Abstract
This study investigated the temperature and salinity parameters associated with waters and oysters linked to food-borne Vibrio vulnificus infections. V. vulnificus was enumerated in oysters collected at three northern Gulf Coast sites and two Atlantic Coast sites from July 1994 through September 1995. Two of these sites, Black Bay, La., and Apalachicola Bay, Fla., are the source of the majority of the oysters implicated in V. vulnificus cases. Oysters in all Gulf Coast sites exhibited a similar seasonal distribution of V. vulnificus: a consistently large number (median concentration, 2,300 organisms [most probable number] per g of oyster meat) from May through October followed by a gradual reduction during November and December to ≤10 per g, where it remained from January through mid-March, and a sharp increase in late March and April to summer levels. V. vulnificus was undetectable (<3 per g) in oysters from the North and South Carolina sites for most of the year. An exception occurred when a late-summer flood caused a drop in salinity in the North Carolina estuary, apparently causing V. vulnificus numbers to increase briefly to Gulf Coast levels. At Gulf Coast sites, V. vulnificus numbers increased with water temperatures up to 26°C and were constant at higher temperatures. High V. vulnificus levels (>103 per g) were typically found in oysters from intermediate salinities (5 to 25 ppt). Smaller V. vulnificus numbers (<102 per g) were found at salinities above 28 ppt, typical of Atlantic Coast sites. On 11 occasions oysters were sampled at times and locations near the source of oysters implicated in 13 V. vulnificus cases; the V. vulnificus levels and environmental parameters associated with these samples were consistent with those of other study samples collected from the Gulf Coast from April through November. These findings suggest that the hazard of V. vulnificus infection is not limited to brief periods of unusual abundance of V. vulnificus in Gulf Coast oysters or to environmental conditions that are unusual to Gulf Coast estuaries.
Between 1989 and 1994, cases of primary septicemia resulting from shellfish-borne Vibrio vulnificus averaged 15 per year, with fatality rates averaging 45% (4). Most cases (85%) occurred between May and October (13, 14, 21), and only oysters harvested from Gulf Coast states have been implicated. Frequently, implicated oysters are traced to one of a small number of harvest locations (4). These facts suggest that oysters harvested from specific areas or harvested from areas with certain environmental conditions (temperature and salinity) may have unusually large numbers of V. vulnificus organisms, thereby increasing the risk of infection for individuals. The persons at greatest risk for infection are those with liver disease (16, 23); however, fewer than 1 in 104 persons from this high-risk group become ill after consuming raw oysters (8).
The highest concentrations of V. vulnificus in Gulf Coast oysters have been reported during the warm months (5, 22). In the Chesapeake Bay, V. vulnificus was recovered from oysters at levels of 103 to 104/g during summer months but was not recovered during winter months (30). V. vulnificus has been recovered, although sporadically and usually in small numbers, from oysters harvested from cooler environments such as the New England Coast (20) and the Pacific Coast (11). These observations point to temperature playing a significant role in controlling the numbers of this organism in oysters. The role of water salinity in the abundance of V. vulnificus is less clear (25, 29).
Understanding the relationship between V. vulnificus and temperature and salinity may help predict the concentrations of V. vulnificus in shellfish. Many of the early studies that gathered this type of information were limited by the narrow geographical scope of sampling (12, 19, 25) or by sporadic or seasonal sampling schedules. Before 1988, the enumeration methodology for V. vulnificus was not standardized. Recent improvements have made enumeration less time-consuming and permit the examination of larger numbers of test samples (18, 27).
Tamplin (24, 26) investigated the relationship of environmental factors and V. vulnificus densities in oysters collected monthly in 14 states. Levels in oysters ranged from none detected (<0.3 per g) to 1,100,000 per g. This information was used to derive a linear regression model based on water temperature and salinity to predict V. vulnificus levels in oysters (28).
This study expands on Tamplin’s research (26) and reports on the seasonal distribution and abundance of V. vulnificus in Northern Gulf and Atlantic Coast oysters in an attempt to further define the relationship between the density of this organism and the temperature and salinity of the water. The selected sampling sites were considered representative of the Gulf Coast shellfish-growing areas with regard to productivity, and they represent more than 90% of the total harvest for Alabama and Florida and ca. 25% for Louisiana. Sites in Louisiana and Florida were also chosen based on their history of shellfish-associated V. vulnificus cases; sites in Alabama and on the Atlantic Coast were picked based in part on their lack of association with cases (4). The intensive and methodical sampling of areas frequently associated with harvest of implicated oysters was expected to provide information on concentrations of V. vulnificus in illness-associated oysters at the time they were harvested. This information may be used to determine the extent to which environmental factors influence the concentration of the pathogen and incidence of the disease.
MATERIALS AND METHODS
Oyster harvesting and handling.
Oysters (Crassostrea virginica) were harvested weekly (25 July 1994 through 25 September 1995) from three Northern Gulf Coast (Apalachicola Bay, Fla., 29°44′25"N, 84°53′10"W; Cedar Point, Ala., 30°18′30"N, 88°07′45"W; and Black Bay, La., 29°36′40"N, 89°34′00"W) and two Atlantic Coast (Folly River, S.C., 32°40′30"N, 79°56′04"W and Newport River, N.C., 34°45′30"N, 76°45′00"W) shellfish-growing areas. Harvesting was reduced to a monthly schedule beginning in November on the Atlantic Coast and in January on the Gulf Coast. Weekly harvesting was resumed in March on both coasts. Collections (lots) consisted of 25 legal-size culled oysters in the shell harvested with either oyster tongs or a dredge. A 50-ml volume of surface water was collected at the harvest site in a screw-cap plastic tube and kept with the oysters for verification of the salinity in the laboratory. Temperature and salinity were measured in the upper 0.5 m of the surface water with a salinometer (Beckman Instruments, Inc., Cedar Grove, N.J.) or with a calibrated bimetallic dial thermometer and a refractometer (Cambridge Instruments, Inc., Buffalo, N.Y.). Immediately after being harvested, the oysters were chilled by contact with bagged ice in a 48-qt ice chest for approximately 2 h. The chilled oysters and the tube of water were shipped in insulated shipping containers (no. 132; FDC Packaging, Inc., Medfield, Mass.) with three frozen cool packs (no. 405; FDC Packaging, Inc.). Bubble wrap was laid between the oysters and the ice packs to prevent direct contact. The oysters were shipped overnight to a laboratory for analysis; the Atlantic Coast oysters were shipped to the Food and Drug Administration (FDA) Southeast Regional Laboratory, Atlanta, Ga., and the Gulf Coast oysters were shipped to the FDA Gulf Coast Seafood Laboratory, Dauphin Island, Ala. Upon receipt, the temperature of the water sample was recorded to ensure that the temperature in the shipping container had remained low enough to prevent the growth of V. vulnificus.
Analysis.
Each lot of oysters was divided into two subsamples of 12 oysters each, and the subsamples were analyzed separately. These subsamples were considered to be replicate oyster samples from the same growing area, harvested at the same time. The oysters were washed, shucked, diluted 1:1 in phosphate-buffered saline (PBS), and homogenized for 90 s in a blender at 14,000 rpm. To prepare the first dilution, 20 g of the homogenate was weighed into a sterile jar and diluted to 100 g with PBS. Subsequent dilutions in PBS were made on a volume basis. V. vulnificus was enumerated by procedures described in the Bacteriological Analytical Manual (7). A three-tube most-probable-number (MPN) series was used for enumeration. Isolates were confirmed by enzyme immunoassay with a monoclonal antibody specific to V. vulnificus (27). Visual readings of the enzyme immunoassay plates were confirmed with a microplate reader (Bio-Tek Instruments, Winooski, Vt.). Normal inoculation sizes for the MPN determinations were 10−1 through 10−6 g, with the exception of the winter months, when the analysis included 1.0-g amounts (Gulf Coast oysters only). An additional 25 g was inoculated into 2,475 ml of alkaline peptone water for a presence/absence test.
Statistical analyses.
For statistical analysis, MPN values that were indeterminate (<0.3 or <3.0) as a result of no positive tubes in any of the series were assigned a value halfway between the maximum value and zero (i.e., 0.15 or 1.5, respectively). MPN counts were converted to base 10 logarithms before being subjected to analysis. Variance due to the three-tube MPN method was estimated by five sets of eightfold replicate measurements; each eightfold set was performed on the same dozen-oyster homogenate (6). Regression analysis and analysis of variance were performed with Statistical Analysis Systems from SAS Institute, Cary, N.C.
Plotting of the V. vulnificus counts against temperature employed a smoothing technique (moving average), which was necessary because in some instances there were few measurements at a particular temperature. This technique involved the use of 3°C increments, derived a geometric mean of the V. vulnificus count for all values corresponding to the three temperatures (e.g., 19, 20, and 21°C), and plotted this against the mean of the temperatures (i.e., 20°C for the above temperatures). A similar procedure was applied with salinity in which 3-ppt salinity increments were used.
RESULTS
Oysters analyzed.
A total of 226 lots of oysters (52 from Alabama, 50 from Louisiana, 47 from Florida, 38 from South Carolina, and 39 from North Carolina) were received and analyzed in duplicate for V. vulnificus within 28 h of harvest. Of these, only 13 lots were received with temperatures above 13°C and none had V. vulnificus counts higher than might be expected based on temperature and salinity at the harvest sites. No oysters were received at temperatures above 19°C.
Oysters from Gulf Coast sites.
Only 16.9% of the paired oyster subsamples from a lot produced identical MPN counts, but in 83.1% of the cases, the MPN counts of the second subsample fell within the 95% confidence limits of the MPN counts of the first. The average variance between Gulf Coast pairs was 0.170. Although the sets of eightfold replicate measurements showed that the MPN method contributes 0.12 to the log variance, the variance due to the difference from one dozen to the next dozen dredged at the same place and time is 0.05 log unit.
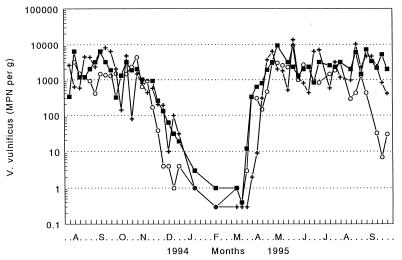
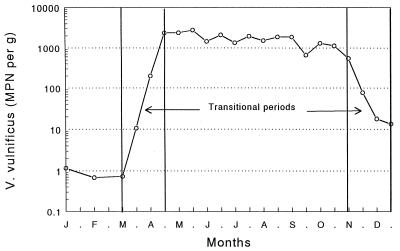
Geometric means were calculated from the paired V. vulnificus values for each lot of oysters and are presented by site in Fig. 1. MPN counts at all three sites appear to be similar and seasonally influenced, ranging from 103 to 104 organisms per g from May to October and falling to <10 per g from late December through mid-March. The seasonal trend is emphasized when the data from all three sites are combined (Fig. 2). Data from oysters collected between days 1 and 15 of each month were combined, as were data from day 16 to the end of each month. The data fell into four clearly defined periods, a cold-weather period (January, February, and early March), a warm-weather period (May through October), and two transitional periods, in the spring (late March and April) and fall (November and December), when counts increased or decreased with time.
FIG. 1.
Weekly densities of V. vulnificus in oysters from Gulf Coast sites in Alabama (+), Florida (○), and Louisiana (▪) during a 14-month period. Each point represents the geometric mean (n = 2) of the MPN of bacteria per gram of oyster meat.
FIG. 2.
Seasonal distribution of V. vulnificus in Gulf Coast oysters. Each point represents the geometric mean of the MPN of V. vulnificus organisms per g of oyster meat for all observations recorded within a half-month period. Transitional periods represent periods of change.
Table 1 presents the summary statistics of V. vulnificus counts in oysters and their associated environmental parameters by period for each site and all Gulf Coast sites. V. vulnificus was recovered from all of the oysters sampled during the warm-weather period, with 70% of the samples exceeding 103 organisms per g. The median value for each of the three sites during the warm-weather period was 2.3 × 103 per g. Only 3.7% of the samples had MPN counts exceeding 104 per g, and none had MPN counts exceeding 4.3 × 104 per g.
TABLE 1.
V. vulnificus counts in oysters and environmental conditions of harvest waters by location and period
| Location and perioda | No. of samples |
V. vulnificus counts (MPN/g)
|
Water temp (°C)
|
Water salinity (ppt)
|
|||
|---|---|---|---|---|---|---|---|
| Range | Log mean (SD)b | Range | Mean (SD) | Range | Mean (SD) | ||
| Alabama | |||||||
| Cold | 8 | <0.3–0.9 | −0.52 (0.29) | 12.6–16.6 | 14.7 (1.9) | 5–18 | 11.5 (6.5) |
| Transitional | 28 | <0.3–43,000 | 1.99 (1.26) | 14.6–23.8 | 20.5 (3.0) | 4–21 | 13.5 (4.8) |
| Warm | 68 | 21–43,000 | 3.26 (0.62) | 22.7–32.4 | 28.0 (2.3) | 5–28 | 17.1 (5.6) |
| All | 104 | <0.3–43,000 | 2.62 (1.36) | 12.6–32.4 | 25.0 (5.1) | 4–28 | 15.7 (5.7) |
| Florida | |||||||
| Cold | 8 | <0.3–2.3 | −0.40 (0.45) | 11.7–17.3 | 14.7 (2.8) | 11.9–26.8 | 19.1 (6.6) |
| Transitional | 26 | <0.3–9,300 | 1.72 (1.18) | 13.4–23.8 | 19.9 (3.2) | 7.5–30.8 | 22.7 (7.0) |
| Warm | 60 | 7.2–9,300 | 3.03 (0.67) | 21.8–32.0 | 27.5 (2.4) | 7.6–30.9 | 19.7 (6.8) |
| All | 94 | <0.3–9,300 | 2.37 (1.33) | 11.7–32.0 | 24.2 (5.2) | 7.5–30.9 | 20.5 (6.8) |
| Louisiana | |||||||
| Cold | 8 | <0.3–9.3 | 0.08 (0.52) | 10.8–16.8 | 13.9 (3.1) | 7.2–15.4 | 11.5 (3.5) |
| Transitional | 24 | 9.3–4,300 | 2.34 (0.80) | 12.7–23.5 | 19.4 (3.9) | 8.5–17.4 | 12.6 (3.0) |
| Warm | 68 | 120–15,000 | 3.38 (0.43) | 19.8–32.4 | 27.9 (2.6) | 5.7–21.6 | 13.0 (3.6) |
| All | 100 | <0.3–15,000 | 2.86 (1.08) | 10.8–32.4 | 24.8 (5.6) | 5.7–21.6 | 12.8 (3.4) |
| All Gulf Sites | |||||||
| Cold | 24 | <0.3–9.3 | −0.30 (0.52) | 10.8–17.3 | 14.4 (2.4) | 5–26.8 | 14.0 (6.3) |
| Transitional | 78 | <0.3–11,000 | 2.00 (1.12) | 12.7–23.8 | 19.9 (3.3) | 4–30.8 | 16.3 (6.9) |
| Warm | 196 | 7.2–43,000 | 3.20 (0.59) | 19.8–32.4 | 27.8 (2.4) | 5–30.9 | 16.5 (6.1) |
| All | 298 | <0.3–43,000 | 2.62 (1.27) | 10.8–32.4 | 24.7 (5.2) | 4–30.9 | 16.3 (6.3) |
| North Carolina | |||||||
| Cold | 30 | <3–15 | 0.38 (0.33) | 6.7–21.7 | 16.1 (4.3) | 6–34 | 24.0 (7.8) |
| Warm | 48 | <3–9,300 | 1.04 (0.89) | 21.7–35.6 | 28.1 (4.0) | 13–37 | 27.9 (7.0) |
| All | 78 | <3–9,300 | 0.77 (0.79) | 6.7–35.6 | 23.3 (7.2) | 6–37 | 26.4 (7.6) |
| South Carolina | |||||||
| Cold | 30 | <3–23 | 0.23 (0.24) | 9.0–23.3 | 17.8 (4.5) | 5–35 | 29.4 (7.2) |
| Warm | 46 | <3–3.6 | 0.19 (0.08) | 21.0–32.0 | 27.2 (3.1) | 30–37 | 33.9 (2.1) |
| All | 76 | <3–23 | 0.22 (0.17) | 9.0–32.0 | 23.3 (5.9) | 5–37 | 32.1 (5.3) |
Gulf Coast states: cold period, January through 15 March; transitional period, 16 through 31 March, April, November, and December; warm period (May through October). Atlantic Coast states: cold period, October through April; warm period, May through September.
SD, standard deviation of the distribution of the data, not the standard error of the mean.
The transitional periods were characterized by water temperatures averaging ≤20°C and V. vulnificus MPN counts averaging 102 per g. The counts increased during the spring and decreased during the fall.
In the cold-weather period, the water temperatures averaged <15°C. V. vulnificus MPN counts in all Gulf Coast oyster samples examined during this period were <10 per g, with a median of <1 per g. Counts of <0.3 per g (i.e., no isolates from tubes inoculated with 1-g portions) were observed in 38% of the cold-weather period samples, but only 2 of 24 were negative for V. vulnificus when 25-g portions were examined. The lowest water temperature at which V. vulnificus was recovered, 10.8°C, was the lowest observed during the study.
Differences among the three Gulf sites with respect to temperature, salinity, and log MPN data were analyzed by a two-way analysis of variance, using the sample week as a blocking factor. There was evidence that the mean log MPN values were not the same at the three sites (P = 0.0003). After all three pairwise comparisons were performed and the Bonferroni adjustment were made, the Louisiana site had a higher mean log MPN value than did the Florida and Alabama sites at the P < 0.05 level. No differences were observed between the Florida and Alabama sites (P < 0.05). A two-way analysis of variance of temperature with the week as a blocking factor did not demonstrate any overall difference among the sites (P = 0.33). The two-way analysis of salinity demonstrated clear evidence of a site effect (P = 0.0001). All three pairwise comparisons had P values well below the Bonferroni critical value of 0.0167. The Florida site had the highest average salinity, and the Louisiana site had the lowest.
The Gulf log MPN data were regressed on temperature and salinity data to determine whether these were good predictors of V. vulnificus concentrations. The linear-regression model which best fits the data is: log MPN = −9.6823 + (0.5855 × temperature) − (0.0092 × temperature2) + (0.6804 × salinity) − (0.0355 × salinity2) + (0.00054 × salinity3). The r2 for the model is 0.705 (r = 0.84). When the site factor is added to this model, the P value indicates that site is not significant (P = 0.43). Thus, after adjustment for temperature and salinity, there is no evidence of any independent effect of the sampling site.
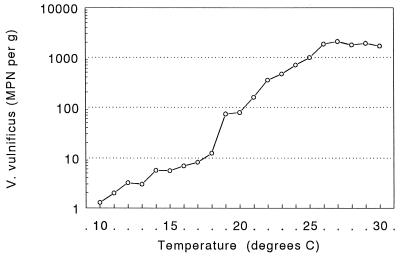
The role of salinity in determining V. vulnificus levels becomes clear when the full model (described above) is compared with one lacking the salinity terms. The model log MPN = −5.5031 + (0.5391 × temperature) − (0.0081 × temperature2) has an r2 of 0.60. (The nature of this association with temperature can be seen in Fig. 3; the numbers of V. vulnificus organisms increased slowly over the range from 10 to 18°C, increased more rapidly from 18 to 26°C, and then stopped increasing above 26°C.)
FIG. 3.
Influence of water temperature on the concentration of V. vulnificus in Gulf Coast oyster meats. Each point represents the geometric mean of all observations recorded within a 3°C temperature range. See Materials and Methods for an explanation of the smoothing technique.
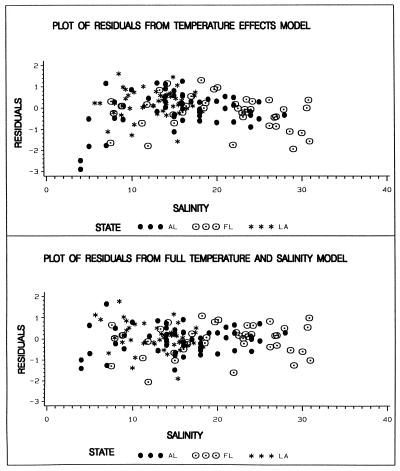
The addition of the salinity terms explains about 10% more of the total variability in the log MPN data and also explains the difference between the sites. Figure 4 shows the residuals [(actual values) − (model predicted values)] from the model lacking salinity terms plotted against salinity. This graph demonstrated that the residuals (from the temperature effects model) are low at both the high and low extremes of salinity and that the three states seem to fall on the same curve. Figure 4 also shows the residuals from the model that includes both temperature and salinity. The evenness and linearity of the remaining residuals are evident from the graph. The model is not intended to represent other estuaries, and it should not be extrapolated beyond the range of temperatures and salinities shown in the data from the three Gulf estuaries.
FIG. 4.
Residuals (measured log V. vulnificus count minus model predictions) plotted against salinity for three Gulf estuaries.
If one makes an arbitrary division of the residuals into two groups at salinities of ≤15 and >15 ppt, there is a positive correlation (r = 0.43, P = 0.0001, n = 74) between salinity and the residuals in the low-salinity group and a negative correlation (r = −0.44, P = 0.0001, n = 71) in the high-salinity group.
Oysters from Atlantic Coast sites.
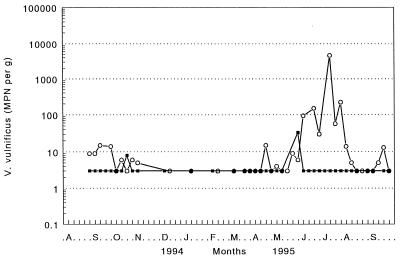
Weekly geometric means of V. vulnificus counts in oysters from the Atlantic Coast sites during the 14-month study period are presented in Fig. 5. MPN counts were below the lower limit of sensitivity for the method (3 per g) in 87% of the South Carolina oysters and 42% of the North Carolina oysters. MPN counts of <10 per g occurred in 86% of the combined set of samples from the two Atlantic Coast sites. Oysters from the North Carolina site collected in July and August had MPN counts exceeding 102 per g on three occasions and exceeding 103 per g once. There was a significant difference (P < 0.05) in the geometric means of the counts in oysters between the North and South Carolina sites and between the Atlantic and Gulf Coast sites.
FIG. 5.
Weekly densities of V. vulnificus in oysters from Atlantic Coast sites in North Carolina (○) and South Carolina (▪) during a 14-month period. Each point represents the geometric mean (n = 2) of the MPN of bacteria per gram of oyster meat.
Seawater temperatures at the Atlantic Coast sites (Table 1) were similar to those recorded at the Gulf Coast sites and, during the warm period, were within the range associated with the recovery of V. vulnificus from shellfish. The water temperatures exceeded 20°C from May through October, and temperatures above 30°C were common during late July and in August.
Water salinities at the Atlantic Coast sites were significantly higher (P < 0.01) than at the Gulf Coast sites and normally ranged from 22 to 36 ppt; 73.4% of samples exceeded a salinity of 26 ppt. The only occasion when V. vulnificus was detected in large numbers occurred during the summer months when water salinities were below normal.
V. vulnificus illnesses.
There were 13 oyster-associated V. vulnificus illnesses during the period of this study in which the most probable harvest site was identified as one of our sampling sites. None of the illnesses were linked to the Atlantic Coast sites. Table 2 presents environmental and bacteriological data for the closest sampling date to the harvest date. Of the 11 salinities associated with the cases, 7 were within 1 standard deviation of the mean for the warm period, 3 were above, and 1 was below. Of the 11 temperatures associated with cases, 9 were within 1 standard deviation of the warm period mean and the other 2 were below. Therefore, the environmental conditions at times implicated by cases were no more conducive to V. vulnificus abundance than were conditions at other times in the warm period.
TABLE 2.
V. vulnificus illnesses that occurred during the study and for which one of the study areas was identified as the most likely harvest area for the implicated oysters
| No.a | Oyster harvest areab | Harvest date (mo/day/yr) | Samplingc
|
|||
|---|---|---|---|---|---|---|
| Date (mo/day/yr) | Temp (°C) | Salinity (ppt) | MPN counts of V. vulnificus (organisms/g)d | |||
| ATL-9827 | Black Bay, La. | 09/21/94 | 09/19/94 | 27.3 | 12.6 | 1,857 |
| NY-Reg | Black Bay, La. | 09/22/94 | 09/19/94 | 27.3 | 12.6 | 1,857 |
| ATL-9823 | Black Bay, La. | 10/23/94 | 10/24/94 | 25.5 | 13.2 | 2,000 |
| ATL-9825 | Black Bay, La. | 11/03/94 | 10/31/94 | 19.8 | 11.4 | 1,016 |
| PHI-59962 | Apalachicola Bay, Fla. | 04/20/95 | 04/17/95 | 23.8 | 24.0 | 462 |
| ORL-7885 | Apalachicola Bay, Fla. | 05/09/95 | 05/08/95 | 26.6 | 24.2 | 2,540 |
| ORL-1566 | Apalachicola Bay, Fla. | 05/15/95 | 05/15/95 | 27.6 | 11.8 | 2,272 |
| NSV-5830 | Apalachicola Bay, Fla. | 05/22/95 | 05/22/95 | 26.5 | 19.7 | 9,300 |
| NSV-5829 | Apalachicola Bay, Fla. | 05/23/95 | 05/22/95 | 26.5 | 19.7 | 9,300 |
| NSV-5736 | Cedar Point, Ala. | 07/15/95 | 07/10/95 | 28.5 | 25.0 | 587 |
| ORL-8324 | Apalachicola Bay, Fla. | 07/24/95 | 07/24/95 | 30.8 | 16.4 | 3,145 |
| FLA-8336 | Black Bay, La. | 09/10/95 | 09/11/95 | 28.3 | 14.9 | 2,175 |
| DAL-6-5000 | Black Bay, La. | 09/30/95 | 09/25/95 | 25.5 | 18.3 | 2,000 |
FDA consumer complaint number.
Most probable harvest location for oysters implicated in illness.
Information on oyster samples collected on the date closest to the harvest date of the implicated oysters.
Geometric mean of the counts from the two subsamples from the lot.
DISCUSSION
Examination of oysters from three geographically distinct estuaries on the northern Gulf Coast demonstrated a similarity in V. vulnificus counts throughout the year. During warm-weather months, when >85% of the shellfish-associated V. vulnificus cases occur, MPN counts were usually 103 to 104 per g. During cold-weather months, when infections have not been reported, MPN counts were <10 per g. Jackson et al. (9) reported similar V. vulnificus densities in Apalachicola Bay oysters during the summer of 1991 but found much higher levels in 1992 and 1993 (>105 per g). These differences may be due to year-to-year variation; we observed much lower counts in Apalachicola Bay oysters in August and September 1995 (10 to 100 per g) than in the same months in 1994. The low counts in 1995 coincided with unusually high salinity. In the present study, we rarely found densities greater than 104 per g. The sample handling procedures used by Jackson et al. before shipment were not specified (9). The multiplication of V. vulnificus in summer harvest oyster shellstock held without refrigeration has been shown to be rapid (2). If shellstock are chilled immediately after harvest and stored at temperatures of ≤13°C, the numbers of V. vulnificus organisms do not differ significantly from those at the time of harvest through 30 h of storage (1). While prolonged storage of shellstock at 0 to 4°C brings about a significant reduction in numbers of V. vulnificus organisms, storage for up to 48 h at these temperatures brings about no reduction in numbers (3). Procedures to handle oysters between harvest and analysis were designed to minimize changes in V. vulnificus density (1). These procedures consisted of chilling oysters immediately after harvest and maintaining them at a low temperature during overnight transport to the analytical laboratory.
This study demonstrates the ubiquitous occurrence of culturable V. vulnificus organisms in Gulf Coast oysters throughout the year (>99% detection in 298 samples). Failure to detect V. vulnificus by the MPN procedure in seven of nine samples collected during the winter was reversed by increasing the sample size eightfold to 25 g. Previous studies reporting a low incidence of V. vulnificus in Gulf Coast oysters during the winter have relied on MPN procedures similar to those used in the present study (6, 9, 25, 29). Since one oyster contains about 25 g of tissue, culturable V. vulnificus would probably overwinter along the Gulf Coast and reseed the estuary when the waters become warmer in the spring. In the present study, densities up to 9.3 per g were observed during January or February. No primary septicemia cases have been reported in these months (4, 8). Further investigation is needed to determine a safe level of V. vulnificus, because levels may change between harvest and consumption and virulence may be seasonal.
Although the presence of V. vulnificus in Gulf Coast estuarine environments is favored by relatively high water temperatures (12, 25), Wright et al. (30) reported no correlation between water temperature and V. vulnificus concentration in Chesapeake Bay waters during the warmer months. Our Gulf Coast data suggests that the number of V. vulnificus organisms in oysters is strongly correlated with water temperature until the temperature reaches 26°C, above which there appears to be no additional increase in the number of bacteria. Investigators developing models for predicting V. vulnificus levels in estuarine environments need to be aware of this break at 26°C, as water temperature normally exceeds 26°C from May through October, when the majority of cases occur.
Seasonal temperature change explains most of the variability in the Vibrio levels in the Gulf. Salinity explains an additional 10% of the variability in these levels and also explains the differences among the three sites. The Louisiana site is in a large estuarine area, remote from direct river discharge and buffered from the salt water of the Gulf of Mexico by barrier islands and marshes. The Florida site is heavily influenced by freshwater from the Apalachicola River during periods of high river flow and by salt water from the wide inlets into the Gulf of Mexico during periods of low river flow. This area showed the largest range in salinities and had the highest salinities. The Alabama site is greatly influenced by the Mobile River; its lowest salinities were also the lowest for the three estuaries. Figure 4 shows that Louisiana experienced neither the lower salinities that suppressed Vibrio levels in Alabama nor the higher salinities that did the same in Florida. These results are consistent with the experience with the Atlantic estuaries in this study, because the higher Atlantic salinities are associated with temperature-driven Vibrio concentrations that are lower than those in the Gulf.
Water temperatures at the Atlantic and Gulf Coast sites were not different, but salinities at the Atlantic Coast sites were higher (P ≤ 0.01) and averaged >26 ppt. Work conducted by Kaspar and Tamplin (10) in seawater microcosms containing pure cultures of V. vulnificus suggested that survival was adversely affected by exposure to elevated salinities (>25 ppt). In a separate study conducted by Motes and DePaola (17), summer Gulf Coast oysters relayed to high-salinity offshore waters (>32 ppt) showed a significant reduction in V. vulnificus numbers after a 2-week period. These results suggest that salinity extremes may play a pivotal role in the survival and growth of V. vulnificus. High salinities for extended periods were not observed at the Gulf Coast sites; however, when salinities increased above 25 ppt, V. vulnificus counts were reduced. A rapid increase in salinity to >25 ppt at the Apalachicola Bay site in mid-August 1994 and again in late August and September 1995 coincided with rapid declines in V. vulnificus levels within the oysters.
A clear relationship between salinity and V. vulnificus counts has not been established. We suggest that within the range of salinities normally encountered in northern Gulf estuaries characterized by high oyster production, salinity plays little role in controlling V. vulnificus numbers. Results obtained with regression models generated from our salinity and temperature data support this observation. However, the majority of observations in this Gulf data were made when the salinity was between 5 and 25 ppt; observations at both the upper and lower ends of the range were minimal. Salinities higher than 25 ppt do have a negative effect on V. vulnificus numbers in oysters. The data from the Atlantic Coast sites support this, as do observations on a few samples from the Florida site which had salinities of >25 ppt.
V. vulnificus has been isolated from oysters from numerous Atlantic Coast sites (19, 20, 30); however, there have not been any documented illnesses associated with Atlantic Coast oysters (4, 21). We observed that V. vulnificus was isolated less frequently and at lower densities from the Atlantic Coast oysters than from the Gulf Coast oysters. During warm-weather months, the levels of V. vulnificus in Atlantic Coast oysters were nearly 2 log units lower than in Gulf Coast oysters. On the other hand, Wright et al. (30) reported that levels of V. vulnificus in Chesapeake Bay oysters were similar to the levels in Gulf Coast oysters during the warm months. If equal levels occurred, it may be expected that Chesapeake Bay oysters would be the source of some cases. It should be noted that the enumeration procedures used in the Chesapeake Bay study employed a gene probe technique. It is possible that the enumerating techniques are measuring different portions of the V. vulnificus population or that the strains of V. vulnificus in the two locations differ in virulence. In addition, the sharp decline in the Chesapeake harvest in recent years (15) might have made it hard to observe this rare type of infection.
In 1994 and 1995, 39 V. vulnificus illnesses were traced to specific oyster harvest areas (4). Of these, 20 were traced to either Black Bay, La., or Apalachicola Bay, Fla. The contribution of these areas to the total Gulf harvest during the warm periods of the year, when most cases occur, is a possible linkage of oyster-associated V. vulnificus cases to these locations. Unfortunately, there is no statistical data to accurately determine the harvest from specific areas and the percentage of oysters from each area that are consumed raw.
The 13 illnesses traced to oysters harvested from the study areas occurred from late April to early November, the times when cases typically occur. Four of these cases occurred in May 1995; May is the month with the highest incidence of reported cases from 1992 to 1996 (4). The median V. vulnificus level in oyster samples harvested in May was 2,300 organisms per g, the same as during other warm months. The V. vulnificus MPN counts (Table 2) obtained from oysters sampled within 5 days of the date when the illness-associated oysters were harvested were not unusually high for the time of year (462 to 9,300 per g) and were consistent with those reported by Jackson et al. (9). Salinity and temperature data did not indicate that any unusual hydrographic or climatic events or conditions coincided with the harvest. However, the system for tracing oysters back to a specific harvest site is not foolproof, and the harvest areas are large and may contain microenvironments in which oysters contain different concentrations of V. vulnificus. Further, the V. vulnificus counts presented here are those present in the oysters at harvest and probably represent only a minimum count because of the possible multiplication that occurs after harvest (1, 2). The total numbers of V. vulnificus may be less relevant than the presence or proportion of virulent strains (9).
In conclusion, the concentration of V. vulnificus in oysters was similar in harvest areas across the northern Gulf Coast. Their abundance was influenced primarily by the water temperature. The numbers were at their highest from May through October, when the majority of shellfish-associated V. vulnificus illnesses occur. The two Atlantic estuaries sampled showed low levels of V. vulnificus even though the temperatures during the warm months were similar to Gulf temperatures; this could be due to the much higher salinity at these sites. The only high levels in the two Atlantic estuaries occurred in North Carolina, when flooding lowered the salinity to levels similar to those in the Gulf. Although salinities below 25 ppt are alike in permitting high V. vulnificus densities, salinities higher than 25 ppt appear to suppress the densities. Conversely, variations in surface water temperature above 26°C have little effect on densities, but the densities decline rapidly as temperatures decline below 26°C. Densities in Gulf Coast oysters at harvest rarely exceed 104 MPN per g, suggesting that oysters with counts higher than this level represent oysters that have been temperature abused. Numbers were <10 MPN per g during January and February, months when no illnesses have been recorded. This research establishes a baseline level for V. vulnificus in northern Gulf Coast oysters at harvest and reinforces findings that salinities of >25 ppt suppress V. vulnificus levels even in warm waters.
ACKNOWLEDGMENTS
We express our appreciation to the following individuals for assistance in sample collection and shipping: B. Perkins and N. Scarborough (Alabama State Health Department, Seafood Division, Mobile, Ala.); S. Moore and J. Shields (Florida Department of Environmental Protection, Bureau of Marine Resources, Regulation and Development, Apalachicola, Fla.); G. Mercadal and R. Anzalone (Louisiana Department of Health and Hospitals, Molluscan Shellfish Program, New Orleans, La.); W. Mobley (North Carolina Department of Environment, Health and Natural Resources, Shellfish Sanitation Branch, Moorehead City, N.C.); T. Yarborough (South Carolina Department of Health and Environmental Control, Shellfish Sanitation, Charleston, S.C.); and T. Previto (FDA, Office of Seafood, Gulf Coast Seafood Laboratory, Dauphin Island, Ala.). We are also grateful to R. Creasy, M. Glatzer, and D. Hesselman (FDA, Office of Regulatory Affairs, State Cooperative Programs, Southeast Region, Atlanta, Ga.) for technical advice and to R. M. McPhearson (FDA, Office of Seafood, Gulf Coast Seafood Laboratory) for reviewing the manuscript.
REFERENCES
- 1.Cook D W. Effect of time and temperature on multiplication of Vibrio vulnificus in postharvest Gulf Coast oysters. Appl Environ Microbiol. 1994;60:3483–3484. doi: 10.1128/aem.60.9.3483-3484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook D W. Refrigeration of oyster shellstock: conditions which minimize the outgrowth of Vibrio vulnificus. J Food Prot. 1997;60:349–352. doi: 10.4315/0362-028X-60.4.349. [DOI] [PubMed] [Google Scholar]
- 3.Cook D W, Ruple A D. Cold storage and mild heat treatment as processing aids to reduce the numbers of Vibrio vulnificus in raw oysters. J Food Prot. 1992;55:985–989. doi: 10.4315/0362-028X-55.12.985. [DOI] [PubMed] [Google Scholar]
- 4.Creasy, R., and M. Glatzer. 1995. Personal communication.
- 5.DePaola A, Capers G M, Alexander D. Densities of Vibrio vulnificus in the intestines of fish from the U.S. Gulf Coast Appl Environ Microbiol. 1994;60:984–988. doi: 10.1128/aem.60.3.984-988.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DePaola A, Motes M L, Cook D W, Veazey J, Garthright W E, Blodgett R. Evaluation of an alkaline phosphatase-labeled DNA probe for enumeration of Vibrio vulnificus in Gulf Coast oysters. J Microbiol Methods. 1997;29:115–120. [Google Scholar]
- 7.Elliot E L, Kaysner C A, Tamplin M L. U.S. Food and Drug Administration bacteriological analytical manual. 7th ed. Arlington, Va: AOAC International; 1992. V. cholerae, V. parahaemolyticus, V. vulnificus, and other Vibrio spp; pp. 111–140. [Google Scholar]
- 8.Hlady W G. Risk assessment and current data. In: Watkins W, McCarthy S, editors. Proceedings of the 1994 Vibrio vulnificus Workshop. Washington, D.C: Office of Seafood; 1994. pp. 27–33. [Google Scholar]
- 9.Jackson J K, Murphree R L, Tamplin M L. Evidence that mortality from Vibrio vulnificus infections results from single strains among heterogeneous populations in shellfish. J Clin Microbiol. 1997;35:2098–2101. doi: 10.1128/jcm.35.8.2098-2101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaspar C W, Tamplin M L. Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl Environ Microbiol. 1993;59:2425–2429. doi: 10.1128/aem.59.8.2425-2429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaysner C A, Abeyta C, Jr, Wekell M M, DePaola A, Jr, Stott R F, Leitch J M. Virulent strains of Vibrio vulnificus isolated from estuaries of the United States West Coast. Appl Environ Microbiol. 1987;53:1349–1351. doi: 10.1128/aem.53.6.1349-1351.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly M T. Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf Coast environment. Appl Environ Microbiol. 1982;44:820–824. doi: 10.1128/aem.44.4.820-824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klontz K C, Lieb S, Schreiber M, Janowski H T, Baldy L M, Gunn R A. Syndromes of Vibrio vulnificus infections: clinical and epidemiologic features in Florida cases, 1981–1987. Ann Intern Med. 1988;109:318–323. doi: 10.7326/0003-4819-109-4-318. [DOI] [PubMed] [Google Scholar]
- 14.Levine W C, Griffin P M the Gulf Coast Vibrio Working Group. Vibrio infections on the Gulf Coast: results of first year of regional surveillance. J Infect Dis. 1993;167:479–483. doi: 10.1093/infdis/167.2.479. [DOI] [PubMed] [Google Scholar]
- 15.MacKenzie C L. The molluscan fisheries of Chesapeake Bay. In: MacKenzie C L, Burrell V G, Rosenfield A, Hobart W L, editors. The history, present conditions and future of the molluscan fisheries of North and Central America and Europe. 1. Atlantic and Gulf Coast. NOAA Technical Report NMFS 127. Seattle, Wash: U.S. Department of Commerce; 1997. pp. 141–169. [Google Scholar]
- 16.Morris J G., Jr Vibrio vulnificus—a new monster of the deep. Ann Intern Med. 1988;109:261–263. doi: 10.7326/0003-4819-109-4-261. [DOI] [PubMed] [Google Scholar]
- 17.Motes M L, DePaola A. Offshore suspension relaying to reduce levels of Vibrio vulnificus in oysters (Crassostrea virginica) Appl Environ Microbiol. 1996;62:3875–3877. doi: 10.1128/aem.62.10.3875-3877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliver J D, Guthrie K, Preyer J, Wright A, Simpson L M, Siebeling R, Morris J G., Jr Use of colistin-polymyxin B-cellobiose agar for isolation of Vibrio vulnificus from the environment. Appl Environ Microbiol. 1992;58:737–739. doi: 10.1128/aem.58.2.737-739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliver J D, Warner R A, Cleland D R. Distribution of Vibrio vulnificus and other lactose-fermenting vibrios in the marine environment. Appl Environ Microbiol. 1983;45:985–998. doi: 10.1128/aem.45.3.985-998.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Neill K R, Jones S H, Grimes D J. Seasonal incidence of Vibrio vulnificus in the Great Bay estuary of New Hampshire and Maine. Appl Environ Microbiol. 1992;58:3257–3262. doi: 10.1128/aem.58.10.3257-3262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rippey S R. Infectious diseases associated with molluscan shellfish consumption. Clin Microbiol Rev. 1994;7:419–425. doi: 10.1128/cmr.7.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruple A D, Cook D W. Vibrio vulnificus and indicator bacteria in shellstock and commercially processed oysters from the Gulf coast. J Food Prot. 1992;55:667–671. doi: 10.4315/0362-028X-55.9.667. [DOI] [PubMed] [Google Scholar]
- 23.Tacket C O, Brenner F, Blake P A. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558–561. doi: 10.1093/infdis/149.4.558. [DOI] [PubMed] [Google Scholar]
- 24.Tamplin M. The ecology of Vibrio vulnificus. In: Watkins W, McCarthy S, editors. Proceedings of the 1994 Vibrio vulnificus Workshop. Washington, D.C: Office of Seafood; 1994. [Google Scholar]
- 25.Tamplin M, Rodrick G E, Blake N J, Cuba T. Isolation and characterization of Vibrio vulnificus from two Florida estuaries. Appl Environ Microbiol. 1982;44:1466–1470. doi: 10.1128/aem.44.6.1466-1470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamplin M L. The seasonal occurrence of Vibrio vulnificus in shellfish, seawater, and sediment of United States coastal waters and the influence of environmental factors on survival and virulence. Final report to Salstonstall-Kennedy Grant Program. Project NA27FD0117-01. U.S. Seattle, Wash: Department of Commerce; 1994. [Google Scholar]
- 27.Tamplin M L, Martin A L, Ruple A D, Cook D W, Kaspar C W. Enzyme immunoassay for identification of Vibrio vulnificus in seawater, sediment, and oysters. Appl Environ Microbiol. 1991;57:1235–1240. doi: 10.1128/aem.57.4.1235-1240.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamplin M L, Murphree R L, Robinson K S, Garrido V M, Gangar V V. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. A linear regression model to predict Vibrio vulnificus levels in U.S. estuaries, abstr. Q-395; p. 455. [Google Scholar]
- 29.Vanoy R W, Tamplin M L, Schwarz J R. Ecology of Vibrio vulnificus in Galveston Bay oysters, suspended particulate matter, sediment and seawater: detection by monoclonal antibody-immunoassay-most probable number procedures. J Ind Microbiol. 1992;9:219–223. [Google Scholar]
- 30.Wright A C, Hill R T, Johnson J A, Roghman M-C, Colwell R R, Morris J G., Jr Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl Environ Microbiol. 1996;62:717–724. doi: 10.1128/aem.62.2.717-724.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]