Abstract
Background
The role of haloperidol as treatment for ICU delirium and related symptoms remains controversial despite two recent large controlled trials evaluating its efficacy and safety. We sought to determine whether haloperidol when compared to placebo in critically ill adults with delirium reduces days with delirium and coma and improves delirium-related sequelae.
Methods
This multi-center double-blind, placebo-controlled randomized trial at eight mixed medical-surgical Dutch ICUs included critically ill adults with delirium (Intensive Care Delirium Screening Checklist ≥ 4 or a positive Confusion Assessment Method for the ICU) admitted between February 2018 and January 2020. Patients were randomized to intravenous haloperidol 2.5 mg or placebo every 8 h, titrated up to 5 mg every 8 h if delirium persisted until ICU discharge or up to 14 days. The primary outcome was ICU delirium- and coma-free days (DCFDs) within 14 days after randomization. Predefined secondary outcomes included the protocolized use of sedatives for agitation and related behaviors, patient-initiated extubation and invasive device removal, adverse drug associated events, mechanical ventilation, ICU length of stay, 28-day mortality, and long-term outcomes up to 1-year after randomization.
Results
The trial was terminated prematurely for primary endpoint futility on DSMB advice after enrolment of 132 (65 haloperidol; 67 placebo) patients [mean age 64 (15) years, APACHE IV score 73.1 (33.9), male 68%]. Haloperidol did not increase DCFDs (adjusted RR 0.98 [95% CI 0.73–1.31], p = 0.87). Patients treated with haloperidol (vs. placebo) were less likely to receive benzodiazepines (adjusted OR 0.41 [95% CI 0.18–0.89], p = 0.02). Effect measures of other secondary outcomes related to agitation (use of open label haloperidol [OR 0.43 (95% CI 0.12–1.56)] and other antipsychotics [OR 0.63 (95% CI 0.29–1.32)], self-extubation or invasive device removal [OR 0.70 (95% CI 0.22–2.18)]) appeared consistently more favorable with haloperidol, but the confidence interval also included harm. Adverse drug events were not different. Long-term secondary outcomes (e.g., ICU recall and quality of life) warrant further study.
Conclusions
Haloperidol does not reduce delirium in critically ill delirious adults. However, it may reduce rescue medication requirements and agitation-related events in delirious ICU patients warranting further evaluation.
Trial registration: ClinicalTrials.gov (#NCT03628391), October 9, 2017.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-023-04692-3.
Keywords: Delirium, Haloperidol, Randomized controlled trial, Antipsychotic, Critical care
Background
Delirium is an acute neuropsychiatric syndrome occurring in up to 50% of critically ill adults [1]. It is associated with longer hospital stay, long-term cognitive dysfunction and increased costs [2–4]. Haloperidol has been the preferred agent to treat delirium for decades [5]. Current guidelines make a conditional recommendation against the routine administration of haloperidol to treat delirium in critically ill adults, only considering its use in case of significant distress or agitation [6]. Instead, these guidelines advocate the use of non-pharmacologic strategies, including the ABCDEF bundle [6]. However, common delirium symptoms, including agitation, hallucinations and delusions, often respond poorly to non-pharmacologic treatments.
Current evidence regarding the use of haloperidol to treat ICU delirium is derived from two recent major trials (MIND-USA and AID-ICU trial [7, 8]) that found haloperidol to be ineffective in reducing delirium duration [9]. Importantly, neither trial rigorously evaluated key delirium-related endpoints including agitation-related sequelae nor the occurrence of psychotic symptoms, although administration of haloperidol is usually targeted at these clinical endpoints in routine practice and these outcomes are likely just as important from both a clinician and patient/family perspective [10].
We conducted a multi-center, double-blind, placebo-controlled, randomized clinical trial to assess the efficacy and safety of haloperidol as treatment for delirium and its associated symptoms and outcomes in critically ill adults.
Methods
Study design, setting and participants
We conducted a multi-center randomized, double-blind, placebo-controlled clinical trial in adults with delirium at eight ICUs in the Netherlands. The study protocol was approved by the Medical Ethics Committees of all participating hospitals and has been published [11]. An independent data and safety monitoring board (DSMB) provided oversight of the trial.
All adult (≥ 18 years) ICU patients were eligible unless they met one or more exclusion criteria: admission to the ICU because of a primary acute neurological condition; pregnancy or breast-feeding; known allergy to haloperidol; history of ventricular arrhythmia (including torsade de pointes); neuroleptic malignant syndrome; parkinsonism; schizophrenia or other psychotic disorder; dementia or an Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) score ≥ 4[12]; expected duration of ICU admission < 24 h; inability to speak the Dutch language or to undergo a valid delirium assessment (e.g., deafness or blindness); or participation in another interventional trial [11]. Eligible patients or their legal representatives were asked for written informed consent as soon as possible after ICU admission to enable randomization as soon as possible after delirium was first diagnosed.
Trained ICU nurses evaluated each patient three times daily (once per 8-h shift) for level of sedation with the Richmond Agitation Sedation Scale (RASS) [13] and for delirium with either the Intensive Care Delirium Screening Checklist (ICDSC) [14] or the Confusion Assessment Method for the ICU (CAM-ICU) [15] in the absence of coma (RASS score − 4 or − 5). Each participating ICU used one of these delirium assessment methods consistently and non-interchangeably [16]. Three ICUs used the ICDSC and five used the CAM-ICU. Once eligible patients with written informed consent developed delirium (a positive CAM-ICU or an ICDSC score ≥ 4), they were randomized if none of the above exclusion criteria were present, and none of the additional criteria for randomization were met: QTc prolongation (QTc > 500 ms), acute alcohol (or substance) withdrawal syndrome, an expected ICU stay < 24 h, or torsade de pointes, neuroleptic malignant syndrome or parkinsonism since ICU admission [11]. During the study, we encountered eligible patients that were missed for randomization and already treated with haloperidol at the ICU > 24 h or received more than 3 doses, and they were also excluded from randomization.
Randomization and masking, study procedures and interventions
Randomization was computer-generated using a block design of eight patients per block (4 haloperidol and 4 placebo in random order), stratified by participating center [11]. The haloperidol and placebo ampoules were identical in appearance. Only the involved site-pharmacists and the trial statistician had access to the contents of each medication kit.
The study drug was administered intravenously, starting with 2.5 mg three times daily (for patients ≥ 80 years 1 mg), and increased up to 5 mg three times daily (for patients ≥ 80 years 2.5 mg) if delirium persisted in the next 8-h shift. The first dose was administered immediately after randomization or at the subsequent regular medication prescription in the electronic patient system (every 8 h). When delirium had resolved (or was not assessable due to coma) for 24 h, study drug was decreased (from 5 to 2.5 mg for patients < 80 years or from 2.5 to 1 mg for patients ≥ 80 years) or stopped (if at a dose of 2.5 mg for patients < 80 years or 1 mg for patients ≥ 80 years). The study drug was restarted if delirium re-occurred within the 14-day intervention period and the patient remained at the ICU. At the discretion of the ICU clinical team, and after consultation with the research team when necessary, doses could be lowered (or held) when safety concerns presumably related to haloperidol were suspected (i.e., drug associated adverse effects, as described in Additional file 1: S1). After the 14-day intervention period, treatment with haloperidol was permitted as per local treatment protocol.
Open-label haloperidol during the 14-day intervention period was strongly discouraged. If agitation did not resolve after potential causes (e.g., acute pain) were identified and treated, it could be treated with an alpha-2 agonist (e.g., dexmedetomidine, clonidine) or GABA agonist (e.g., propofol, benzodiazepine). Patients appearing to be in distress (e.g., from hallucinations) could be treated with a low-dose atypical antipsychotic. Standard clinical practice was followed according to clinical protocols, based on the clinical practice guidelines from the Society of Critical Care Medicine that were implemented in a previous multi-center implementation study [6, 16, 17]. At the start of the study, all centers were subjected to a qualitative survey on perceived adherence to components of the ABCDE (awakening and breathing coordination, choice of sedation, delirium monitoring and management, and early mobility) bundle (Additional file 1: S2). Quality ascertainment of delirium assessments with spot checks assured accurateness of > 90% (Additional file 1: S2).
Outcomes
The primary outcome was the number of delirium- and coma-free days (DCFDs) while alive and admitted to the ICU up to 14 days after randomization. A delirium day was defined as at least one positive delirium assessment on that calendar day where the RASS score was ≥ − 3. A patient was considered to be comatose when any RASS score on that day was − 4 or − 5 in the absence of documented delirium or if delirium was indicated to be not assessable due to coma [7]. Patients who died within the intervention period were considered to have 0 DCFDs for the whole intervention period, in line with previous intervention trials [18, 19]. Patients who were discharged from the ICU during the intervention period were considered to be delirium- and coma-free after ICU discharge, regardless of their delirium status at discharge [7, 20]. We conducted prespecified subgroup analyses on motor subtype, presence of hallucinations or delusions, delirium severity, and delirium phenotype [11].
All outcomes refer to the 14-day intervention period, which started after randomization. Predefined secondary outcomes were daily RASS scores, maximum mobility level, sleep quality, use of “escape medication” for hallucinations and/or agitation (including atypical antipsychotics, alpha-2 agonists [clonidine and dexmedetomidine], GABA agonists [benzodiazepines and propofol], opiates and ‘open-label’ haloperidol), daily study drug dose corrected for body weight (mg/kg), self-extubation rate and patient-initiated removal of invasive devices, (serious) adverse drug associated events (prolonged QTc by EKG, muscle rigidity and other associated movement disorders [Simpson Angus Scale] [21], ventricular arrhythmias including torsade de pointes), blood pressure after first study drug dose, daily respiratory status, time from randomization to first resolution of delirium, time to readiness for discharge from the ICU and 28-day mortality [11].
We also assessed the following prospectively collected post hoc exploratory outcomes: number of days with study drug administration, and number of days with delirium, coma, agitation (defined as a RASS score + 2 or more at least once on that day[18]) or hallucinations/delusions, auto-removal of urinary catheter, physical restraint and (almost) fell or stepped out of bed.
Secondary predefined long-term outcomes after hospital discharge were patient and family member memories and experiences at hospital discharge and 3 months after randomization, post-traumatic stress disorder (PTSD) symptoms and burden experienced by the family at 3 months after randomization, anxiety, depression, cognition, and quality of life at 3 and 12 months after randomization, and 12-month mortality.
Data collection and definitions of all subgroup analyses and outcomes are described in detail in Additional file 1: S1 and in the EuRIDICE study protocol [11].
Sample size calculation and statistical analyses
We aimed to include 742 patients (371 in each group) to provide 90% power to detect a one-day difference in DCFDs (alpha level of 0.05, with non-parametric testing) assuming an average prevalence of 3.2 DCFDs in the control group and 4.2 in the haloperidol group (standard deviation [SD] in both groups 4.2); the estimates were based on a previous study by our group [16].
Categorical data were presented as numbers (percentages) and continuous data as means (SD) or median (interquartile range [IQR]). Categorical data were compared using Chi-square tests or Fisher’s Exact Tests. For continuous data, independent sample t-tests or Mann Whitney-U tests were used, all when appropriate.
For the primary outcome, we analyzed differences between the groups with a Poisson or negative binomial mixed effects model, depending on the presence of overdispersion. We performed adjusted analyses based on significant differences in baseline characteristics between treatment groups (when present) as covariates and hospital as a random effect.
For the secondary outcomes, we adjusted analyses similarly. For data with a Poisson distribution, we used a Poisson or negative binomial mixed effects model. For other continuous secondary outcomes, linear mixed effects models were used. We applied a mixed effects Cox proportional hazards model for time to ICU discharge, censoring at death, withdrawal of consent before ICU discharge, and loss to follow-up (i.e., transferred to another ICU not participating in the trial). Additionally, categorical secondary outcomes were analyzed with logistic mixed effects models. Mortality risk was assessed as a binary endpoint at 28-days after randomization. One-year mortality was expressed as time until death, calculated as time from randomization until death, and was analyzed with a mixed-effect Cox proportional model using hospital as a random effect and censoring if death had not occurred during the 1-year follow-up, if informed consent was withdrawn or if patients were lost to follow-up during the 1-year follow-up. We analyzed all data using an intention-to-treat-principle.
We used SPSS (version 25) and R software (version 1.3.1073). A p value < 0.05 (two-sided) was considered statistically significant. The results are reported using the CONSORT statement [22].
Interim analysis and trial termination
Interim analyses, focused on safety and futility, were pre-specified by a DSMB charter to occur at the one-third and two-third enrolment benchmarks or every 6 months, whichever occurred first. Recruitment started in February 2018. Due to slow recruitment, the steering committee and DSMB agreed to decrease the statistical power from 90 to 80% in April 2019, lowering the intended sample size to 554 patients. Based on a subsequent pre-planned interim analyses conducted on October 31, 2019 (n = 118), the DSMB advised to stop recruitment on December 19, 2019, because of expected futility of being able to reach a significant one-day difference between treatment groups in the primary outcome of DCFD in the intended sample size of 554 patients. Patient recruitment for the trial was terminated on January 22, 2020, at 26% of the intended sample size (n = 142).
Results
Patient characteristics and interventions
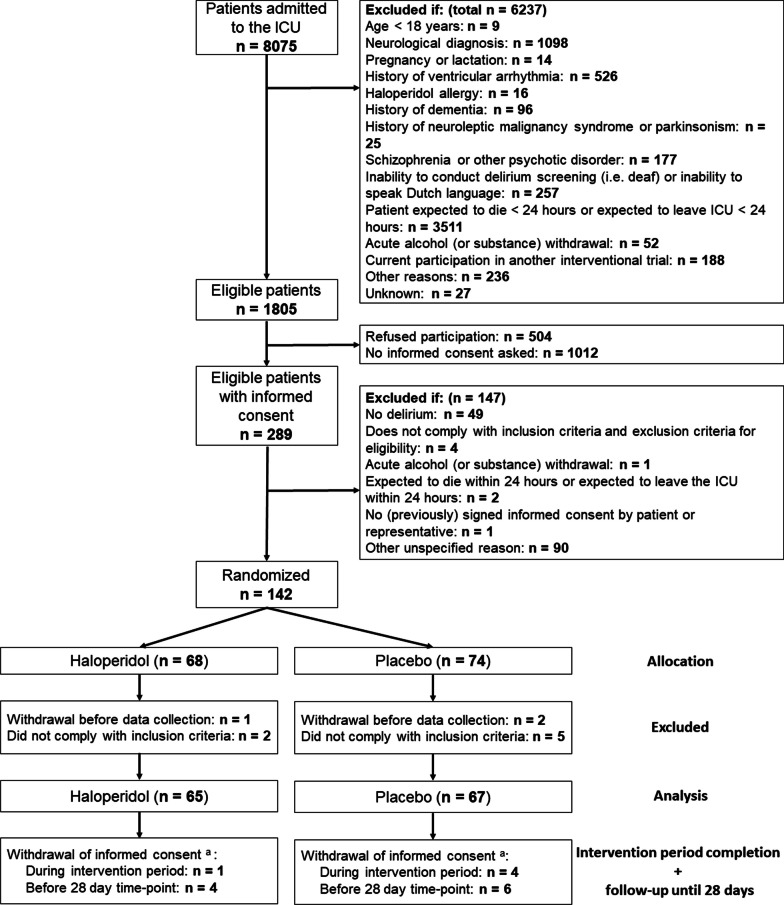
From February 21, 2018 to January 22, 2020, we screened 8075 ICU patients. Follow-up was completed on April 12, 2021, 15 months after randomization was halted based on the DSMB advice of further futility. Written informed consent was obtained from 289 (16%) of 1805 eligible patients (Fig. 1). Subsequently, 142 patients developed delirium and were randomized. Ten randomized patients were withdrawn before first study drug administration, because they did not comply with the inclusion criteria or withdrew informed consent. Consequently, we analyzed data from 132 patients (65 haloperidol, 67 placebo). A total of 91 patients were eligible for the follow-up period (45 haloperidol, 46 placebo; details provided in Additional file 1: S3).
Fig. 1.
Flow of participants in the EuRIDICE trial. aNone of the patients who withdrew informed consent rejected the use of their yet collected data. They, however, refused further participation in the trial
The mean age of included patients was 64 years (SD 15.3), and 90 (68%) were male. The overall mean APACHE IV score was 73.1 (SD 33.9). The groups were similar at baseline, except for the median modified SOFA score (mSOFA) at randomization, which was significantly lower in the haloperidol group (p = 0.03), Table 1 and Additional file 1: Table E1. Information related to delirium screening instrument used in included patients is shown in Additional file 1: Table E2.
Table 1.
Baseline characteristics of the patients
| Characteristic | Haloperidol (n = 65) | Placebo (n = 67) |
|---|---|---|
| Age, median (IQR), year | 66 (57–75.5) | 68 (60–74) |
| Male, n (%) | 48 (74) | 42 (63) |
| Location before ICU admission | ||
| Hospital ward, n (%) | 29 (45) | 31 (46) |
| Operation room/recovery, n (%) | 17 (26) | 18 (27) |
| ED, n (%) | 12 (19) | 11 (16) |
| Other, n (%) | 7 (11) | 7 (10) |
| Type of ICU admission | ||
| Medical, n (%) | 36 (55) | 39 (58) |
| Emergency surgery, n (%) | 17 (26) | 17 (25) |
| Elective surgery, n (%) | 12 (19) | 11 (16) |
| ICU admission diagnosis | ||
| Respiratory, n (%) | 24 (37) | 28 (42) |
| Gastrointestinal, n (%) | 14 (22) | 10 (15) |
| Cardiovascular, n (%) | 9 (14) | 16 (24) |
| Transplant, n (%) | 7 (11) | 5 (8) |
| Trauma, n (%) | 5 (8) | 1 (2) |
| Other, n (%) | 6 (9) | 7 (10) |
| APACHE IV score, median (IQR) | 70 (57–90.5) | 77 (55–99) |
| mSOFA score at randomization, median (IQR) | 5 (3–7)* | 6 (4–9) |
| Baseline QTc at randomization, mean (SD) | 428.9 (29.9) | 421.3 (32.1) |
| No. of days from ICU admission to randomization, median (IQR) | 4 (2–10.5) | 6 (3–9) |
APACHE acute physiology and chronic health evaluation, ED emergency department, ICU intensive care unit, mSOFA modified sequential organ failure assessment (without the central nervous system component), SOFA sequential organ failure assessment, Yr year
*Significantly differed from the placebo group (p < 0.05)
Primary outcome: delirium- and coma-free days
The median number of DCFDs was not different between the haloperidol (9 [IQR 3–12]) and placebo group (9 [IQR 2–11]), p = 0.66 (Table 2). After adjusting for mSOFA at randomization and a random effect for hospital, the number of DCFDs remained similar (adjusted RR [aRR] 0.98 [95% CI 0.73–1.31], p = 0.87, Table 2 and Additional file 1: Figures E1 and E2).
Table 2.
Comparison of delirium- and coma-free days and predefined secondary outcomes between the haloperidol and placebo group
| Outcome | Haloperidol (n = 65) | Placebo (n = 67) | Adjusted difference (95%CI) | Adjusted relative risk (95% CI)a | p value |
|---|---|---|---|---|---|
| Primary outcome: delirium- and coma-free days | |||||
| No. of DCFDs, median (IQR)b | 9 (3–12) | 9 (2–11) | 0.98 (0.73–1.31) | 0.871 | |
| Predefined secondary outcomes: clinical outcomes | |||||
| Daily RASS score, median (IQR) | − 0.5 (− 0.9 to − 0.1) | − 0.3 (− 0.6 to − 0.1) | − 9.9% (− 55.1% to 80.6%)c | 0.777 | |
| Maximum mobility level, median (IQR)d | 4 (1.5–5) | 3 (1.8–5) | − 0.03 (− 0.8 to 0.74) | 0.938 | |
| Sleep quality as assessed by nurse, mean (SD)d | 4.2 (1.3) | 4.4 (1.2) | − 0.26 (− 0.7 to 0.18) | 0.251 | |
| Sleep quality according to patient (RCSQ), mean (SD)d | 4.1 (1.4) | 4.4 (1.5) | − 0.27 (− 0.92 to 0.38) | 0.416 | |
| Mechanical ventilation, n (%) | 50 (77) | 51 (76) | OR: 1.17 (0.51–2.71) | 0.707 | |
| No. of days, median (IQR) | 2 (1–7.5) | 2 (1–7) | 1.17 (0.77–1.78) | 0.465 | |
| Time to first resolution of delirium in days, median (IQR) | 2 (1–3) | 2 (1–4) | − 16.6% (− 35.5% to 7.7%)c | 0.168 | |
| Median time to ICU discharge alive, days (95% CI)d,e | 18.1 (9.8–26.4) | 15.5 (12.3–18.7) | HR: 0.69 (0.47–1.02) | 0.061 | |
| 28-day mortality, n (%)d | 10 (16) | 13 (21) | 0.79 (0.31–2.01) | 0.622 | |
| Predefined secondary outcomes: medication-related outcomes | |||||
| Daily study drug corrected for body weight (mg/kg), median (IQR) | 0.08 (0.05–0.11) | 0.10 (0.08–0.13) | − 0.01 (− 0.03 to 0.00) | 0.101 | |
| Use of “escape medication”, n (%) | 64 (99) | 64 (96) | OR: 3.59 (0.43–75.62) | 0.283 | |
| Open-label haloperidol, n (%) | 4 (6) | 9 (13) | OR: 0.43 (0.12–1.56) | 0.201 | |
| Open-label haloperidol, mean 24 h dose in mg, mean (SD)c | 3.6 (3.2) | 2.6 (1.1) | 1.32 (− 0.9 to 3.54) | 0.295 | |
| Atypical antipsychotic, n (%)f | 23 (35) | 32 (48) | OR: 0.63 (0.29–1.32) | 0.223 | |
| Clonidine, n (%) | 26 (40) | 34 (51) | OR: 0.61 (0.28–1.30) | 0.198 | |
| Dexmedetomidine, n (%) | 13 (20) | 16 (24) | OR: 0.87 (0.31–2.46) | 0.787 | |
| Benzodiazepine, n (%) | 37 (57) | 49 (73) | OR: 0.41 (0.18–0.89) | 0.028 | |
| Propofol, n (%) | 39 (60) | 38 (57) | OR: 1.43 (0.67–3.07) | 0.357 | |
| Opioid, n (%) | 57 (88) | 60 (90) | OR: 0.94 (0.31–2.88) | 0.919 | |
| Other sedatives, n (%) | 3 (5) | 6 (9) | OR: 0.42 (0.08–1.79) | 0.256 | |
| Predefined secondary outcomes: safety outcomes | |||||
| Self-extubation or removal of invasive devices, ever, n (%) | 6 (9.2) | 10 (14.9%) | OR: 0.70 (0.22–2.18) | 0.539 | |
| QTc prolongation, n (%) | 3 (5) | 6 (9) | OR: 0.62 (0.12–2.71) | 0.535 | |
| Muscle rigidity and associated movement disorders, n (%) | 3 (5) | 1 (2) | OR: 4.52 (0.53–97.33) | 0.211 | |
| Ventricular arrhythmia, n (%) | 4 (6) | 1 (2) | OR: 5.21 (0.71–105.98) | 0.153 | |
| Systolic blood pressure change after first study drug dose, median (IQR) | − 5 (− 21 to 9.25) | 2 (− 4.5 to 10) | − 12.41 (− 19.63 to 5.18) | 0.001 | |
| Diastolic blood pressure change after first study drug dose, median (IQR) | − 3 (− 9 to 1) | 2 (− 2 to 6.5) | − 7.96 (− 11.78 to − 4.20) | < .001 | |
CI confidence interval, DCFD delirium- and coma-free day, IQR interquartile range, OR odds ratio, RR relative risk
aRR unless mentioned otherwise. The placebo group was used as a reference. The RR may be interpreted as follows: the number of DCFDs in the haloperidol group is 0.98 times the number in the placebo group
bThe mean number of DCFDs in the haloperidol group was 7.4 (SD 4.7) and in the placebo group 7.2 (SD 4.8). In order to increase comparability and to assess the impact of assumptions related to the definition of DCFD, post hoc analyses were performed (Additional file 1: Online Supplement 4)
cAfter log transformation due to non-normality and/or heteroscedasticity, and needs to be interpreted as adjusted percent change for the haloperidol vs. placebo group. For example, compared to placebo, haloperidol decreases the median time to first resolution of delirium in days by 16.6 percent (adjusted percent change for haloperidol vs. placebo is − 16.6%, not significant) if all other variables are kept constant
dData were missing for some patients: maximum mobility 1 (0.8%), mean sleep quality as assessed by nurse 5 (3.9%), mean sleep quality according to patient 50 (37.9%), systolic blood pressure change after first study drug administration 15 (11.4%), diastolic blood pressure change after first study drug administration 15 (11.4%), time to ICU discharge 3 (2.3%), 28-day mortality 10 (7.6%). No missing data were present for the other outcomes, study drug or covariates (mSOFA and hospital)
eUnadjusted differences were estimated with the Kaplan–Meier method
fOlanzapine or quetiapine
Predefined secondary outcomes
The results of the secondary outcomes are presented in Table 2 and Additional file 1: Table E3. Significantly fewer haloperidol-treated (vs. placebo) patients ever received a benzodiazepine (57% vs. 73%, adjusted OR [aOR] 0.41 [95%CI 0.18–0.89], p = 0.03; both continuous infusion and intermittent, Additional file 1: Table E4). The patients in the haloperidol group had significantly lower systolic and diastolic blood pressure after the first study drug dose than the placebo group (adjusted difference in systolic blood pressure change: − 12.41 [95% CI − 19.63 to 5.18], p = 0.001). No statistically significant differences in adverse drug associated events were observed. There was no statistically significant difference in duration of ventilation and 28-day mortality. Effect measures of secondary outcomes related to agitation (use of open label haloperidol [aOR 0.43 (95% CI 0.12–1.56)] and other antipsychotics [aOR 0.63 (95% CI 0.29–1.32)] and self-extubation or invasive device removal [aOR 0.70 (95% CI 0.22–2.18)]) appeared consistently more favorable with haloperidol treatment, although the confidence interval also included no effect. The results of the long-term outcomes are provided in Additional file 1: S5. Patients randomized to haloperidol experienced fewer intrusive memories than the placebo group at hospital discharge (adjusted OR 0.40, 95% CI 0.40–0.40, p < 0.001). At 3 months after randomization, the haloperidol group was less likely to remember their ICU admission (adjusted OR 0.20, 95% CI 0.06–0.72, p = 0.014), and perceived their general health as better than the placebo group (adjusted difference 8.75, 95% CI 1.03–16.47, p = 0.032).
Prespecified subgroup analyses
The prespecified subgroup analyses for motor subtype, presence of hallucinations or delusions, delirium severity, and delirium phenotype showed no statistically significant differences between groups (Additional file 1: Table E5).
Post hoc outcomes
Patients who received haloperidol were less likely to fall or step out of bed than the placebo group (9% vs. 27%, aOR 0.32 [95% CI 0.11–0.84], p = 0.03), Table 3 and Additional file 1: Table E6. No other statistically significant differences were observed.
Table 3.
Comparison of post hoc exploratory secondary outcomes between the haloperidol and placebo group
| Outcome | Haloperidol (n = 65) | Placebo (n = 67) | Adjusted relative risk (95% CI)a | p value |
|---|---|---|---|---|
| No. of days with study drug, median (IQR) | 4 (3–8) | 6 (3–9) | 0.96 (0.79–1.16) | 0.66 |
| No. of delirium days, median (IQR) | 3 (2–6.5) | 3 (2–5) | 1.05 (0.81–1.37) | 0.722 |
| No. of coma days, median (IQR) | 0 (0–2) | 0 (0–1) | 1.5 (0.85–2.66) | 0.164 |
| Agitation (RASS > 1), n (%) | 25 (39) | 30 (45) | OR: 0.84 (0.4–1.75) | 0.638 |
| No. of days, median (IQR) | 0 (0–1) | 0 (0–1) | 0.77 (0.43–1.39) | 0.388 |
| Hallucinations/delusions, n (%) | 55 (85) | 51 (76) | OR: 1.75 (0.72–4.4) | 0.220 |
| No. of days, median (IQR) | 2 (1–3) | 3 (1–4.5) | 0.74 (0.53–1.03) | 0.075 |
| Removal of urinary catheter, n (%) | 5 (8) | 9 (13) | OR: 0.48 (0.14–1.52) | 0.226 |
| Physical restraint, n (%) | 48 (74) | 48 (72) | OR: 1.19 (0.54–2.64) | 0.660 |
| (Almost) fell or stepped out of bed, n (%) | 6 (9) | 18 (27) | OR: 0.32 (0.11–0.84) | 0.026 |
CI confidence interval, IQR interquartile range, NA not applicable, OR odds ratio, RR relative risk
aRR unless otherwise noted. The placebo group is used as reference
Discussion
This multi-center randomized double-blind, placebo-controlled trial aiming to assess the effect of haloperidol on DCFDs within 14 days after randomization was preliminarily halted, per DSMB advice upon planned interim analysis because of futility of reaching the predefined difference of one day in DCFDs.
Our primary findings are in line with the MIND-USA and AID-ICU trial [7, 8]. However, the EuRIDICE trial differs from these prior trials in that it specifically assessed delirium outcomes related to agitation, which have not been previously reported in a therapeutic setting. Fewer haloperidol-treated patients required the use of a benzodiazepine, suggesting a benzodiazepine-sparing effect with haloperidol. Albeit non-significantly, other predefined secondary outcomes showed the same direction: study drug, rescue haloperidol, atypical antipsychotics and alpha-2 agonists use was lower in haloperidol-treated patients. The use of haloperidol was also significantly associated with the post hoc outcome of reduced patient harm associated with agitation (significantly fewer falls out of bed). The Hope-ICU trial, a trial assessing prophylactic effect of haloperidol, found that haloperidol reduced agitation [18], which is in line with our findings. An explanation could be that the EuRIDICE trial had a higher rate of agitated patients as compared to other trials (mixed delirium 73% in EuRIDICE versus 45% in AID-ICU [defined as RASS > 0] and 37% in MIND-USA with only 11% with agitated delirium upon inclusion) and included patients with lower disease severity (SOFA 5/6 vs. 11 in MIND-USA and mortality 16–21% as opposed to around 40% in the other two trials) [7, 8, 23].
Our findings, combined with those of Hope-ICU, encourage further research, focusing on delirium-associated symptoms, specifically those related to agitation, and the possible rescue medication-sparing effect of haloperidol rather than on delirium as a the main outcome of interest. More importantly, we feel that our results indicate that abandoning haloperidol from clinical practice to manage delirium symptoms at the ICU may not currently be warranted before further studies have definitely excluded such effects. Our findings and those from the Hope-ICU trial appear in line with current clinical experience, indicating that haloperidol might help reduce agitation in ICU patients with delirium.
Interestingly, no differences were observed regarding psychotic symptoms. It is unclear whether this points to haloperidol’s intrinsic lack of effect on psychotic features in ICU delirium, or the fact that more rescue atypical antipsychotics were administered in the control group. Another explanation may be the trial’s lack of statistical power.
Safety issues such as extrapyramidal symptoms, prolonged QTc interval and torsade de pointes occurred infrequently and did not differ between the two groups, consistent with the previous trials [7, 8]. We did, however, notice a significant decrease in blood pressure after haloperidol administration, but this safety measure did not appear to be clinically significant given the small drop in blood pressure.
A strength of this trial is that delirium assessments were performed three times daily. This may better facilitate the assessment of any response of fluctuating delirium symptoms to haloperidol compared with other studies [7, 24]. Other specific differences of EuRIDICE as opposed to MIND-USA and AID-ICU were: the possibility to use rescue atypical antipsychotics next to the study drug which was prohibited in both MIND-USA and AID-ICU; the exclusion of patients with possible alcohol related delirium, which was not specified in MIND-USA; the halting of study drugs when the patients were comatose (similar to MIND-USA), which was not advised in AID-ICU; and the assessment of many more relevant secondary outcomes related to delirium which is in line with a recent ICU delirium research core outcomes set [25]. Further, to our knowledge this is the first intervention study of haloperidol, administered to treat delirium while in the ICU, to report on a variety of patient-oriented long-term secondary outcomes. However, these findings are very preliminary as they concerned secondary outcomes from a preliminary halted, and consequently, underpowered clinical trial. However, our results may pave the way for further prospective research to determine possible efficacy of haloperidol to decrease the burden of recall of troublesome ICU experiences and memories and quality of life.
Our study has several limitations. First, this trial was prematurely terminated as advised by the DSMB partly because of randomization challenges due to the informed consent requirement (as compared to deferred consent in the AID-ICU trial), and therefore, in general all findings related to secondary outcomes should be viewed as hypothesis generating. Second, approximately 75% of all screened patients were deemed ineligible according to our exclusion criteria, which may limit external validity. Third, we did not assess actual adherence to the ABCDEF bundle during the intervention periods but only assessed estimates on adherence by the local PI’s [26]. Delirium assessments were performed by trained ICU nurses rather than dedicated study personnel. This may have influenced delirium diagnosis and management [27]. However, this trial was conducted in ICUs of which most participated in a large implementation study of delirium management [16], and in ICUs involved in a large delirium prevention study [28]. Therefore, these ICUs had sufficiently implemented routine delirium-oriented practices and are representative of real world clinical practice, supporting external validity of our study.
Conclusion
This trial, that was stopped early, did not show evidence that haloperidol reduces delirium and coma in critically ill patients with delirium. The beneficial effects on some agitation-related outcomes and lower sedative requirements reported in a clinical trial are novel, clinically relevant and in line with clinical use. Together with some other signals of possible benefit in not previously reported (secondary) outcomes, these findings argue for additional effectiveness research of haloperidol for ICU delirium.
Supplementary Information
Additional file 1. Online Data Supplement.
Acknowledgements
We would like to thank all the members of the EuRIDICE study group, including local research nurses: E. Berger (Franciscus Gasthuis, Department of Intensive Care, Rotterdam, The Netherlands); A. Bouman (Jeroen Bosch Hospital, Department of Intensive Care Medicine, 's-Hertogenbosch, The Netherlands); D. van Duijn (Erasmus MC-University Medical Centre, Department of Intensive Care Adults, Rotterdam, The Netherlands); H. van Embden—van Donk (Ikazia Hospital, Department of Intensive Care, Rotterdam, The Netherlands); D. van de Graaf (IJsselland Hospital, Department of Intensive Care, Capelle aan den Ijssel, The Netherlands); J. van Holten (Ikazia Hospital, Department of Intensive Care, Rotterdam, The Netherlands); E. Hoogendoorn (Albert Schweitzer Hospital, Department of Intensive Care, Dordrecht, The Netherlands); P. Ormskerk (Erasmus MC-University Medical Centre, Department of Intensive Care Adults, Rotterdam, The Netherlands); N. Roovers (Radboud University Medical Centre, Department of Intensive Care Medicine, Nijmegen, The Netherlands); E. Toscano (Maasstad Hospital, Department of Intensive Care, Rotterdam, The Netherlands); A. Vileito (Erasmus MC-University Medical Centre, Department of Intensive Care Adults, Rotterdam, The Netherlands); T. van Zuylen (Jeroen Bosch Hospital, Department of Intensive Care Medicine, 's-Hertogenbosch, The Netherlands). We would also like to thank all the intensive care teams for their contribution to this trial. Additionally, we thank other EuRIDICE study group members: C. Exler and R. Bouamar (Erasmus MC-University Medical Centre, Department of Pharmacy, Rotterdam, The Netherlands); E. van den Berg (Erasmus MC-University Medical Centre, Department of Neuropsychology, Rotterdam, The Netherlands); E. Ista (Erasmus MC—Sophia Children's Hospital University Medical Centre, Department of Pediatric Surgery, Intensive Care Unit, Rotterdam, The Netherlands); J. van Meeteren (Erasmus MC-University Medical Centre, Department of Rehabilitation Medicine, Rotterdam, The Netherlands); M. Koopmanschap (Erasmus School of Health Policy & Management, Department of Health Economics and HTA, Rotterdam, The Netherlands). We further acknowledge the contributions of D. Nieboer, and patient representatives: I. Nutma and E. Kuijper, and also thank the DSMB members: prof. dr. R.C. van der Mast, dr. H.F. Lingsma, and dr. P. Spronk. We would like to thank K. Hagoort and S. Dijkland for their assistance in translation of the questionnaires. Our thanks also goes to M. Alvarez, M. de Groot and I. Kriens for performing neurocognitive tests. Last, we thank all the participants of the EuRIDICE trial and their family members for their dedication.
Author contributions
LS was the trial coordinator, analyzed and interpreted the data, and wrote the first draft of the manuscript. AS, JD and RO were members of the steering committee, and contributed to study design. ZT contributed to the study design and patient recruitment. NH advised on pharmacy related matters. HP was involved as a member of the steering committee and local principal investigator, contributing to patient acquisition. AB, JS, KS, MB, JL and DB were local principal investigators in participating ICUs involved in patient acquisition. WR was involved as a trial statistician, and advised on study design, data analysis and interpretation. MJ was the principal investigator, responsible for funding, study design and execution, patient recruitment, and drafting the manuscript. All authors revised, read and approved the final manuscript.
Funding
Netherlands Organisation for Health Research and Development (ZonMw), 848041001.
Availability of data and materials
All de-identified individual participant data that underlie the results reported in this article, and the data dictionary will be shared to investigators with the purpose of individual participant data meta-analysis after approval from the EuRIDICE steering committee and a signed data access agreement. All requests should be sent to m.vanderjagt@erasmusmc.nl. The study protocol is publicly available online.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Medical Ethics Committees of all participating hospitals and has been published [11]. The study was conducted according to the principles of the Declaration of Helsinki and in accordance with the Medical Research Involving Human Subjects Act (WMO) and other guidelines, regulations and Acts. Eligible patients or their legal representatives were asked for written informed consent as soon as possible after ICU admission to enable randomization as soon as possible after delirium was first diagnosed.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilson JE, Mart MF, Cunningham C, Shehabi Y, Girard TD, MacLullich AMJ, Slooter AJC, Ely EW. Delirium. Nat Rev Dis Prim. 2020;6(1):90. doi: 10.1038/s41572-020-00223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandharipande PP, Girard TD, Ely EW. Long-term cognitive impairment after critical illness. N Engl J Med. 2014;370(2):185–186. doi: 10.1056/NEJMc1313886. [DOI] [PubMed] [Google Scholar]
- 3.Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, Truman B, Bernard GR, Dittus RS, Ely EW. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962. doi: 10.1097/01.CCM.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 4.Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, Truman B, Dittus R, Bernard R, Inouye SK. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morandi A, Piva S, Ely EW, Myatra SN, Salluh JIF, Amare D, Azoulay E, Bellelli G, Csomos A, Fan E, et al. Worldwide survey of the “assessing pain, both spontaneous awakening and breathing trials, choice of drugs, delirium monitoring/management, early exercise/mobility, and family empowerment” (ABCDEF) Bundle. Crit Care Med. 2017;45(11):e1111–e1122. doi: 10.1097/CCM.0000000000002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 7.Girard TD, Exline MC, Carson SS, Hough CL, Rock P, Gong MN, Douglas IS, Malhotra A, Owens RL, Feinstein DJ, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506–2516. doi: 10.1056/NEJMoa1808217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen-Ranberg NC, Poulsen LM, Perner A, Wetterslev J, Estrup S, Hastbacka J, Morgan M, Citerio G, Caballero J, Lange T, et al. Haloperidol for the treatment of delirium in ICU patients. N Engl J Med. 2022;387(26):2425–2435. doi: 10.1056/NEJMoa2211868. [DOI] [PubMed] [Google Scholar]
- 9.Andersen-Ranberg NC, Poulsen LM, Perner A, Hastbacka J, Morgan M, Citerio G, Collet MO, Weber SO, Andreasen AS, Bestle M, et al. Haloperidol versus placebo for the treatment of delirium in ICU patients: a pre-planned, secondary Bayesian analysis of the AID-ICU trial. Intensive Care Med. 2023;49(4):411–420. doi: 10.1007/s00134-023-07024-9. [DOI] [PubMed] [Google Scholar]
- 10.Marcantonio ER. Haloperidol for treatment of ICU delirium: progress or setback? N Engl J Med. 2022;387(26):2464–2465. doi: 10.1056/NEJMe2214417. [DOI] [PubMed] [Google Scholar]
- 11.Smit L, Trogrlic Z, Devlin JW, Osse RJ, Ponssen HH, Slooter AJC, Hunfeld NGM, Rietdijk WJR, Gommers D, van der Jagt M, et al. Efficacy of halopeRIdol to decrease the burden of delirium in adult critically ill patiEnts (EuRIDICE): study protocol for a prospective randomised multi-centre double-blind placebo-controlled clinical trial in the Netherlands. BMJ Open. 2020;10(9):e036735. doi: 10.1136/bmjopen-2019-036735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorm AF, Jacomb PA. The informant questionnaire on cognitive decline in the elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19(4):1015–1022. doi: 10.1017/S0033291700005742. [DOI] [PubMed] [Google Scholar]
- 13.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 14.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 15.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 16.Trogrlic Z, van der Jagt M, Lingsma H, Gommers D, Ponssen HH, Schoonderbeek JFJ, Schreiner F, Verbrugge SJ, Duran S, Bakker J, et al. Improved guideline adherence and reduced brain dysfunction after a multicenter multifaceted implementation of ICU delirium guidelines in 3930 patients. Crit Care Med. 2019;47(3):419–427. doi: 10.1097/CCM.0000000000003596. [DOI] [PubMed] [Google Scholar]
- 17.Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 18.Page VJ, Ely EW, Gates S, Zhao XB, Alce T, Shintani A, Jackson J, Perkins GD, McAuley DF. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2013;1(7):515–523. doi: 10.1016/S2213-2600(13)70166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colantuoni E, Dinglas VD, Ely EW, Hopkins RO, Needham DM. Statistical methods for evaluating delirium in the ICU. Lancet Respir Med. 2016;4(7):534–536. doi: 10.1016/S2213-2600(16)30138-2. [DOI] [PubMed] [Google Scholar]
- 20.Girard TD, Pandharipande PP, Carson SS, Schmidt GA, Wright PE, Canonico AE, Pun BT, Thompson JL, Shintani AK, Meltzer HY, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med. 2010;38(2):428–437. doi: 10.1097/CCM.0b013e3181c58715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 22.Schulz KF, Altman DG, Moher D. Group C: CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenser RB. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2019;380(18):1778–1780. doi: 10.1056/NEJMc1901272. [DOI] [PubMed] [Google Scholar]
- 24.Pisani MA, Araujo KL, Murphy TE. Association of cumulative dose of haloperidol with next-day delirium in older medical ICU patients. Crit Care Med. 2015;43(5):996–1002. doi: 10.1097/CCM.0000000000000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose L, Burry L, Agar M, Campbell NL, Clarke M, Lee J, Marshall JC, Devlin JW, Blackwood B, Needham DM, et al. A core outcome set for research evaluating interventions to prevent and/or treat delirium in critically ill adults: an international consensus study (Del-COrS) Crit Care Med. 2021;49(9):1535–1546. doi: 10.1097/CCM.0000000000005028. [DOI] [PubMed] [Google Scholar]
- 26.Marra A, Ely EW, Pandharipande PP, Patel MB. The ABCDEF bundle in critical care. Crit Care Clin. 2017;33(2):225–243. doi: 10.1016/j.ccc.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Eijk MM, van den Boogaard M, van Marum RJ, Benner P, Eikelenboom P, Honing ML, van der Hoven B, Horn J, Izaks GJ, Kalf A, et al. Routine use of the confusion assessment method for the intensive care unit a multicenter study. Am J Respir Crit Care Med. 2011;184(3):340–344. doi: 10.1164/rccm.201101-0065OC. [DOI] [PubMed] [Google Scholar]
- 28.van den Boogaard M, Slooter AJC, Bruggemann RJM, Schoonhoven L, Beishuizen A, Vermeijden JW, Pretorius D, de Koning J, Simons KS, Dennesen PJW, et al. Effect of Haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA. 2018;319(7):680–690. doi: 10.1001/jama.2018.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Online Data Supplement.
Data Availability Statement
All de-identified individual participant data that underlie the results reported in this article, and the data dictionary will be shared to investigators with the purpose of individual participant data meta-analysis after approval from the EuRIDICE steering committee and a signed data access agreement. All requests should be sent to m.vanderjagt@erasmusmc.nl. The study protocol is publicly available online.