Abstract
Different authors have recently described a subtype of lipoma characterized by variation of adipocyte size, single cell fat necrosis, and a subset with minimal to mild nuclear atypia, and termed these as anisometric cell/dysplastic lipoma (AC/DL). These lipomas follow a benign course and rarely recur. In 3 examples, AC/DL has occurred in patients with childhood retinoblastoma (RB). We report another such example where multiple AC/DL occurred in the neck and back of a 30-year-old male who had germline RB1 gene deletion and bilateral RB in infancy. On excision, all tumors histologically showed similar morphology of adipocyte anisometry, focal single cell necrosis with surrounding binucleated or multinucleated histiocytes, hyperchromatic and minimally atypical lipocyte nuclei, vacuolated Lockhern change, rare foci of fibromyxoid change, occasional mononuclear cell clusters around capillaries, and loss of RB1 immunostaining. Unequivocal atypical cells, lipoblasts, floret-nucleated or multinucleated giant cells were absent. Molecular analysis of tumor cells showed monoallelic RB1 gene loss without amplification of MDM2 and CDK4 genes. Short-term follow up did not show tumor recurrence. AC/DLs in RB survivors are characterized by multiplicity, unifying histology, and benign course. Their biology appears distinct from ordinary lipomas, spindle cell lipomas, and atypical lipomatous tumors.
Keywords: lipoma, dysplastic lipoma, RB1, MDM2, retinoblastoma
Introduction
In 2015, Dr Evans described 13 cases of a unique lipomatous tumor and labeled it as “anisometric cell lipoma.” 1 This was followed by additional comprehensive descriptions of a number of cases by Agaimy 2 and Michal et al. 3 The common features of these benign lipomatous tumors were presence of significant size variation in tumor lipocytes, minimal to mild nuclear atypia, and foci of necrotic single adipocytes—ultimately termed as “dysplastic” lipoma. Interestingly a clinical association with retinoblastoma in infancy was reported in rare instances.1,3,4 This association was again documented recently by den Bakker et al. 5 We report another such case of an anisometric cell/dysplastic lipoma (AC/DL) in a retinoblastoma survivor. We present, to the best of our knowledge, the fourth case in the literature of this extremely rare association and review the previously documented examples.
Case Report
A 30-year-old male presented with complaint of soft tissue masses located on the posterior back and neck, noticed 6 months ago due to continued growth and mild pain. His medical history was significant for bilateral retinoblastoma diagnosed at 2 weeks of life and treated with orbital radiation and cryotherapy, and embryonal rhabdomyosarcoma of the nasopharynx at 4 years of age, treated with surgical resection and neoadjuvant chemoradiation therapy. A detailed family history revealed cancer history in several relatives, including grandparents, but not in biological parents. Germline (saliva) testing showed a heterozygous pathogenic RB1 exon 11 variant c.1072C > T (p.Arg358*) gene sequence, encoding a premature translational stop codon expected to result in an absent or disrupted protein (loss-of-function mutation).
He remained symptom-free until his most recent presentation. Physical examination showed dense, mobile masses on the upper back and posterior neck. Magnetic resonance imaging showed multiple ill-defined subcutaneous lesions demonstrating T1 hyperintensity and postcontrast enhancement with features suggestive of fat components (Figure 1A). The largest lesion was present in the cervical neck subcutaneous tissue and measured 6 cm. Excision was performed of multiple neck and back tumors (6 nodules) with no immediate or late postoperative sequelae. A skin cyst from the right lower back was also excised.
Figure 1.
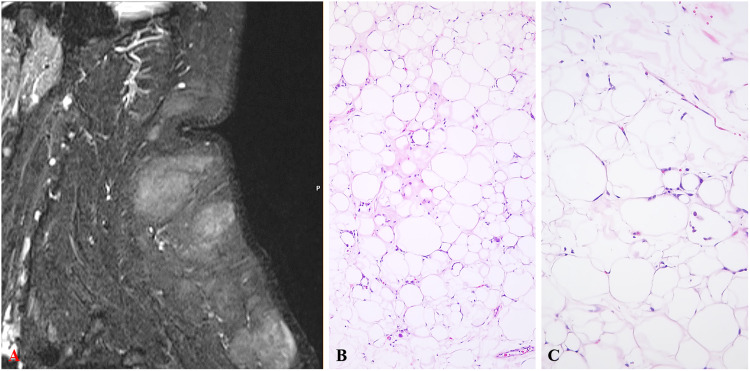
(A) MRI showing multiple subcutaneous masses in the neck and back with postcontrast enhancement. (B) Low-power and intermediate-power showing striking variation in adipocyte size with a slight fibrous stroma in the center. (C) Minimal to mild hyperchromasia of the adipocyte nuclei is present with single cell fat necrosis (H&E). Original magnification (H&E) A: ×50, B: ×100. Abbreviations: H&E, hematoxylin and eosin; MRI, magnetic resonance imaging.
Grossly, multiple lobulated lesions with a translucent, thin capsule were examined. The largest excised lesion measured 5 cm. Histologically, all tumors were composed of adipocytes with striking size variation appreciable at low power (Figure 1B-C). On high power, the adipocytes showed focal single cell necrosis with binucleated or multinucleated histiocytes surrounding fat vacuoles, hyperchromatic and occasionally enlarged lipocyte nuclei, vacuolated Lockhern change, rare foci of fibromyxoid change, and occasional blood vessels with surrounding mononuclear population (Figure 2A-E). No unequivocal atypical stromal cells, lipoblasts, floret-nucleated or multinucleated giant cells were identified. By immunohistochemistry (IHC), P53 (clone DO-7; 1:60; Dako) was positive in ∼5% of adipocytic nuclei (Figure 2F). Retinoblastoma-1 antigen (RB1, clone 1F-8; 1:50; Thermo Fisher Scientific) showed loss of nuclear staining in nearly all adipocytes with retained expression in mononuclear and endothelial cells (Figure 3A). Fluorescence in situ hybridization (FISH) performed on formalin-fixed paraffin embedded tissue for RB1 (RB1/13q14; Vysis LSI 13, Abbott Labortaories) showed >50% cells with monoallelic loss in probe signal, confirming retained heterozygosity of RB1 (Figure 3B). FISH for mouse double minute 2 homolog gene (MDM2/12q15; Kreatech Repeat-Free Poseidon, Leica Biosystems) and cyclin dependent kinase 4 gene (CDK4/12q13; Kreatech RF Poseidon, Leica Biosystems) did not show gene amplification (Figure 3C).
Figure 2.
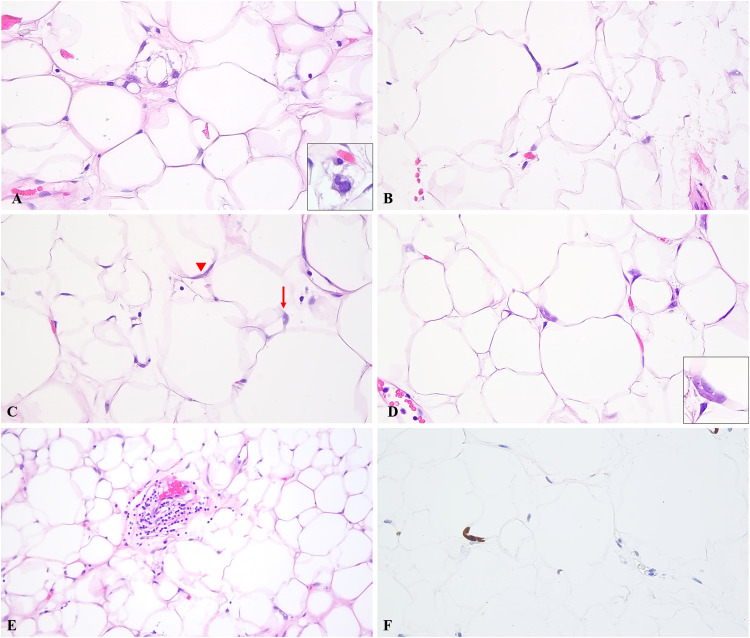
(A) Single cell fat necrosis with a binucleated histiocyte (inset). (B) Minimal atypia of adipocyte nuclei characterized by nuclear hyperchromasia. (C) Binucleation of adipocyte nucleus (arrowhead) with focal nuclear vacuolation (arrow). (D) In addition to hyperchromasia, rare adipocytes showed multinucleation (inset). (E) Background capillaries with a cuff of mononuclear cells and surrounding adipocytic anisometry (H&E). (F) P53 immunohistochemical stain showing rare nuclear staining (∼5% of adipocytes). Original magnification: A–D, F: ×400, E: ×200. Abbreviation: H&E, hematoxylin and eosin.
Figure 3.
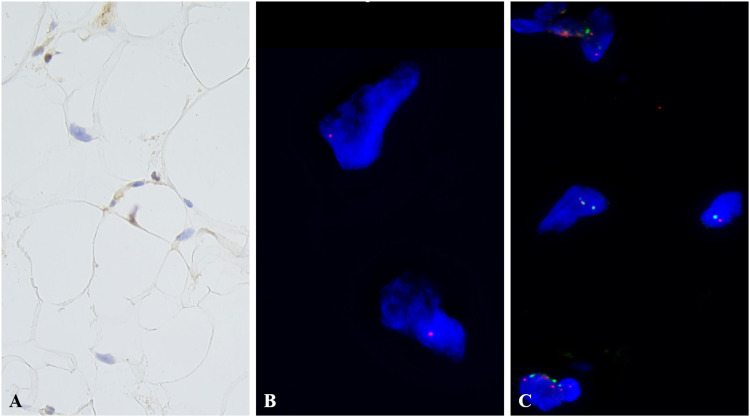
(A) RB1 immunohistochemistry showing loss of nuclear staining in the adipocyte nuclei (×400). (B) FISH for RB1 shows monoallelic signal indicative of heterozygous RB1 gene deletion. (C) FISH for MDM2 showing nonamplified normal signals. Abbreviation: FISH, fluorescence in situ hybridization; RB, retinoblastoma.
The patient was scheduled for a yearly follow up. No recurrence was reported on the short 3-month interval.
We searched our hospital database from 2005 to 2022 for additional fatty tumors reported in retinoblastoma survivors. We came across only one other case of an atypical lipomatous tumor arising in the orbit (radiation field); no benign adipocytic tumors were reported during this period to incorporate in this study.
Discussion
RB1 is located on chromosome 13q14.2 and encodes a tumor suppressor protein that controls the cell cycle. Absence of RB1 results in unchecked cell proliferation. Germline RB1 deletions are associated with bilateral retinoblastoma. Incidence of lipomas is higher in survivors of hereditary retinoblastoma (3% to 18%) as opposed to sporadic cases (<1%), albeit it is still rare.6–8 In our database search for fatty tumors in retinoblastoma survivors, we came across one other case of an atypical lipomatous tumor arising in the orbit (radiation field), which underscores the fact that adipocytic tumors are indeed rare events in retinoblastoma patients.
Somatic RB1 deletions genetically underpin a group of soft tissue tumors that contain fat as an integral component. 9 This group includes spindle cell/pleomorphic lipoma, atypical spindle cell/pleomorphic lipomatous tumor, pleomorphic liposarcoma, myofibroblastoma, cellular angiofibroma, and acral fibromyxoma. 9 These tumors have not been described in association with retinoblastoma so far. In addition, deletions of the RB1 gene locus (13q) were also noted in 6 of 87 ordinary lipomas. 10
In Evans’ description of 14 anisometric-cell lipomas (characterized by adipocyte anisometry, single cell foci of fat necrosis, and slight to minimal nuclear atypia), one occurred in a patient with childhood history of bilateral retinoblastoma. 1 Subsequently, Michal et al (in their cohort of 66 cases) and den Bakker et al reported 2 more tumors in patients with bilateral retinoblastoma (summarized in Table 1)—both authors adopted the term “dysplastic” lipoma for the tumors that showed increased nuclear size, coarse chromatin, and frequent intranuclear vacuoles.3,5 It is presumed that dysplastic lipomas may harbor TP53 mutations 5 ; we did not perform genetic testing for TP53 mutation and P53 IHC was equivocal in our case. In our practice, we have adhered to the combined nomenclature of “anisometric cell/dysplastic lipoma” since the distinction is unclear at this time. We however note the suggestions made by den Bakker. 5
Table 1.
Clinical, Pathological, and Molecular Genetic Features of All Reported AC/DL in Retinoblastoma Survivors.
| Author | Age/sex | Location/size of lipoma(s) | Histological and IHC features | Molecular features | Treatment/recurrence |
|---|---|---|---|---|---|
| Evans 1 | 36 years / male | Left scrotum/15 cm; right scrotum/11 cm; left inguinal/10 cm; right buttock/7.1 cm | Lipocyte anisometry, single cell fat necrosis, slightly enlarged, and hyperchromatic adipocyte nuclei, focal intranuclear vacuolation; IHC details N/A | Negative for CPM gene amplification | Multiple lipomas, excisions since age 24 years / NA |
| Michal et al 3 | 34 years / male | Neck/trunk, upper arm, bilateral scrotal area/all less than 9 cm | Lipocyte anisometry, single cell fat necrosis, other features not specified; negative MDM2 and CDK4 IHC; RB1 IHC partially lost | Negative for MDM2 amplification by FISH | Multiple excisions since 26 years / NER |
| den Bakker et al 5 | 42 years / female | Left shoulder, right neck, left neck, right upper arm, right shoulder/1-2 cm, each | Lipocyte anisometry, single cell fat necrosis in 5 of 6 tumors; 1 of 5 showed dispersed atypical adipocytes, with enlarged, irregular, lobulated or angulated, and variably hyperchromatic nuclei; 1 of 6 showed features of angiolipoma; RB1 IHC lost in all, P53 IHC positive in 90% nuclei in 1 of 6 tumors, p16 IHC negative in all |
RB1 FISH showed polysomy with loss in 1 of 6, possible loss in 1 of 6, and no loss in 4 of 6; MDM2 FISH showed polysomy in 2 of 6 and no amplification in 4 of 6; RB1 SNP array showed LOH (4 of 6) and RH (2 of 6); loss of wild-type gene by Sanger sequencing in 4 of 6; TP53 mutation [c.1009C > T (p.R337C)] by NGS in case with P53 positive IHC; no fusions on NGS fusion panel |
Excisions/NER (on limited follow up) |
| Current case | 30 years / male | Right back and neck, multiple (6 tumors)/5 cm, size of the largest | Lipocyte anisometry, single cell fat necrosis with binucleated histiocytes, hyperchromatic, and occasionally enlarged lipocyte nuclei, vacuolated fat cells; RB1 IHC lost in fat cell nuclei, P53 IHC positive in ∼5% | RB1 FISH showed monoallelic loss in >50% cells nuclei, MDM2 and CDK4 negative for gene amplification | Excisions/NER (short follow up) |
Abbreviations: AC/DL, anisometric cell/dysplastic lipoma; FISH, fluorescent in situ hybridization; IHC, immunohistochemistry; LOH, loss of heterozygosity; NA, not available; NER, no evidence of recurrence; NGS, next-generation sequencing; RB, retinoblastoma; RH, retained heterozygosity; SNP, single nucleotide polymorphism.
Overlapping features between AC/DL and spindle cell lipoma, such as predilection for the shoulder and back, histological features, and underlying genetic aberrations begs the question: are both these entities the same? As Agaimy pointed out for anisometric cell lipoma (not dysplastic lipoma), these can be considered a “fat-only” spindle cell lipoma, 2 although we agree that consistent paucity or absence of fibromyxoid stroma in AC/DL argues against this. In addition, spindle cell lipomas on the pleomorphic spectrum show much more profound atypia than seen in the minimally atypical cells of AC/DL (and hence the earlier term of lipomas with minimal atypia by Evans). 4 These interesting points were also communicated between Creytens and den Bakker, separately.11,12 Creytens et al also commented that at least some dysplastic lipomas could represent “fat-rich” or “spindle cell-poor” variants of atypical spindle cell lipomatous tumor, another recognized tumor with RB1 deletion. 13 We recommend separation of AC/DL from spindle cell lipoma to improve recognition by pathologists and thereby provide additional follow-up accumulation.
In our review of the literature, all previously reported retinoblastoma-associated AC/DL showed multiplicity. In contrast, among all 83 patients reported to have AC/DL outside the history of retinoblastoma,1–3 11 (13%) showed more than 1 fatty tumors; 4 (5%) of these 83 patients were confirmed to be multiple AC/DL. In the remaining, either the additional fatty tumors were not available for review or turned out to be ordinary lipomas. Although, the number is too small to draw any conclusion, it does suggest that germline RB1 deletion predisposes to multiple AC/DL. Additional studies of larger cohorts are needed at this time for insight to the underlying biology.
Two histological differentials that warrant mention are fat necrosis and atypical lipomatous tumor/well-differentiated liposarcoma (ALT/WDLS). The single cell lipocyte necrosis encountered in AC/DL is distinctly different than that seen in traumatized ordinary lipomas. For example, binucleated or multinucleated histiocytes surround a necrotic fat cell without damage to any adjacent foci. Further, the reactive fibrosis is limited (or even absent) and does not form a discrete area that is conventionally seen in fat necrosis. We hypothesize that RB1-mediated cell cycle dysregulation leads to adipocytic dysplasia with spontaneous apoptosis 14 leading to anisometry as well as the single cell necrosis. Further studies are needed to determine this.
The atypical cells seen in ALT/WDLS tend to be present in traversing fibrous septae that courses near a blood vessel. In contrast, the minimally atypical nucleus in AC/DL is always noted in a fat cell and not in a fibrous band. MDM2 IHC is a useful screening tool to distinguish between the 2, however a small subset can show weak (1+ or 2+) staining 3 ; MDM2 amplification is consistently negative by FISH. 3
Histopathological descriptions of lipomas in retinoblastoma survivors are limited in the literature and as speculated before, 2 many reported such associations may very well represent AC/DL. These tumors tend to present as multiple subcutaneous masses usually as the survivor approaches their third decade of life. Adipocyte anisometry and single cell fat necrosis appear to be unifying features, with minimal atypia, lipocyte binucleation, and hyperchromasia seen in a subset of cases. Although these tumors may apparently be on a spectrum with spindle cell lipomas, the multicentricity, especially in germline RB mutations provides a counterargument. The histological features can be alarming and therefore ancillary techniques to detect MDM2 amplification are an invaluable adjunct. Larger studies of these rare tumors are needed to fully understand the biology, as well as to establish if these are different from AC/DL presenting outside the history of germline RB1 mutation.
Footnotes
Author Contributions: FM and KC were involved in acquisition, analysis, interpretation of data, and manuscript drafting and editing.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
ORCID iD: Faizan Malik https://orcid.org/0000-0001-8597-5074
References
- 1.Evans HL. Anisometric cell lipoma: a predominantly subcutaneous fatty tumor with notable variation in fat cell size but not more than slight nuclear enlargement and atypia. AJSP Rev Rep. 2016;21(4):195‐199. [Google Scholar]
- 2.Agaimy A. Anisometric cell lipoma: insight from a case series and review of the literature on adipocytic neoplasms in survivors of retinoblastoma suggest a role for RB1 loss and possible relationship to fat-predominant (“fat-only”) spindle cell lipoma. Ann Diagn Pathol. 2017;29:52‐56. doi: 10.1016/j.anndiagpath.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Michal M, Agaimy A, Contreras AL, et al. Dysplastic lipoma: a distinctive atypical lipomatous neoplasm with anisocytosis, focal nuclear atypia, p53 overexpression, and a lack of MDM2 gene amplification by FISH; a report of 66 cases demonstrating occasional multifocality and a rare association with retinoblastoma. Am J Surg Pathol. 2018;42(11):1530‐1540. doi: 10.1097/PAS.0000000000001129. [DOI] [PubMed] [Google Scholar]
- 4.Evans H. Subcutaneous minimally atypical lipomatous tumors with variable fat cell size a study of 13 cases. NATURE PUBLISHING GROUP 75 VARICK ST, 9TH FLR, NEW YORK, NY 10013-1917 USA; 2015:17A-17A.
- 5.Den Bakker MA, Den Toom DT, Damen THC, et al. Anisometric cell and dysplastic lipomas in a retinoblastoma patient. Int J Surg Pathol. 2020;28(7):793‐798. doi: 10.1177/1066896920917220. [DOI] [PubMed] [Google Scholar]
- 6.Van Hoefen Wijsard M, Schonfeld SJ, van Leeuwen FE, et al. Benign tumors in long-term survivors of retinoblastoma. Cancers (Basel). 2021;13(8):1773. doi: 10.3390/cancers13081773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo KI, Harbour JW. Review of 676 second primary tumors in patients with retinoblastoma: association between age at onset and tumor type. Arch Ophthalmol. 2010;128(7):865‐870. doi: 10.1001/archophthalmol.2010.126. [DOI] [PubMed] [Google Scholar]
- 8.Li FP, Abramson DH, Tarone RE, Kleinerman RA, Fraumeni JF, Jr, Boice JD, Jr. Hereditary retinoblastoma, lipoma, and second primary cancers. J Natl Cancer Inst. 1997;89(1):83‐84. doi: 10.1093/jnci/89.1.83. [DOI] [PubMed] [Google Scholar]
- 9.Libbrecht S, Van Dorpe J, Creytens D. The rapidly expanding group of RB1-deleted soft tissue tumors: an updated review. Diagnostics (Basel). 2021;11(3):430. doi: 10.3390/diagnostics11030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher CD, Akerman M, Dal Cin P, et al. Correlation between clinicopathological features and karyotype in lipomatous tumors. A report of 178 cases from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. Am J Pathol. 1996;148(2):623‐630. [PMC free article] [PubMed] [Google Scholar]
- 11.Creytens D. “Dysplastic lipoma” is probably not a separate entity but rather belongs to the morphological spectrum of atypical spindle cell/pleomorphic lipomatous tumor. Int J Surg Pathol. 2020;28(8):929‐930. doi: 10.1177/1066896920939657. [DOI] [PubMed] [Google Scholar]
- 12.den Bakker MA. Response to “dysplastic lipoma” is probably not a separate entity but rather belongs to the morphological spectrum of atypical spindle cell/pleomorphic lipomatous tumor. Int J Surg Pathol. 2020;28(8):931. doi: 10.1177/1066896920946174. [DOI] [PubMed] [Google Scholar]
- 13.Creytens D, Mentzel T, Ferdinande L, Van Gorp J, Van Dorpe J, Flucke U. “Fat-rich” (spindle cell-poor) variants of atypical spindle cell lipomatous tumors show similar morphologic, immunohistochemical and molecular features as “dysplastic lipomas”: are they related lesions? Comment on Michal et al. (2018). Am J Surg Pathol. 2019;43(2):288‐289. doi: 10.1097/PAS.0000000000001156. [DOI] [PubMed] [Google Scholar]
- 14.Indovina P, Pentimalli F, Casini N, Vocca I, Giordano A. RB1 dual role in proliferation and apoptosis: cell fate control and implications for cancer therapy. Oncotarget. 2015;6(20):17873‐17890. doi: 10.18632/oncotarget.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]