Abstract
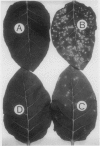
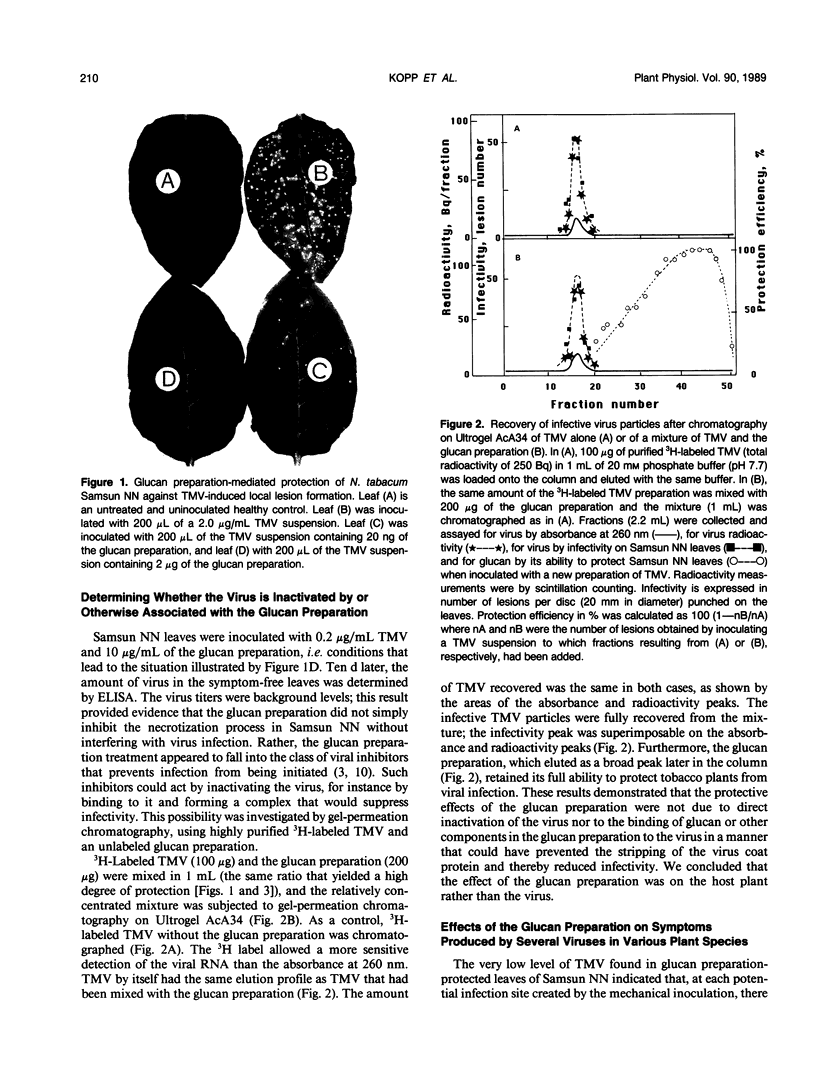
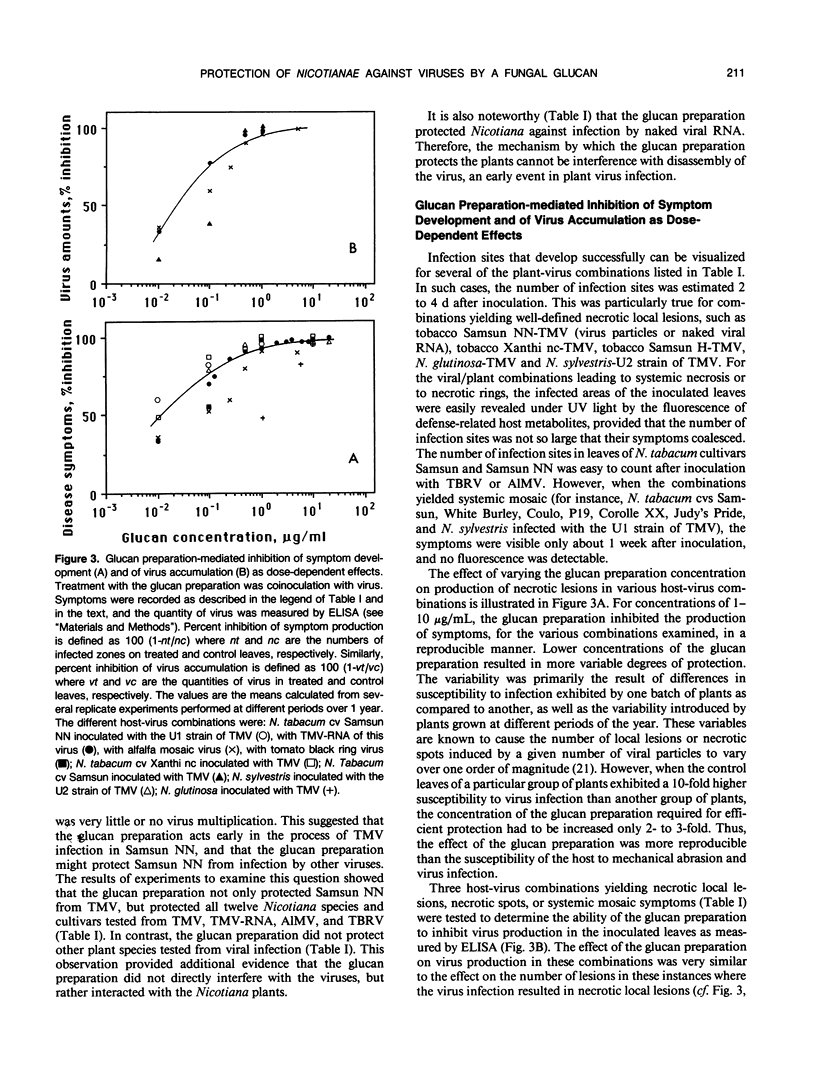
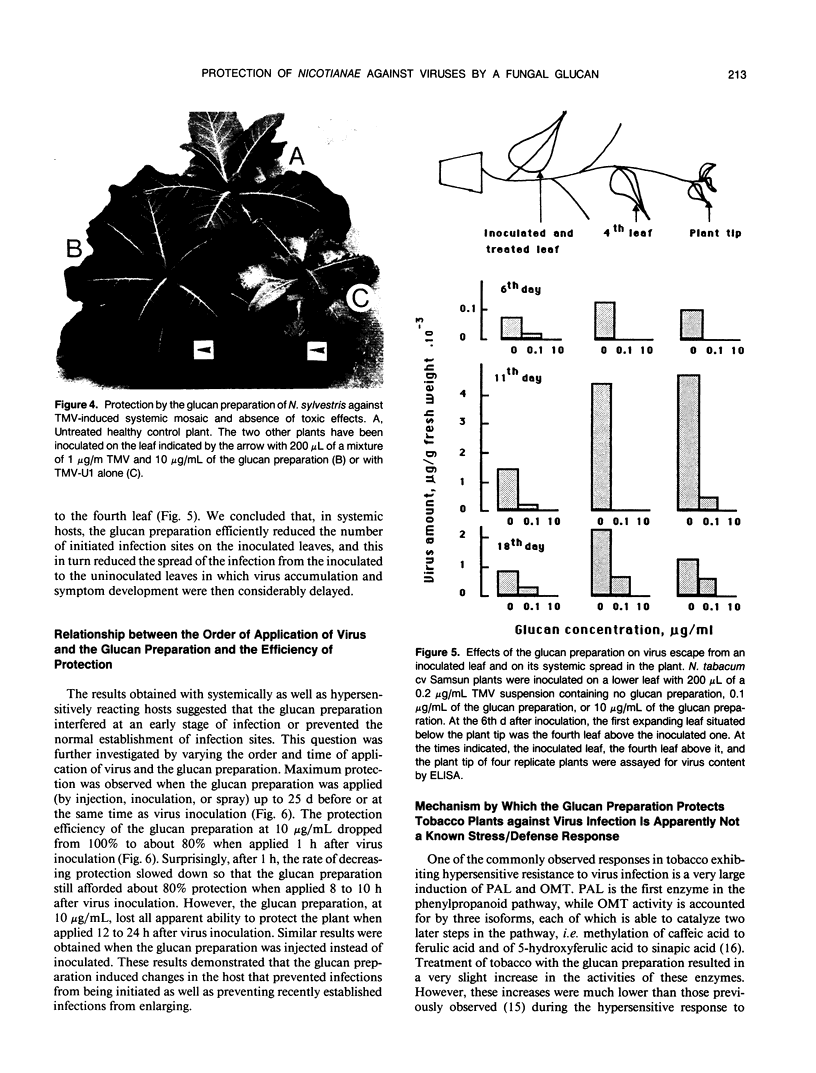
A glucan preparation obtained from the mycelial walls of the fungus Phytophthora megasperma f.sp. glycinea and known as an elicitor of phytoalexins in soybean was shown to be a very efficient inducer of resistance against viruses in tobacco. The glucan preparation protected against mechanically transmitted viral infections on the upper and lower leaf surfaces. Whether the glucan preparation was applied by injection, inoculation, or spraying, it protected the plants if applied before, at the same time as, or not later than 8 hours after virus inoculation. At concentrations ranging from 0.1 to 10 micrograms per milliliter, the glucan preparation induced protection ranging from 50 to 100% against both symptom production (necrotic local lesions, necrotic rings, or systemic mosaic) and virus accumulation in all Nicotiana-virus combinations examined. However, no significant protection against some of the same viruses was observed in bean or turnip. The host plants successfully protected included N. tabacum (9 different cultivars), N. sylvestris, N. glutinosa, and N. clevelandii. The viruses belonged to several taxonomic groups including tobacco mosaic virus, alfalfa mosaic virus, and tomato black ring virus. The glucan preparation did not act directly on the virus and did not interfere with virus disassembly; rather, it appeared to induce changes in the host plant that prevented infections from being initiated or recently established infections from enlarging. The induced resistance does not depend on induction of pathogenesis-related proteins, the phenylpropanoid pathway, lignin-like substances, or callose-like materials. We believe the induced resistance results from a mechanism that has yet to be described.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel P. P., Nelson R. S., De B., Hoffmann N., Rogers S. G., Fraley R. T., Beachy R. N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science. 1986 May 9;232(4751):738–743. doi: 10.1126/science.3457472. [DOI] [PubMed] [Google Scholar]
- Albersheim P., Valent B. S. Host-pathogen interactions in plants. Plants, when exposed to oligosaccharides of fungal origin, defend themselves by accumulating antibiotics. J Cell Biol. 1978 Sep;78(3):627–643. doi: 10.1083/jcb.78.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAWDEN F. C. Inhibitors and plant viruses. Adv Virus Res. 1954;2:31–57. doi: 10.1016/s0065-3527(08)60528-x. [DOI] [PubMed] [Google Scholar]
- Gupta B. M., Chandra K., Verma H. N., Verma G. S. Induction of antiviral resistance in Nicotiana glutinosa plants by treatment with Trichothecium polysaccharide and its reversal by actinomycin D. J Gen Virol. 1974 Jul;24(1):211–213. doi: 10.1099/0022-1317-24-1-211. [DOI] [PubMed] [Google Scholar]
- Kauffmann S., Legrand M., Geoffroy P., Fritig B. Biological function of ;pathogenesis-related' proteins: four PR proteins of tobacco have 1,3-beta-glucanase activity. EMBO J. 1987 Nov;6(11):3209–3212. doi: 10.1002/j.1460-2075.1987.tb02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konate G., Fritig B. Extension of the ELISA method to the measurement of the specific radioactivity of viruses in crude cellular extracts. J Virol Methods. 1983 Jun;6(6):347–356. doi: 10.1016/0166-0934(83)90057-5. [DOI] [PubMed] [Google Scholar]
- Legrand M., Kauffmann S., Geoffroy P., Fritig B. Biological function of pathogenesis-related proteins: Four tobacco pathogenesis-related proteins are chitinases. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6750–6754. doi: 10.1073/pnas.84.19.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson A., von Wechmar M. B., van Regenmortel M. H. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol Commun. 1980;9(5):475–493. doi: 10.3109/08820138009066010. [DOI] [PubMed] [Google Scholar]
- Sharp J. K., Valent B., Albersheim P. Purification and partial characterization of a beta-glucan fragment that elicits phytoalexin accumulation in soybean. J Biol Chem. 1984 Sep 25;259(18):11312–11320. [PubMed] [Google Scholar]