interstitial pH: its application to in vivo H+ compartmentation and cellular regulation during hypoxic/ischemic conditions. Neurochem Res 25, 1385–1396. [DOI] [PubMed] [Google Scholar]
- Konig M, Thinnes A and Klein J (2018) Microdialysis and its use in behavioural studies: Focus on acetylcholine. J Neurosci Methods 300, 206–215. [DOI] [PubMed] [Google Scholar]
- Kucherenko DY, Kucherenko IS, Soldatkin OO, Topolnikova YV, Dzyadevych SV and Soldatkin AP (2019) A highly selective amperometric biosensor array for the simultaneous determination of glutamate, glucose, choline, acetylcholine, lactate and pyruvate. Bioelectrochemistry 128, 100–108. [DOI] [PubMed] [Google Scholar]
- Kuhl DE, Koeppe RA, Minoshima S, Snyder SE, Ficaro EP, Foster NL, Frey KA and Kilbourn MR (1999) In vivo mapping of cerebral acetylcholinesterase activity in aging and Alzheimer’s disease. Neurology 52, 691–699. [DOI] [PubMed] [Google Scholar]
- Laplante F, Sibley DR and Quirion R (2004) Reduction in Acetylcholine Release in the Hippocampus of Dopamine D5 Receptor-Deficient Mice. Neuropsychopharmacology 29, 1620–1627. [DOI] [PubMed] [Google Scholar]
- Lerner TN, Shilyansky C, Davidson TJ et al. (2015) Intact-Brain Analyses Reveal Distinct Information Carried by SNc Dopamine Subcircuits. Cell 162, 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yu B, Sun Q et al. (2018) Generation of a whole-brain atlas for the cholinergic system and mesoscopic projectome analysis of basal forebrain cholinergic neurons. Proc Natl Acad Sci U S A 115, 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E and Johansen JP (2019) Neuromodulation in circuits of aversive emotional learning. Nat Neurosci 22, 1586–1597. [DOI] [PubMed] [Google Scholar]
- Lindner M, Bell T, Iqbal S, Mullins PG and Christakou A (2017) In vivo functional neurochemistry of human cortical cholinergic function during visuospatial attention. PLoS One 12, e0171338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffelholz K, Klein J and Koppen A (1993) Choline, a precursor of acetylcholine and phospholipids in the brain. Prog Brain Res 98, 197–200. [DOI] [PubMed] [Google Scholar]
- Logothetis NK (2008) What we can do and what we cannot do with fMRI. Nature 453, 869–878. [DOI] [PubMed] [Google Scholar]
- Lohani S, Moberly AH, Benisty H, Landa B, Jing M, Li Y, Higley MJ and Cardin JA (2022) Spatiotemporally heterogeneous coordination of cholinergic and neocortical activity. Nat Neurosci 25, 1706–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma KG and Qian YH (2019) Alpha 7 nicotinic acetylcholine receptor and its effects on Alzheimer’s disease. Neuropeptides 73, 96–106. [DOI] [PubMed] [Google Scholar]
- Meriney SD and Fanselow EE (2019) Synaptic transmission. Academic Press, London; San Diego, CA. [Google Scholar]
- Mineur YS, Cahuzac EL, Mose TN, Bentham MP, Plantenga ME, Thompson DC and Picciotto MR (2018) Interaction between noradrenergic and cholinergic signaling in amygdala regulates anxiety- and depression-related behaviors in mice. Neuropsychopharmacology 43, 2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Eibl C, Young G, Kochevar C, Papke RL, Gundisch D and Picciotto MR (2009) Cytisine-based nicotinic partial agonists as novel antidepressant compounds. J Pharmacol Exp Ther 329, 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Einstein EB, Seymour PA, Coe JW, O’Neill B T, Rollema H and Picciotto MR (2011) alpha4beta2 nicotinic acetylcholine receptor partial agonists with low intrinsic efficacy have antidepressant-like properties. Behav Pharmacol 22, 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Mose TN, Maibom KL, Pittenger ST, Soares AR, Wu H, Taylor SR, Huang Y and Picciotto MR (2022a) ACh signaling modulates activity of the GABAergic signaling network in the basolateral amygdala and behavior in stress-relevant paradigms. Mol Psychiatry 27, 4918–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Mose TN, Vanopdenbosch L et al. (2022b) Hippocampal acetylcholine modulates stress-related behaviors independent of specific cholinergic inputs. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS and Picciotto MR (2021) The role of acetylcholine in negative encoding bias: Too much of a good thing? Eur J Neurosci 53, 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi SZ, Beitollahi H and Tajik S (2018) Nonenzymatic coated screen-printed electrode for electrochemical determination of acetylcholine. Micro and Nano Systems Letters 6, 9. [Google Scholar]
- Moran SP, Maksymetz J and Conn PJ (2019) Targeting Muscarinic Acetylcholine Receptors for the Treatment of Psychiatric and Neurological Disorders. Trends Pharmacol Sci 40, 1006–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhin AG, Gundisch D, Horti AG et al. (2000) 5-Iodo-A-85380, an alpha4beta2 subtype-selective ligand for nicotinic acetylcholine receptors. Mol Pharmacol 57, 642–649. [DOI] [PubMed] [Google Scholar]
- Mulholland GK, Wieland DM, Kilbourn MR, Frey KA, Sherman PS, Carey JE and Kuhl DE (1998) [18F]fluoroethoxy-benzovesamicol, a PET radiotracer for the vesicular acetylcholine transporter and cholinergic synapses. Synapse 30, 263–274. [DOI] [PubMed] [Google Scholar]
- Murphy KZ, Haile E, McTigue A, Pierce AF and Donaldson ZR (2023) PhAT: A flexible open-source GUI-driven toolkit for photometry analysis. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BT and Kang MJ (2019) Application of Capillary Electrophoresis with Laser-Induced Fluorescence to Immunoassays and Enzyme Assays. Molecules 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle MW (2002) Myasthenia gravis. Neurologist 8, 2–21. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V and Sarter M (2007) Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron 56, 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Pomerleau F, Huettl P, Gerhardt GA, Sarter M and Bruno JP (2004) Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur J Neurosci 20, 1545–1554. [DOI] [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI and Kellar KJ (2002) Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem 82, 468–481. [DOI] [PubMed] [Google Scholar]
- Perry M, Li Q and Kennedy RT (2009) Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal Chim Acta 653, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps ME (2000) Positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci U S A 97, 9226–9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ and Mineur YS (2012) Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76, 116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K and Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391, 173–177. [DOI] [PubMed] [Google Scholar]
- Ravasi L, Tokugawa J, Nakayama T, Seidel J, Sokoloff L, Eckelman WC and Kiesewetter DO (2012) Imaging of the muscarinic acetylcholine neuroreceptor in rats with the M2 selective agonist [18F]FP-TZTP. Nucl Med Biol 39, 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CH and Finnerty NJ (2017) Long Term Amperometric Recordings in the Brain Extracellular Fluid of Freely Moving Immunocompromised NOD SCID Mice. Sensors (Basel) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeberg NT, Sandberg SG, Johnson JA, Phillips PE and Wightman RM (2017) Hitchhiker’s Guide to Voltammetry: Acute and Chronic Electrodes for in Vivo Fast-Scan Cyclic Voltammetry. ACS Chem Neurosci 8, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Guanowsky V, Mineur YS, Shrikhande A, Coe JW, Seymour PA and Picciotto MR (2009) Varenicline has antidepressant-like activity in the forced swim test and augments sertraline’s effect. Eur J Pharmacol 605, 114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford AL, Morton SW, Whitehouse KL, Oara HM, Lugo-Morales LZ, Roberts JG and Sombers LA (2010) Voltammetric detection of hydrogen peroxide at carbon fiber microelectrodes. Anal Chem 82, 5205–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saricicek A, Esterlis I, Maloney KH et al. (2012) Persistent beta2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J Psychiatry 169, 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuvailo ON, Dzyadevych SV, El’skaya AV, Gautier-Sauvigne S, Csoregi E, Cespuglio R and Soldatkin AP (2005) Carbon fibre-based microbiosensors for in vivo measurements of acetylcholine and choline. Biosens Bioelectron 21, 87–94. [DOI] [PubMed] [Google Scholar]
- Sherathiya VN, Schaid MD, Seiler JL, Lopez GC and Lerner TN (2021) GuPPy, a Python toolbox for the analysis of fiber photometry data. Sci Rep 11, 24212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirzewski M, Princz-Lebel O, German-Castelan L et al. (2022) Continuous cholinergic-dopaminergic updating in the nucleus accumbens underlies approaches to reward-predicting cues. Nat Commun 13, 7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H and Seidman S (2001) Acetylcholinesterase--new roles for an old actor. Nat Rev Neurosci 2, 294–302. [DOI] [PubMed] [Google Scholar]
- Stangler LA, Kouzani A, Bennet KE, Dumee L, Berk M, Worrell GA, Steele S, Burns TC and Howe CL (2021) Microdialysis and microperfusion electrodes in neurologic disease monitoring. Fluids Barriers CNS 18, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey EM (2006) Henry Dale and the discovery of acetylcholine. C R Biol 329, 419–425. [DOI] [PubMed] [Google Scholar]
- Tracey KJ (2007) Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR and Wylie KP (2019) Alpha7 Nicotinic Receptors as Therapeutic Targets in Schizophrenia. Nicotine Tob Res 21, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi C, Chauhan N, Tripathi A, Jain U and Avasthi DK (2019) Voltammetric measurements of neurotransmitter-acetylcholine through metallic nanoparticles embedded 2-D material. Int J Biol Macromol 140, 415–422. [DOI] [PubMed] [Google Scholar]
- Valuskova P, Forczek ST, Farar V and Myslivecek J (2018) The deletion of M(4) muscarinic receptors increases motor activity in females in the dark phase. Brain Behav 8, e01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler I, Scheuer B and Kilbinger H (1987) [3H]acetylcholine release from the phrenic nerve is increased or decreased by activation or desensitization of nicotine receptors. Eur J Pharmacol 135, 85–87. [DOI] [PubMed] [Google Scholar]
- Wightman RM (2006) Detection technologies. Probing cellular chemistry in biological systems with microelectrodes. Science 311, 1570–1574. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M and Duman RS (2016) GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest 126, 2482–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Kim J et al. (2013) PET imaging of high-affinity alpha4beta2 nicotinic acetylcholine receptors in humans with 18F-AZAN, a radioligand with optimal brain kinetics. J Nucl Med 54, 1308–1314. [DOI] [PubMed] [Google Scholar]
- Yang M, Yang Y, Yang Y, Shen G and Yu R (2005) Microbiosensor for acetylcholine and choline based on electropolymerization/sol–gel derived composite membrane. Analytica Chimica Acta 530, 205–211. [Google Scholar]
- Yu LF, Zhang HK, Caldarone BJ, Eaton JB, Lukas RJ and Kozikowski AP (2014) Recent developments in novel antidepressants targeting alpha4beta2-nicotinic acetylcholine receptors. J Med Chem 57, 8204–8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Bognar C, Zhang X, Gaehle GG, Moerlein SM, Perlmutter JS and Tu Z (2016) Automated production of [(1)(8)F]VAT suitable for clinical PET study of vesicular acetylcholine transporter. Appl Radiat Isot 107, 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackheim JA and Abercrombie ED (2003) HPLC/EC detection and quantification of acetylcholine in dialysates. Methods Mol Med 79, 433–441. [DOI] [PubMed] [Google Scholar]
- Zapata A, Chefer VI, Shippenberg TS and Denoroy L (2009) Detection and quantification of neurotransmitters in dialysates. Curr Protoc Neurosci Chapter 7, Unit 7 4 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Han Y, Zhang P, Zheng Y and Cheng A (2023) Comparison of fluorescence biosensors and whole-cell patch clamp recording in detecting ACh, NE, and 5-HT. Front Cell Neurosci 17, 1166480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WT, Chao TH, Yang Y et al. (2022) Spectral fiber photometry derives hemoglobin concentration changes for accurate measurement of fluorescent sensor activity. Cell Rep Methods 2, 100243. [DOI] [PMC free article] [PubMed] [Google Scholar]


