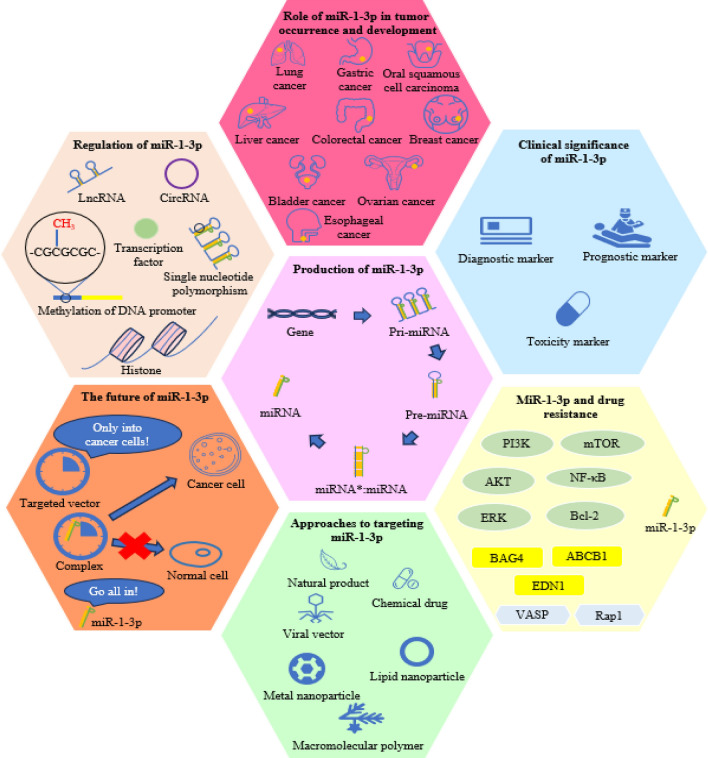
Abstract
Cancer is a malignant tumor that seriously threatens human life and health. At present, the main treatment methods include surgical resection, chemotherapy, radiotherapy, and immunotherapy. However, the mechanism of tumor occurrence and development is complex, and it produces resistance to some traditional treatment methods, leading to treatment failure and a high mortality rate for patients. Therefore, exploring the molecular mechanisms of tumor occurrence, development, and drug resistance is a very important task. MiRNAs are a type of non-coding small RNA that regulate a series of biological effects by binding to the 3′-UTR of the target mRNA, degrading the mRNA, or inhibiting its translation. MiR-1-3p is an important member of them, which is abnormally expressed in various tumors and closely related to the occurrence and development of tumors. This article introduces miR-1-3p from multiple aspects, including its production and regulation, role in tumor occurrence and development, clinical significance, role in drug resistance, and approaches for targeting miR-1-3p. Intended to provide readers with a comprehensive understanding of the important role of miR-1-3p in tumors.
Graphical Abstract
Keywords: miR-1-3p, Cancer, Mechanism, Chemotherapy sensitivity, Function, Targeted therapy
Introduction
Cancer is a serious threat to human health worldwide. The latest cancer data reports that 1,958,310 new cancer cases and 609,820 cancer deaths are expected in the United States this year [1]. Meanwhile, China has also recently released its 2016 cancer data report. In 2016, it is estimated that there were about 4,064,000 new cases (crude incidence rate of 293.91/100,000) and 2,413,500 deaths (crude mortality rate of 174.55/100,000) in China [2]. The burden of cancer remains very severe. Currently, common cancer treatments include surgical removal, radiation therapy, and chemotherapy [3–6]. Surgical resection can directly remove the tumor site, but it may cause various postoperative complications, and some patients lose the opportunity for surgery when diagnosed with cancer. Although radiotherapy and chemotherapy have the ability to kill cancer cells, they have limitations and non-selectivity, respectively. More importantly, due to the complex mechanisms of tumor occurrence and development, drug resistance and recurrence often occur, leading to treatment failure and high mortality rates for patients [7, 8]. Therefore, it is very important to study the molecular mechanisms underlying the occurrence and development of tumors and the generation of therapeutic resistance. This may contribute to the development of molecularly targeted drugs.
MicroRNA (miRNA) is a class of non-coding RNA with a length of about 22 nt, which degrades mRNA or inhibits mRNA translation by binding to 3′-UTR of target mRNA, and regulates gene expression at the post-transcriptional level [9]. MiRNAs play an important role in maintaining normal cell metabolism. The abnormal expression of miRNAs may be related to the occurrence and development of various diseases, such as cardiovascular disease, neurodegenerative disease, cancer, diabetes, fibrotic disease, and inflammation. miR-1-3p is a very important member of the miRNA family, encoded by the miR-1–2 gene located on chromosome 18q11.2. Initial studies found that miR-1-3p was abundantly expressed in cardiac and skeletal muscle and is involved in their development [10–12]. miR-1-3p is able to directly regulate muscle differentiation regulators, including serum response factor, myogenic differentiation antigen (MyoD), and myocyte enhancer factor 2 (Mef2) [10]. Heart and neural crest derivatives-expressed transcript 2 (Hand2, a transcription factor that promotes ventricular cardiomyocyte expansion) has also been shown to be a target for miR-1-3p [10]. This miRNA also regulates myocardial physiological functions, and its aberrant expression has been associated with a variety of cardiac diseases, such as heart failure, myocardial infarction, cardiac hypertrophy, and arrhythmia. In recent years, miR-1-3p has been found to be highly conserved and consistently down-regulated in various tumors, and thus has attracted the attention of researchers. miR-1-3p is considered to be a tumor suppressor with great potential because of its ability to effectively inhibit a variety of tumors and improve the sensitivity of some anticancer drugs. In addition, miR-1-3p also plays a role in tumor diagnosis and prognosis. In the future, the function and mechanism of miR-1-3p still need to be further investigated, which will be beneficial to provide a solid theoretical foundation for clinical translation.
This article introduces miR-1-3p from various aspects, including the generation process and regulatory factors, its role in tumorigenesis and development, clinical significance, drug resistance, and targeted approaches.
Production and regulation of miR-1-3p
Production of miR-1-3p
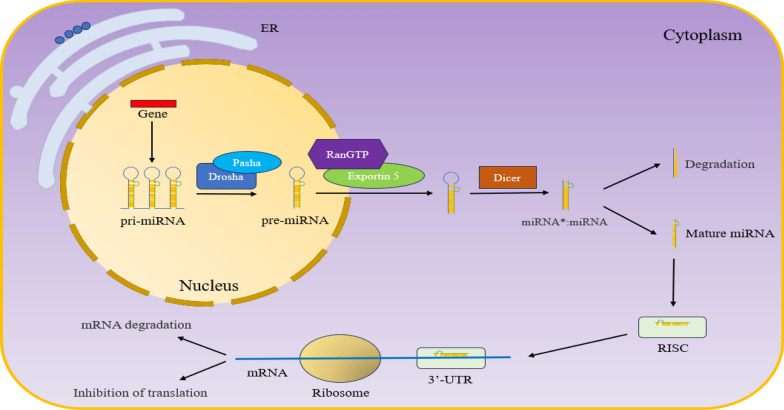
The gene encoding miR-1-3p is located in the intron region of the gene encoding protein MIB1 on chromosome 18q11.2 [13, 14]. First, the gene encoding miR-1-3p in the nucleus is transcribed into primary miRNA (pri-miRNA) under the action of RNA polymerase II [15, 16]. Under the action of Ribonuclease (RNase) Drosha and cofactor Pasha, pri-miRNA was cut into precursor miRNA (pre-miRNA) with hairpin structure, which was about 70 nt [17, 18]. Subsequently, pre-miRNA is transported from the nucleus to the cytoplasm through the RanGTP/exportin 5 transport mechanism [19]. The pre-miRNA in the cytoplasm is cut into double-stranded miRNA (combination of miRNA and miRNA*, miRNA* refers to a strand with very low or no expression) by another RNase III Dicer [20]. Afterward, miRNA and miRNA* are separated, where miRNA* is degraded, while mature miRNA enters the RNA-induced silencing complex (RISC) and binds to the 3′-UTR of the target mRNA, thereby degrading mRNA or inhibiting mRNA translation (Fig. 1) [21–23].
Fig. 1.
Process of miRNA production and processing. The gene encoding miRNA is transcribed into pri-miRNA, cleaved into pre-miRNA under the action of Drosha and Pasha, and then transported to the cytoplasm by RanGPT/exportin 5. Dicer in the cytoplasm further cleaves pre-miRNA into double-stranded miRNA (miRNA*:miRNA, miRNA* refers to a strand with very low or no expression). Subsequently, the double-stranded miRNA dissociates, with mature miRNA entering RISC and binding to the 3′-UTR of mRNA to degrade mRNA or inhibit translation
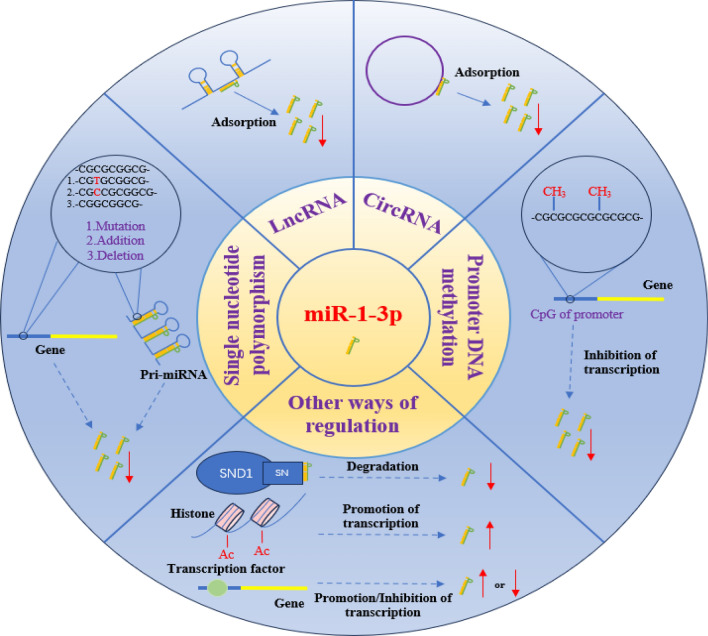
Regulation of miR-1-3p
LncRNA
Long non-coding RNA (lncRNA) is a kind of non-coding RNA with a length of more than 200 nt. It can interact with DNA, RNA, and protein, thus participating in a series of biological processes [24]. As a competitive endogenous RNA (ceRNA), lncRNA can bind and silence corresponding miRNAs, thereby upregulating downstream mRNA and participating in a series of cellular biological processes. This is also one of the mechanisms that has been extensively studied. For example, lncRNA TUG1 can bind and silence miR-1-3p, thereby promoting the proliferation of liver cancer cells [25]. LncRNA MALAT1 can regulate migration and invasion in prostate cancer cells and survival and metastasis in esophageal cancer cells by targeting miR-1-3p [26, 27]. The exosomes secreted by breast cancer cells contain high expression levels of MALAT1, which can be transferred to surrounding breast cancer cells to silence the miR-1-3p in the cells and promote the metastasis of breast cancer cells and chemotherapy resistance [28]. In addition, RMRP, LINC00242, LINC01518, and DANCR have also been reported to silence miR-1-3p in non-small cell lung cancer, gastric cancer, esophageal squamous cell carcinoma, and glioma cells, respectively, promoting the malignant phenotype of tumor cells [29–32]. In summary, lncRNA is an important molecule that regulates miRNA levels within cells, and changes in its expression can cause changes in cellular function.
CircRNA
Circular RNA (circRNA) is a non-coding RNA that is covalently closed between the 3′ and 5′ ends, formed by reverse splicing through a special splicing method [33, 34]. Because circRNA is a circular structure with no polyadenylated tail at the 3′ end and no cap structure at the 5′ end, it is difficult to be degraded by nucleic acid exonuclease, so it is relatively stable in cells [35, 36]. The way circRNA regulates miRNA is similar to that of lncRNA, and it also acts as ceRNA binding to miRNA, thereby blocking the inhibition of miRNA on mRNA. For example, CircAGO2 can bind and silence miR-1-3p, thereby upregulating the expression of RBBP4. RBBP4 can deacetylate histones in the HSPB8 promoter region and inhibit HSPB8 transcription, thereby promoting the proliferation and invasion of colorectal cancer cells [37]. In addition, cHP1BP3 can also bind and silence miR-1-3p, upregulate the expression of C1GALT1, and promote the proliferation and migration of bladder cancer cells [38]. This indicates that changes in miRNA levels may also be caused by changes in circRNA expression.
Promoter DNA methylation
The dinucleotide structure formed by cytosine and guanine through phosphate linkage is called CpG. The DNA region rich in CpG is called the CpG island, typically between 200 and 1400 bp in length [39, 40]. CpG island is mainly located near the transcription start site of the gene promoter, which is an important occurrence area of DNA methylation [41]. High methylation at the CpG island site can lead to gene transcription silencing, while low methylation at the CpG island site promotes gene transcription [42–44]. Research has found that hypermethylation of the miR-1-2 gene (the gene coding miR-1-3p) promoter reduces the expression of miR-1-3p in prostate cancer. The decrease in miR-1-3p expression promotes the invasive ability of prostate cancer cells, which may be related to targeting downstream genes GOLPH3 and JUP [45]. Zhou et al. found that circSKA3 could increase the methylation of the miR-1 gene in glioblastoma, thereby reducing the expression of miR-1, and promoting the proliferation of glioblastoma cells [46]. During tumor development, high methylation of the CpG island of tumor suppressor genes is often observed. The methylation of the miR-1-3p gene leads to a decrease in its expression, which in turn promotes the occurrence and development of tumors, which is consistent. In summary, the expression of miR-1-3p is influenced by the methylation status of the coding gene, which is a regulatory factor worth paying close attention to.
Single nucleotide polymorphism
Single nucleotide polymorphism (SNP) is a common heritable variation, which refers to the DNA sequence polymorphism caused by the variation of a single nucleotide in a gene. SNP can be caused by the conversion or reversal of individual bases, as well as the insertion or deletion of bases, with single base conversion being the most common. Li et al. found that the serum miR-1-3p expression level in patients with abdominal aortic aneurysm (AAA) of rs2155975 AG + GG or rs4591246 AG + AA genotype (two SNPs located in pri-miR-1-3p) was significantly reduced, which was related to postoperative all-cause mortality and overall survival rate [47]. In addition, it was found that the SNP rs4591246 in pri-miR-1-3p also downregulated the expression of mature miR-1-3p in abdominal aortic aneurysm tissue, and then promoted the transformation of cell phenotype by upregulating TLR4, which was closely related to the risk of AAA patients [48].
Other ways of regulation
SND1 (Staphylococcal Nuclease and Tudor Domain Containing 1) is an RNA binding protein, which is reported to play the role of nuclease in RISC, and also has the function of degrading hyperedited pre-miRNA and mediating the degradation of a group of mature miRNAs [49–52]. Recent reports have shown that SND1 can bind and degrade specific miRNAs through the SN domain, and its activity is related to the template [51]. The inhibition of SND1 can increase the expression level of miR-1-3p in colon cancer cells and enhance the sensitivity of tumor cells to the Bcl-2 family inhibitor navitoclax [52]. In addition, acetylation of histones, various mutations in coding genes, and changes in transcription factors may all affect the levels of miR-1-3p (Fig. 2).
Fig. 2.
Regulation of miR-1-3p. The regulation of miR-1-3p is mainly influenced by factors such as lncRNA, circRNA, DNA promoter methylation, SNP, etc. LncRNA and circRNA can bind and silence miR-1-3p. DNA promoter methylation can inhibit the transcription of the miR-1-3p gene. SNP can affect the binding of related enzymes, thereby affecting the generation and processing of miR-1-3p. SND1 can bind and degrade miR-1-3p through the SN domain. In addition, factors such as transcription factors, histone acetylation, and gene mutations also regulate the expression level of miR-1-3p
Role of miR-1-3p in tumor occurrence and development
Gastric cancer
Gastric cancer (GC) is a common tumor of the digestive tract. The incidence rate of gastric cancer ranks fifth among all kinds of tumors, and the mortality rate ranks fourth [53–55].
Research has found that miR-1-3p was low expressed in gastric cancer tissues and cells, and was closely related to the size of the tumor. Overexpression of miR-1-3p inhibits the proliferation and invasion of gastric cancer cells by targeting stanniocalcin 2 (STC2) or centromere protein F (CENPF), in which CENPF is also associated with migration [56, 57]. Interestingly, miR-1-3p can target glucose-6-phosphate dehydrogenase (G6PD) to affect the Warburg effect (aerobic glycolysis) of gastric cancer cells. It can reduce glucose uptake, lactate production, and ATP production, inhibit cell proliferation, and promote cell apoptosis [30]. G6PD is a key rate-limiting enzyme in the pentose phosphate pathway (PPP), and how it participates in the regulation of the Warburg effect still needs further study [58]. It is worth noting that the G6PD-mediated PPP pathway is the main way to generate NADPH, which is the common reduction equivalent in the four major defense systems of ferroptosis (GPX4/GSH, FSP1/CoQH2, GCH1/BH4, and DHODH/CoQH2) [59–62]. The lack of NADPH can lead to ferroptosis, which is considered as a biomarker of ferroptosis sensitivity. However, it has also been reported that excessive NADPH can generate reactive oxygen species (ROS) under the action of NADPH oxidase (NOX), thus promoting the occurrence of ferroptosis [63]. Therefore, the role of NADPH in ferroptosis has a dual role, which needs specific analysis in different situations.
Colorectal cancer
MiR-1-3p exhibits low expression in colorectal cancer tissues and cells. In primary colorectal cancer, the expression of miR-1-3p is closely related to tumor grade and overall survival in CRC patients [64]. MiR-1-3p can significantly inhibit the proliferation and invasion of CRC cells, which is related to targeting tyrosine 3/tryptophan 5 monooxygenase activation protein zeta (YWHAZ). YWHAZ can promote the epithelial mesenchymal transition (EMT) process in CRC cells, increasing β-catenin and N-cadherin, while reducing the expression of E-cadherin [65]. Ye et al. found that propofol could inhibit the proliferation of CRC cells and promote their apoptosis. The mechanism is that propofol can upregulate the expression level of miR-1-3p in CRC cells, thereby targeting insulin-like growth factor 1 (IGF1) and inhibiting the activation of the AKT/mTOR signaling axis [66]. In previous studies, it has also been shown that IGF1 can bind to insulin-like growth factor 1 receptor (IGF1R), promoting the activation of the AKT/mTOR signaling pathway, thereby affecting cell proliferation, apoptosis, and metastasis [67–69]. Lv et al. found that nicotinamide phosphoribosyl transferase (NAMPT) was highly expressed in tumor tissues of CRC patients and is closely related to invasion, TNM staging, and low overall survival rate. NAMPT can activate transforming growth factor-β (TGF-β) signal pathways (upregulation of Smad 2, Smad 3, Smad 4, p-Smad 2, p-Smad 3 levels) promote the secretion of transforming growth factor-β1 (TGF-β1). And TGF-β1 can upregulate the level of miR-1-3p, which targets and silences NAMPT, ultimately forming a negative feedback pathway [70]. Targeting miR-1-3p to intervene in the negative feedback pathway for the treatment of CRC may also be a novel perspective.
Lung cancer
Lung cancer is the cancer with the highest mortality rate worldwide. Lung cancer mainly includes two types, one is non-small cell lung cancer (NSCLC), and the other is small cell lung cancer, with NSCLC accounting for over 85% of lung cancer [71]. NSCLC includes lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), and large-cell carcinoma [72].
MiR-1-3p is downregulated in LUAD tissue, and upregulation of miR-1-3p levels demonstrates the ability to inhibit proliferation and migration in LUAD cells. In LUAD and adjacent tissues, there is a significant negative correlation between miR-1-3p and CENPF expression. CENPF expression is elevated in LUAD, with higher expression in the late stage (II + III + IV) compared to the early stage (I). There is a strong correlation between CENPF and poor prognosis of patients [73]. Therefore, the miR-1-3p/CENPF axis may have an important regulatory effect on LUAD and be a potential therapeutic target. Liu et al.'s study showed that family with sequence similarity 83 member A (FAM83A) is overexpressed in lung cancer cells and is associated with low survival rates in patients. The silencing of FAM83A can inhibit the proliferation, invasion and migration of lung cancer cells, which may be related to the inhibition of epidermal growth factor receptor (EGFR)/mitogen activated protein kinase (MAPK)/choline kinase α (CHKA) signal transduction and activation. The overexpression of FAM83A is believed to be related to the downregulation of miR-1-3p expression level. Overexpression of miR-1-3p can reduce the expression of FAM83A, thereby exerting potential anti-tumor effects [74].
Miao et al. found that overexpression of miR-1-3p could inhibit the proliferation, migration, and invasion ability of lung cancer cells, which was related to targeting cadherin EGF LAG seven-pass G-type receptor 3 (CELSR3) [75]. In exploring the regulatory function of lncRNA RMRP in NSCLC, it was found that RMRP achieved its cancer promoting effect by silencing miR-1-3p [29]. This also indicates once again that low expression of miR-1-3p is an important factor in promoting lung cancer progression, and increasing the expression level of miR-1-3p will be a potential targeted treatment approach. In addition, the miR-1-3p-PAICS axis has also been reported to be involved in the glycolysis and nucleotide metabolism of NSCLC cells, thereby affecting the progression of NSCLC [76].
Bladder cancer
Bladder cancer (BLCA) is one of the ten most common cancers in the world [77–79]. At present, the occurrence of BLCA is believed to be highly correlated with smoking [80–82].
Low expression of miR-1-3p was observed in BLCA tissues and cells. Increasing the expression level of miR-1-3p in BLCA cells can inhibit cell proliferation, colony formation, migration and invasion, promote mitosis to stagnate in the G0/G1 phase, and increase the ratio of apoptosis. This process is related to miR-1-3p targeting C–C motif chemokine ligand 2 (CCL2) [83]. The role of CCL2 has also been extensively studied in BLCA. CCL2 is highly expressed in BLCA, and CCL2 staining results in BLCA cells and immune cells are considered as prognostic biomarkers for BLCA patients [84]. In the study of the mechanism of heat shock protein 47 (HSP47) promoting angiogenesis in BLCA, it was found that the induction of CCL2 and the activation of the ERK pathway were the causes of HSP47-induced angiogenesis [85]. In the functional study of lncRNA LNMAT1, it was found that it could promote BLCA related lymphangiogenesis and lymphatic metastasis. The mechanism is that LNMAT1 can recruit hnRNPL to the CCL2 promoter, leading to an increase in H3K4 trimethylation, thereby activating the expression of CCL2. The increased CCL2 is secreted into the tumor microenvironment, promoting the recruitment of tumor associated macrophages (TAM), and then promoting lymphatic metastasis through the secretion of vascular endothelial growth factor C (VEGF-C) [86]. In summary, the miR-1-3p/CCL2 axis is a highly promising therapeutic target in BLCA and deserves the focus of researchers.
Core 1 beta1,3-galactosyltransferase 1 (C1GALT1) has the function of regulating the O-glycosylation of tumor related proteins. Changes in the expression of C1GALT1 can lead to changes in the glycosylation of glycoproteins on the cell membrane, including mucins, growth factor receptors, adhesion molecules, etc. This change can cause a shift in the interaction between cell membrane surface molecules and ligands, ultimately affecting the biological behavior of tumor cells [87]. Tan et al. found that the expression of C1GALT1 and product T antigen was highly expressed in BLCA and promotes malignant behaviors such as proliferation, colony formation, migration, and invasion of BLCA cells. Mucin16 (MUC16) has been identified as a C1GALT1 target glycoprotein in BLCA, and its silencing inhibits the proliferation and migration ability of BLCA cells. With further research, it has been found that the role of C1GALT1 in BLCA was regulated by the cHP1BP3/miR-1-3p axis. Therefore, the cHP1BP3/miR-1-3p axis is a potential diagnostic marker and therapeutic target for BLCA [38].
Zhang et al. found that miR-1-3p could inhibit the proliferation, migration, and invasion of BLCA cells by targeting glutaminase (GLS) [88]. In addition to the enhanced glycolysis process, the enhancement of glutamine decomposition is also a characteristic of tumor cells, and GLS is a key enzyme in the glutamine decomposition process. GLS can decompose glutamine (Gln) into glutamic acid (Glu), and then Glu generates α-ketoglutarate (α-KG) under the action of glutamic acid transaminase 1 (GOT1) [89, 90]. The α-KG is an important intermediate product of the tricarboxylic acid (TCA) cycle, and the increase of α-KG can promote the TCA cycle to produce more energy, nucleotides, lipids, amino acids and other substances required by cells, which is conducive to cell growth and survival [91–93].
In addition, miR-1-3p can also inhibit the proliferation, migration, invasion ability of BLCA cells and promote their apoptosis by targeting the BDNF-TrkB signaling axis [94]. In summary, low expression of miR-1-3p in BLCA demonstrates a promoting effect on cancer development, and restoring or even overexpressing miR-1-3p levels is a potential therapeutic approach for BLCA.
Liver cancer
Liver cancer is a deadly malignant tumor, and although treatment methods are constantly improving, the five-year survival rate of patients is still very low [95]. Liver cancer is divided into three categories: hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and mixed cancer. HCC accounts for the vast majority of liver cancer (approximately 90%) [96]. The high-risk factors for HCC mainly include hepatitis B virus/hepatitis C virus (HBV/HCV) infection, long-term alcohol consumption, non-alcoholic fatty liver disease (NAFLD), type 2 diabetes, aflatoxin, liver cirrhosis, obesity, etc. [53, 97, 98].
MiR-1-3p is downregulated in HCC cells, and overexpression of miR-1-3p can inhibit the proliferation of HCC cells and promote their apoptosis, which is related to targeting sex-determining region Y-box 9 (SOX9) [99]. SOX9, as a transcript factor, has also been further studied in HCC. Research has found that SOX9 could directly bind to the promoter region to induce C-X-C motif chemokine 5 (CXCL5) expression, then activate signal transduction of PI3K-AKT and ERK1/2, ultimately promoting the proliferation and invasion of HCC cells. In addition, the SOX9/CXCL5 axis also facilitates the infiltration of macrophages and neutrophils in tumor tissue [100]. According to reports, SOX9 can bind to the promoter region and stimulate the expression of lncRNA-MKLN1-AS, thereby promoting the proliferation, invasion, and EMT process of HCC cells [101]. It is worth noting that the expression and stability of SOX9 are related to maintaining tumor stem cell characteristics [102].
Chen et al.’s study showed that miR-1-3p is low expressed in HCC tissues and cells, while overexpression of miR-1-3p can inhibit HCC cell proliferation, migration, and invasion, and induce cell cycle arrest and apoptosis. This is related to targeting origin recognition complex bundle 6 (ORC6) [103]. ORC6 is associated with the I-IV phase, overall survival (OS), and relapse-free survival (RFS) of HCC and plays a crucial role in the initiation of DNA replication, DNA metabolism, cell cycle and other processes [104, 105].
High vascularity is one of the important characteristics of HCC and plays an important role in tumor growth and metastasis. Anti-tumor angiogenesis is considered an effective treatment for advanced HCC [106, 107]. Some scholars have found that thymoquinone (TQ) could inhibit diethylnitrosamine (DEN) induced angiogenesis and metastasis of HCC, which may be related to upregulating the expression level of miR-1-3p [108]. This means that miR-1-3p may become a potential target for inhibiting angiogenesis in HCC and may provide promising treatment options for HCC patients.
In addition, in the research of Tang et al., it is also proved that miR-1-3p can inhibit the proliferation of HCC cells and promote apoptosis, and more HCC cells stay in G0/G1 phase. The LncRNA TUG1/miR-1-3p/IGF1 axis has also been proven to exist in HCC cells, but further research is needed on its effects on HCC cells [25].
Prostate cancer
Prostate cancer (PCa) is a common malignant tumor in men. In the United States, prostate cancer has become the leading malignant tumor with the highest number of new cases and the second highest number of deaths [95]. The high-risk factors for prostate cancer mainly include age, genetics, dietary fat, obesity, androgen levels, and so on [109, 110].
The expression level of miR-1-3p is downregulated in prostate cancer tissues and cells, and is associated with poor prognosis in patients. MiR-1-3p can inhibit the proliferation and colony forming ability of PCa cells, decrease the expression levels of cyclin-dependent kinase 2 (CDK2) and cyclin-dependent kinase 4 (CDK4), and make more cells stay in the G0/1 phase. This indicates that miR-1-3p may affect cell proliferation by intervening in the cell cycle process. Further research has identified E2F transcription factor 5 (E2F5) and PFTAIRE protein kinase 1 (PFTK1) as targets for miR-1-3p to function [111]. E2F5 is an important member of the E2F family and has been reported to promote cell cycle progression and proliferation [112]. PFTK1 is a new member of the CDK family and has been reported to accelerate the G0/G1-S phase transition, thereby regulating cell cycle processes [113]. During the experiment, it was once again confirmed that E2F5 and PFTK1 have a promoting effect on proliferation and cell cycle in PCa. In addition, miR-1-3p can significantly inhibit tumor volume in PCa bearing nude mice, and reduced expression of E2F5 and PFTK1 was detected in tumor tissue [111].
Dai et al. found that silencing of lncRNA MALAT1 could inhibit the expression of coronin 1C (CORO1C) by reducing the adsorption of miR-1-3p. This process inhibits the migration, invasion, and EMT progression of PCa cells [26]. Guo et al.’s study showed that miR-1-3p is not only associated with promoting the proliferation and migration of PCa cells, but also with bone metastasis (BM) of Gleason 3+4 PCa. LIM and SH3 protein 1 (LASP1) have been identified as a target for miR-1-3p, which may be involved in activating Wnt signaling through interactions with β-catenin [114].
Esophageal cancer
Esophageal cancer (EC) ranks seventh in the world in incidence rate and sixth in mortality and about 70% of cases occur in males [53]. EC is mainly divided into esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). In developing countries, ESCC is the main type of EC, and its high-risk factors may be overheated food and beverages, smoking, alcohol abuse, dietary composition, etc. [53, 115]. In developed countries, EAC has become the main type of EC. The high-risk factors for EAC may be overweight, gastroesophageal reflux, etc. [48]. In the future, the proportion of EAC in EC worldwide will continue to increase, and overweight may become an increasingly important factor [116].
MiR-1-3p was detected to be downregulated in EC tissues and cells, indicating a close correlation with EC [27, 31, 117]. Quercetin is a Natural product, which can inhibit the proliferation, colony formation, invasion and promote apoptosis of EC cells. In the study of its mechanism, it was found that the activation of miR-1-3p/ transgelin2 (TAGLN2) axis in EC cells induced by quercetin was an important factor for its anti-tumor function [117]. In the study of the mechanism of silencing lncRNA LINC01518 against ESCC, it was found that the silencing of LINC01518 could upregulate miR-1-3p, thereby inhibiting the PIK3CA/Akt pathway [31]. In addition, it was also found that silencing lncRNA MALAT1 could inhibit the migration and invasion of EC cells by upregulating miR-1-3p. This may be related to miR-1-3p inhibiting the downstream CORO1C/ tropomyosin 3 (TPM3) axis [27].
Oral squamous cell carcinoma
The incidence rate of oral cancer ranks eighth, and about 95% of oral cancer is oral squamous cell carcinoma (OSCC) [1, 118]. The main risk factors are smoking, drinking and oral human papilloma virus (HPV) infection, and the number of HPV related oral cancer cases is growing every year [1, 80]. However, in regions such as South Asia, East Asia, and the Pacific Island, one of the main risk factors is excessive chewing of betel nuts [119].
The expression of miR-1-3p was significantly downregulated in OSCC tissues and cells [120]. Overexpression of miR-1-3p can inhibit the proliferation, migration, and invasion of OSCC cells, block the transition from G0/G1 phase to S phase, and induce cell apoptosis, which is related to targeted silencing of dickkopf homolog 1 (DKK1) [120].
Ovarian cancer
Ovarian cancer (OA) is one of the common gynecological malignancies. Icariin is the main active ingredient of Epimedium, which can inhibit the proliferation of OA cells, induce cell cycle arrest in the G1/S phase, and promote cell apoptosis. The mechanism is that icariin upregulates the expression level of miR-1-3p, thereby inhibiting the transduction of the TNKS2/Wnt/β-catenin signaling pathway [121]. Qu et al. showed that miR-1-3p was able to block cell cycle progression and inhibit proliferation, migration and invasion of OA cells by targeting c-Met [122]. Importantly, miR-1-3p can increase the sensitivity of OA cells to ferroptosis by targeting FZD7 [123]. This means that it may be possible to improve the efficacy of some anti-tumor drugs. For example, cisplatin induces not only apoptosis but also ferroptosis in tumor cells [124].
Although, there have been some studies showing that MiR-1-3p has the ability to resist OA. However, it has also been suggested that the inhibitory effect of miR-1-3p in OA is very limited [125]. This indicates that the signaling or effector cascade of miR-1 has been dysregulated in OA.
Breast cancer
Breast cancer (BC) is the most common cancer in women worldwide, ranking second in the number of cancer deaths in women [1]. According to the expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2), BC can be divided into hormone receptor positive breast cancer (ER + or/and PR +), HER2 positive breast cancer (ER-, PR-, HER2 +) and triple negative breast cancer (ER −, PR −, HER2 −) [126].
Many studies have shown that miR-1-3p is expressed in low levels in BC tissues and cells [127]. For HR + BC, miR-1-3p can inhibit the proliferation, migration and invasion of MCF-7 and ZR-7530 BC cells, and promote their apoptosis, which may be related to targeting Bcl-2 [127]. Liu et al. showed that miR-1-3p inhibited MCF-7 cell proliferation and motility and promoted apoptosis, mainly by targeting K-Ras and lncRNA MALAT1 [128]. Meanwhile, miR-1-3p can increase the sensitivity of MCF-7 cells to cisplatin and paclitaxel [127]. For HER2 + BC, miR-1-3p can inhibit the malignant phenotype of SKBR3 cells, which is also related to targeting K-Ras and MALAT1 [128]. More importantly, compared to SKBR3 cells, the expression level of miR-1-3p in SKBR3-LR cells (lapatinib-resistant cell lines) is lower [128]. Restoration of miR-1-3p partially reverses resistance to lapatinib in SKBR3-LR cells [128]. For triple-negative BC, miR-1-3p was able to inhibit the proliferation of MDA-MB-231 cells, which was thought to be mainly caused by a significant increase in apoptosis rate [129]. Unsurprisingly, miR-1-3p was also inhibitory for migration and invasion. These phenotypic changes may be related to targeting the MEK/ERK pathway, but further validation is needed [129]. For potential clinical value, miR-1-3p was effective in increasing the sensitivity of MDA-MB-231 cells to cisplatin [129]. MiR-1-3p also plays an important role in regulating breast cancer stem cells. Wu et al. showed that miR-1-3p was able to target ecotropic viral integration site-1 (EVI-1) to inhibit proliferation and EMT-related genes in BCSCs and promote apoptosis [130]. Interestingly, miR-1-3p was able to trigger mitochondrial damage and promote mitochondrial autophagy in BCSCs, which was associated with targeting mitochondrial inner membrane organizing system 1 (MINOS1), glycerol-3-phosphate dehydrogenase 2 (GPD2), and interacting with leucine-rich pentatricopeptide-repeat containing (LRPPRC) proteins [131]. However, this phenomenon did not occur in tumor non-stem cells [131]. This provides new insights into the role of MiR-1-3p for mitochondria.
In conclusion, miR-1-3p plays an important inhibitory role for different subtypes of BC as well as breast cancer stem cells. Moreover, it plays a positive role in improving drug sensitivity. Therefore, miR-1-3p may have a very promising clinical potential.
Other cancer
Renal cell carcinoma (RCC) is a common malignant tumor of the urinary system, which originates from the epithelial system of the renal parenchyma urinary tubules and accounts for the vast majority of renal malignant tumors [132]. MiR-1-3p exhibits low expression in RCC tissues and cells, and is associated with clinical pathological parameters such as capsule, lymph node metastasis, and vascular invasion. MiR-1-3p can inhibit the EMT process of RCC cells and weaken the ability of migration and invasion, which is related to targeting and silencing Fibronectin 1. The same results were also obtained in RCC xenograft tumor mice [133]. In previous studies, it was reported that Fibronectin 1 has the ability to promote tumor cell migration and invasion [134]. Therefore, the miR-1-3p/Fibronectin 1 axis is a target worthy of attention for inhibiting the migration and invasion of RCC.
Osteosarcoma (OS) is a common malignant tumor of bone that occurs mostly in adolescents [135, 136]. miR-1-3p is lowly expressed in OS tissues and cells. Overexpression of miR-1-3p is able to inactivate the Wnt/β-catenin pathway by targeting cyclin-dependent kinase 14 (CDK14), thereby inhibiting cell proliferation and cell cycle progression while promoting apoptosis [137]. Cell cycle-dependent kinases (CDKs) are a class of key regulatory enzymes that drive cell cycle transitions and are considered to be critical targets for regulating cancer progression [138–140]. CDK14 is an important member of the CDK family. It has been reported that miR-330-3p, miR-139, miR-216a, miR-1182, and miR-223 can all inhibit OS development by targeting CDK14 [141–145]. This also reflects the importance of CDK14 in regulating OS. Wnt/β-catenin is a very classical signaling pathway that initiates the transcription of a series of downstream target genes (such as c-myc, cyclin D1, etc.) [146]. Its aberrant activation promotes the proliferation and survival of OS cells [146].
miR-1-3p also plays an important regulatory role in brain tumors. For example, miR-1-3p was able to inhibit the proliferation and migration of glioblastoma (GBM) by targeting fibronectin and increase the sensitivity of GBM cells to temozolomide [147]. Zhang et al. found that lncRNA HOTAIR promoted the malignant phenotype of medulloblastoma, which was associated with targeting miR-1-3p/Yin Yang 1 (YY1) [148]. For pituitary tumors, miR-1-3p was able to inhibit NADPH production and glycolytic processes in pituitary tumor cells by targeting G6PD, causing inhibition of proliferation and promotion of apoptosis (Fig. 3) (Table 1) [149].
Fig. 3.
Role of miR-1-3p in tumor occurrence and development. Gastric cancer: STC2, stanniocalcin 2; CENPF, centromere protein F; G6PD, glucose-6-phosphate dehydrogenase. Colorectal cancer: YWHAZ, tyrosine 3/tryptophan 5 monooxygenase activation protein zeta; IGF1, insulin-like growth factor 1; mTOR, mammalian target of rapamycin; NAMPT, nicotinamide phosphoribosyl transferase; TGF-β1, transforming growth factor-β1. Lung cancer: CENPF, centromere protein F; FAM83A, family with sequence similarity 83 member A; EGFR, epidermal growth factor receptor; MAPK, mitogen activated protein kinase; CHKA, choline kinase α; PAICS, phosphoribosylaminoimidazole carboxylase. CELSR3, cadherin EGF LAG seven-pass G-type receptor 3; Bladder cancer: BDNF, brain-derived neurotrophic factor; TrkB, tyrosine kinase receptor B; C1GALT1, core 1 beta1,3-galactosyltransferase 1; MUC16, mucin16; HSP47, heat shock protein 47; ERK, extracellular regulated protein kinases; CCL2, C–C motif chemokine ligand 2; VEGF-C, vascular endothelial growth factor C; GLS, glutaminase; Liver cancer: SOX9, sex-determining region Y-box 9; CXCL5, C-X-C motif chemokine 5; PI3K, phosphatidylinositol-3-kinase; ERK1, extracellular regulated protein kinases 1; ERK2, extracellular regulated protein kinases 2; ORC6, origin recognition complex bundle 6; IGF1, insulin-like growth factor 1. Esophageal cancer: CORO1C, coronin 1C; TPM3, tropomyosin 3; TAGLN2, transgelin2; PIK3CA, phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform. Oral squamous cell carcinoma: DKK1, dickkopf homolog 1. Ovarian cancer: TNKS2, tankyrase 2; c-Met, cellular-mesenchymal epithelial transition factor. Breast cancer: Bcl-2, B-cell lymphoma 2; ERK, extracellular regulated protein kinases; EVI-1, ecotropic viral integration site-1; MINOS1, mitochondrial inner membrane organizing system 1; GPD2, glycerol-3-phosphate dehydrogenase 2; LRPPRC, leucine-rich pentatricopeptide-repeat containing. Other cancer: CDK14, cyclin-dependent kinase 14; YY1, Yin Yang 1; G6PD, glucose-6-phosphate dehydrogenase
Table 1.
Role of miR-1-3p in tumor occurrence and development
| Target | Research object | Effect on tumor | References |
|---|---|---|---|
| STC2 | Gastric cancer | Inhibit the proliferation and invasion | [56] |
| CENPF | Gastric cancer | Inhibit the proliferation, migration, and invasion | [57] |
| G6PD | Gastric cancer |
Inhibit the Warburg effect Inhibit cell proliferation and promote cell apoptosis |
[30] |
| YWHAZ | Colorectal cancer | Inhibit the proliferation and invasion | [65] |
| IGF1 | Colorectal cancer |
Inhibit the proliferation and promote their apoptosis |
[66] |
| NAMPT | Colorectal cancer | Form a negative feedback pathway | [70] |
| CENPF | Lung adenocarcinoma | Inhibit proliferation and migration | [73] |
| FAM83A | Lung cancer | Inhibit the proliferation, invasion, and migration | [74] |
| CELSR3 | Lung adenocarcinoma | Inhibit the proliferation, migration, and invasion | [75] |
| / | Non-small cell lung cancer | Inhibit the proliferation | [29] |
| PAICS | Non-small cell lung cancer | Involved in the glycolysis and nucleotide metabolism | [76] |
| CCL2 | Bladder cancer | Inhibit cell proliferation, colony formation, migration, and invasion, promote mitosis to stagnate in the G0/G1 phase, and increase the ratio of apoptosis | [83] |
| C1GALT1 | Bladder cancer | Inhibit the proliferation and migration | [38] |
| GLS | Bladder cancer | Inhibit the proliferation, migration, and invasion | [88] |
| BDNF/TrkB | Bladder cancer | Inhibit the proliferation, migration, invasion | [94] |
| SOX9 | Hepatocellular carcinoma | Inhibit the proliferation of HCC cells and promote their apoptosis | [99] |
| ORC6 | Hepatocellular carcinoma | Inhibit HCC cell proliferation, migration, and invasion, and induce cell cycle arrest and apoptosis | [103] |
| / | Hepatocellular carcinoma | Inhibit angiogenesis | [108] |
| IGF1 | Hepatocellular carcinoma | Inhibit the proliferation of HCC cells and promote apoptosis, and more HCC cells stay in G0/G1 phase | [25] |
| E2F5 and PFTK1 | Prostate cancer | Inhibit the proliferation and colony forming and make more cells stay in the G0/1 phase | [111] |
| CORO1C | Prostate cancer | Inhibit the migration, invasion, and EMT progression | [26] |
| LASP1 | Prostate cancer | Inhibit the proliferation and migration and bone metastasis (BM) of Gleason 3 + 4 PCa | [114] |
| TAGLN2 | Esophageal cancer | Inhibit the proliferation, colony formation, invasion and promote apoptosis | [117] |
| PIK3CA/AKT pathway | Esophageal cancer | Inhibit the proliferation and promote apoptosis | [31] |
| CORO1C/TPM3 | Esophageal cancer | Inhibit the migration and invasion | [27] |
| DKK1 | Oral squamous cell carcinoma | Inhibit the proliferation, migration, and invasion, block the transition from the G0/G1 phase to the S phase and induce cell apoptosis | [120] |
| Fibronectin 1 | Renal cell carcinoma | Inhibit the EMT process and weaken the ability of migration and invasion | [133] |
| TNKS2/Wnt/ β-catenin signaling pathway | Ovarian cancer | Inhibit the proliferation, induce cell cycle arrest in the G1/S phase, and promote cell apoptosis | [121] |
| c-Met | Ovarian cancer | Block cell cycle progression and inhibit proliferation, migration, and invasion of OA cells | [122] |
| Bcl-2 | Breast cancer | Inhibit proliferation, migration, invasion and promote cell apoptosis | [127] |
| K-Ras and lncRNA MALAT1 | Breast cancer | Inhibited cell proliferation and motility and promoted apoptosis | [128] |
| MEK/ERK | Breast cancer | Inhibit proliferation, migration, invasion | [129] |
| BVI-1 | Breast cancer | Inhibit proliferation and EMT-related genes in BCSCs and promote apoptosis | [130] |
| MINOS1, GPD2, LRPPRC | Breast cancer | Trigger mitochondrial damage and promote mitochondrial autophagy in BCSCs | [131] |
| CDK14 | Osteosarcoma | Inhibit cell proliferation and cell cycle progression while promoting apoptosis | [137] |
| Fibronectin 1 | Glioblastoma | inhibit the proliferation and migration of cells | [147] |
| YY1 | Medulloblastoma | Promote malignant phenotypes | [148] |
| G6PD | Pituitary tumors |
Inhibit NADPH production and glycolytic processes; Inhibit proliferation and promote apoptosis |
[149] |
| VASP/Rap1 axis | Breast cancer | Inhibit the malignant phenotype and chemotherapy resistance of BC cells | [28] |
In conclusion, miR-1-3p has been found to be down-regulated in a variety of tumors and closely associated with tumor development. Overexpression of miR-1-3p exhibited tumor suppression in a variety of tumor cells and animal models. Although, it is not clear whether these experimental results can be reproduced in the human body, this lays a preliminary theoretical foundation for clinical translation.
Clinical significance of miR-1-3p
Diagnostic marker
The study found that the expression level of miR-1-3p in serum was different between benign and malignant OA patients [150]. This seems to be helpful for the diagnosis of OA, but its diagnostic significance is lower than that of tumor marker C125 [150]. This limits the role of miR-1-3p in the diagnosis of OA. The research of Chen et al. shows that the expression level of miR-1-3p is significantly low in the serum of stomach adenocarcinoma (STAD) patients, and is closely related to the TNM stage and invasion depth of the patients. The level of miR-1-3p in serum has a certain degree of diagnostic ability. If combined with miR-125b-5p, miR-196a-5p, and miR-149-5p in serum, it can significantly improve the sensitivity and specificity of diagnosing STAD patients [151]. In addition, miR-1-3p in serum also plays an important role in the diagnosis of CRC [152]. Compared with the control group, the expression level of miR-1-3p in the serum of CRC patients was significantly reduced, and they had better predictive ability than carcinoembryonic antigen (CEA) and carcinoembryonic antigen 211 (CA211) [153]. In conclusion, the potential of miR-1-3p in tumor diagnosis still needs to be further developed. The combination of miR-1-3p and a variety of miRNAs may further improve the specificity and sensitivity of diagnosis, which will be conducive to clinical transformation.
Prognostic marker
MiR-1-3p has good potential as a prognostic marker. The study found that patients with low serum miR-1-3p levels had higher all-cause mortality after abdominal aortic aneurysm (AAA) surgery [47]. Detecting the levels of miR-1-3p in patients before and after AAA surgery may help doctors determine the prognosis of AAA patients after surgery, in order to take appropriate intervention measures. The study of Wei et al. found that in patients who underwent radical prostatectomy for PCa, the expression level of miR-1-3p was significantly lower in the tumor tissues of the patients in the recurrence group compared with that in the no recurrence group. miR-1-3p was considered to be the only independent factor for prostate cancer recurrence [154]. This conclusion is similarly supported by the study of Karatas et al. [155]. Therefore, detecting the expression level of miR-1-3p in tumor tissues of PCa patients after radical prostatectomy for prostate cancer can help to provide physicians with information about the likelihood of the patient's cancer recurrence, so that relevant interventions can be prepared. In addition, NSCLC may lead to leptomeningeal metastases (LM), which is a terrible consequence. During the process of intrathecal chemotherapy for NSCLC-LM patients, the expression levels of miR-1-3p in the cerebrospinal fluid exosomes (CSF) of patients with partial response (PR) continuously increased compared to patients with progressive disease (PD) [156]. This suggests that miR-1-3p in CSF extracellular vesicles may become a biomarker for evaluating the efficacy of intrathecal chemotherapy in NSCLC-LM patients.
Currently, the study of miR-1-3p in the prognosis of tumors still needs a lot of exploration. In general, decreased expression levels of miR-1-3p are associated with poor prognosis, while the opposite is true for increased expression levels. However, long-term use of cardiotoxic drugs can also cause elevated serum levels of miR-1-3p, which requires special attention.
Toxicity marker
Doxorubicin (DOX) is a common and potent anticancer drug, but its cytotoxicity is not specific. As a result, it also damages normal cells, such as myocardial cells, which is considered one of the main side effects of DOX [157]. MiR-1-3p was previously considered a specific miRNA in myocardial and skeletal muscles, which is released into the bloodstream during myocardial and skeletal muscle injuries. Rigaud et al. found that the increased expression of miR-1-3p in serum was closely related to cardiac dysfunction in BC patients who used DOX continuously. The ability of serum miR-1-3p to differentiate between patients with DOX-related myocardial injury and those without myocardial injury was superior to the common myocardial injury marker cardiac troponin I (cTnI) (Table 2) [158].
Table 2.
Clinical significance of miR-1-3p
| Function | Effect | Sample | Notes | References |
|---|---|---|---|---|
| Diagnostic marker | Distinguishing between benign and malignant OA | Plasma | The diagnostic significance is lower than that of tumor marker C125 | [150] |
| Diagnostic marker | Diagnosing patients with STAD | Plasma | Combine with miR-125b-5p, miR-196a-5p, and miR-149-5p in serum | [151] |
| Diagnostic marker | Diagnosing patients with CRC | Plasma | The predictive ability is better than CEA and CA211 | [152, 153] |
| Prognostic marker | Predicting the risk of postoperative all-cause mortality in AAA patients | Plasma | / | [47] |
| Prognostic marker | Predicting the risk of recurrence after radical resection in patients with Pca | Tissue | The only independent factor for prostate cancer recurrence | [154, 155] |
| Prognostic marker | Evaluating the efficacy of intrathecal chemotherapy in patients with NSCLC-LM | Cerebrospinal fluid | / | [156] |
| Toxicity marker | Evaluation of DOX-induced cardiotoxicity in the treatment of BC patients | Plasma | The ability to distinguish between patients with DOX-related myocardial injury and those without myocardial injury is superior to cTnI | [158] |
MiR-1-3p and drug resistance
The emergence of drug resistance in tumors involves complex mechanisms, including changes in the expression levels of non-coding RNAs (such as miRNAs) [159–161]. The change of miRNA expression contributes to tumor cell survival and resistance to chemotherapy by regulating a series of downstream genes related to proliferation, cell cycle, invasion, metastasis, DNA repair and programmed cell death.
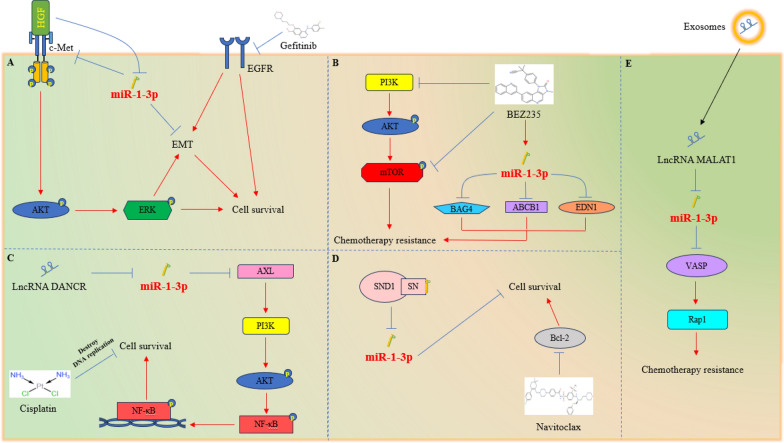
Gefitinib is a tyrosine kinase inhibitor (TKI), which has good reactivity to advanced NSCLC patients with epidermal growth factor receptor (EGFR) mutations. Unfortunately, shortly after the use of drugs, the emergence of drug resistance led to treatment failure. It is reported that hepatocyte growth factor (HGF) is overexpressed in about 61% of patients with acquired drug resistance [162, 163]. HGF can reduce the expression of miR-1-3p in cells and induce EGFR mutant NSCLC cells to be resistant to gefitinib. Overexpression of miR-1-3p can target c-Met (HGF receptor), thereby inhibiting the AKT/ERK signaling pathway and EMT process, ultimately restoring the sensitivity of cells to gefitinib [164].
The abnormal activation of the PI3K/AKT/mTOR pathway can promote the proliferation of tumor cells and endow various malignant tumors, including acute myeloid leukemia (AML), with resistance to chemotherapy. The PI3K/mTOR dual inhibitor BEZ235 can inhibit the proliferation and migration of multidrug-resistant AML cell lines (HL-60/VCR and K562/ADR), and improve their sensitivity to VCR and ADR. The mechanism is that BEZ235 can upregulate miR-1-3p, and then silence BAG4, EDN1, and ABCB1 (key regulators of cell apoptosis, migration, and multidrug resistance), and ultimately sensitize multidrug-resistant AML cells [165].
Cisplatin is one of the most commonly used anticancer drugs in clinical practice and can be used as an adjuvant chemotherapy drug for malignant gliomas. LncRNA DANCR has been found to be associated with cisplatin sensitivity in malignant gliomas. DANCR upregulates AXL by targeting five miRNAs, including miR-1-3p, thereby activating the transduction of the PI3K/Akt/NF-κB signaling pathway, ultimately endowing malignant glioma cells with resistance to cisplatin [32].
Navitoclax is a powerful Bcl-2 protein family inhibitor with anti-tumor activity against various tumor cells. As previously mentioned, SND1 can bind and degrade specific miRNAs through the SN domain. Inhibiting SND1 can enhance the sensitivity of colon cancer cells to navitoclax by upregulating the levels of miR-1-3p [52].
In addition, the exosomes derived from BC cells transmit lncRNA MALAT1 to surrounding BC cells, which can silence miR-1-3p and activate the vasodilator-stimulated phosphoprotein (VASP)/RAS-associated protein 1 (Rap1) signaling axis, ultimately endowing BC cells with chemotherapy resistance (Fig. 4) [28].
Fig. 4.
Role of miR-1-3p in chemotherapy sensitivity. A HGF can reduce the expression level of miR-1-3p and promote the resistance of NSCLC cells with EGFR sensitive mutations to Gefitinib. Overexpression of miR-1-3p can target c-Met, thereby inhibiting the AKT/ERK signaling pathway and EMT process, and ultimately restoring the sensitivity of NSCLC cells to Gefitinib. B The abnormal activation of the PI3K/AKT/mTOR signaling pathway endows AML cells with chemotherapy resistance. The dual inhibitor BEZ235 of PI3K and mTOR can upregulate the expression of miR-1-3p, thereby inhibiting BAG4, EDN1, and ABCB1, ultimately enhancing the chemotherapy sensitivity of AML cells. C LncRNA DANCR upregulates AXL by targeting five miRNAs, including miR-1-3p, thereby activating PI3K/Akt/NF- κB transduction of the signaling pathway, ultimately endowing GBM cells with resistance to cisplatin. D The SN domain of SND1 can bind and degrade miR-1-3p, giving colon cancer cells resistance to navitoclax. E BC cells endow surrounding BC cells with chemotherapy resistance through paracrine exosomes containing high expression levels of lncRNA MALAT1
Approaches to targeting miR-1-3p
Conventional drug-targeted methods
Conventional drugs including chemical drugs and natural drugs can target miR-1-3p and change its expression level. This is a very simple targeting method.
Propofol is a commonly used anesthetic in clinical settings. Ye et al. found that propofol could upregulate miR-1-3p in CRC cells, thereby inhibiting the activation of IGF1 and the AKT/mTOR axis, which was able to inhibit cell proliferation and promote apoptosis. Tumor growth in propofol-treated CRC xenograft nude mice was inhibited and upregulation of miR-1-3p could be detected, whereas silencing miR-1-3p reversed the efficacy of propofol [66]. However, propofol is strictly controlled due to its specific pharmacological effects and psychiatric dependence, which makes it difficult to be used as an agonist of miR-1-3p in the clinic. As research progresses, chemical agonists of miR-1-3p will continue to be discovered. Generally, chemical agonists are relatively inexpensive, but may also have more side effects.
Natural products have attracted the attention of many researchers because of their relatively low toxicity. Studies have found that quercetin could activate the miR-1-3p/TAGLN2 signaling axis in EC cells, thereby inhibiting cell proliferation and invasion and inducing apoptosis [117]. In addition, icariin was able to target the miR-1-3p/tankyrase 2 (TNKS2)/Wnt/β-catenin axis to inhibit the proliferation of OA cells and induce apoptosis [121]. Based on this theoretical basis, Fu et al. injected icariin into the peritoneum of OA xenograft nude mice, which effectively inhibited tumor growth. And in experiments, icariin was found to have less toxicity than cisplatin [121].
Although several chemical drugs and natural products are able to target miR-1-3p, they all share some common limitations. First, they have poor specificity and a rather large number of targets. Second, the mechanism of targeting miR-1-3p is also unclear [90]. Therefore, in order to better target the target molecule, gene drugs will become a hot spot for future research.
Nano-delivery methods
MiR-1-3p expression is down-regulated in a variety of tumors, and delivery of miR-1-3p to tumor tissues is a promising gene therapy. However, miRNAs are negatively charged and not easily taken up by cells. In addition, they are very unstable in body fluids and are easily degraded by enzymes. Therefore, miRNA delivery is highly dependent on carriers. Vectors for the delivery of nucleic acids mainly include viral vectors and non-viral vectors. Viral vectors can effectively deliver miRNA into cells but are difficult to be further used for in vivo delivery due to factors such as biosafety risk, immune response, and small loading volume. Currently, non-viral vector delivery systems have become a research hotspot because of their diversity and modifiability. The same nanocarrier can deliver different miRNAs to target cells. Because there is currently little research on delivering miR-1-3p, the next step will be to introduce common miRNA delivery vectors and methods through other studies of miRNA delivery.
Lipid nanoparticle
Lipid nanoparticles (LNPs) are simple to prepare, have a large loading capacity, and are easy to produce on a large scale. LNP can increase their stability and targeting ability with some modifications.
Doxorubicin (DOX) is a broad-spectrum antitumor antibiotic for the treatment of HCC. However, its use is largely limited due to toxicity and chemotherapy resistance. miR-375 was able to inhibit the development of HCC by reducing the expression of Yes-associated protein 1 (YAP1), autophagy-related protein 7 (ATG7,) and astrocyte elevated gene-1 (AEG-1). It can also target multidrug resistance gene 1 (MDR1) and significantly inhibit DOX resistance. Fan et al. employed liposomal encapsulation of miR-375 and DOX to construct the L-miR-375/DOX NP complex. The complex was able to more effectively inhibit tumor growth in HCC-transplanted tumor-bearing mice and attenuated the cardiotoxicity and hepatotoxicity of DOX compared to DOX alone. In addition, the complex did not produce significant toxicity to the lungs, spleen, and kidneys. Xu et al. constructed a miR-101/DOX-L complex using liposome-encapsulated miR-101 and DOX, and used it for the treatment of HCC-transplanted tumor-bearing nude mice, and obtained similar results.
It has been reported that miR-603 expression was significantly reduced in GBM patients after radiotherapy, while the suppression of IGF1 and IGF1R expression was partially lifted, thus promoting cancer stem cell status and radiotherapy resistance [166]. Shabana et al. encapsulated the complex formed by miR-603 and polyethylenimine (PEI) with polyethylene glycol (PEG) and PR_b-modified liposomes. In this complex, PEG enhances the water solubility and biocompatibility of liposomes. PR_b is a fibronectin-mimetic peptide that can achieve targeting by specifically binding to integrin α5β1. PEI is a cationic polymer that helps miRNA escape from endosomes and lysosomes of cells. This complex can effectively elevate miR-603 in GBM cells and inhibit the expression of IGF1, thereby increasing the sensitivity to radiotherapy. This may be an effective strategy to improve radiotherapy resistance in GBM patients.
Metal nanoparticles
Inorganic metal nanoparticles are widely used in nucleic acid delivery studies, mainly including gold nanoparticles, superparamagnetic iron oxide nanoparticles (SPIONs), and mesoporous silica nanoparticles (MSNs).
Gold nanoparticles have unique optical properties, easy control of shape and size, good biocompatibility, and low cytotoxicity. Gold nanoparticles can be modified with PEI, PEG, lipoic acid, folic acid (FA), and other groups to enhance their encapsulation, biocompatibility, and targeting ability. Guo et al. constructed gold nanoparticles loaded with miR-21-3p (miR-21-3p-AuNp) and injected the complex into melanoma graft-tumor-bearing mice, which showed a significant increase in miR-21-3p in tumor tissues. MiR-21-3p was able to increase sensitivity to anti-PD-1 immunotherapy by promoting ferroptosis. The nanoparticles had low immunogenicity and did not significantly damage tissues such as heart, liver, spleen, lungs and kidneys, which demonstrated the high safety of gold nanoparticles [167]. In addition, gold nanoparticles have a strong near-infrared absorption capacity and can act as an anticancer photothermal agent in their own right. Huang et al. constructed anti-miR-181b/PTPAuNCs complexes using PEI-, PEG-, LA-, and FA-modified gold nanocages loaded with anti-miR-181b, a tumor suppressor. The complex was injected into HCC hormonal mice and irradiated the tumor site with near-infrared light, which was able to achieve the combination of gene therapy and photothermal therapy, thus significantly inhibiting tumor growth [168].
SPIONs are magnetically responsive nanoparticles that possess good biocompatibility, modifiability, low cytotoxicity, and degradability [169]. SPIONs can be directed to aggregate in tumor tissues under an applied magnetic field, and the magnetic field increases the nanoparticle's ability to penetrate the cell membrane and blood–brain barrier [170, 171]. It was reported that SPIONs loaded with miR-374a and SPIONs loaded with miR-326 were able to inhibit tumorigenicity in human glioma stem cell xenograft tumor mice and human endometrial cancer stem cell xenograft tumor mice, respectively [172, 173]. SPIONs can gather in the capillaries of tumor tissues under the action of the local magnetic field, thus blocking the blood supply of tumor tissues. For normal tissues in non-magnetic field areas, SPIONs are dispersed and do not block the blood vessels of normal tissues. In addition, SPIONS have magnetothermal effects and capabilities of magnetic resonance imaging (MRI) [174, 175]. After being localized in tumor tissues, SPIONs are able to gradually generate heat and warm up under the action of an alternating magnetic field, thus causing devastating damage to tumor cells [176]. This temperature controllability also enables the ability to achieve controlled drug release. However, this type of magnetothermal therapy is not suitable for combination with nucleic acid delivery, which may lead to degradation of the nucleic acid drug, and is therefore more suitable for combination with chemotherapeutic agents. As an excellent contrast agent, another property of SPIONs has the ability of MRI, which facilitates the diagnosis of cancer and visualization of nucleic acid delivery.
MSNs are solid nanoparticles with porous structure and large specific surface area. MSNs have good modifiability, biocompatibility, thermal stability, biodegradability, and are a good carrier for controlled release [177–179]. Garrido-Cano et al. constructed the MSN-PEI-miR200c-HA complex by wrapping MSNs with PEI to form a cationic surface to adsorb the negatively charged miR-200c-3p, and then wrapping a layer of hyaluronic acid (HA) around the outer layer. Among them, PEI mediated lysosomal escape and HA was able to bind CD44, which was highly expressed on BC cells, thus conferring targeting properties to the complex. Injection of the complex into BC xenograft tumor-bearing mice was able to accumulate in the tumor tissue and significantly increase the level of miR-200c-3p. miR-200c-3p was able to inhibit the expression of Zinc finger E-box binding homeobox 1 (ZEB1) and Zinc finger E-box binding homeobox 2 (ZEB2), which ultimately inhibited the ability of BC to grow [180].
Macromolecular polymer
PEIs, as mentioned previously, are cationic polymers with positively charged amino groups on their straight and branched chains capable of binding to negatively charged phosphate groups on miRNAs. Upon entering cancer cells, it is able to effectively escape from the lysosome, thus aggregating in the cytoplasm and releasing miRNAs [181]. However, PEI also has some limitations. Firstly, PEI is difficult to biodegrade in cells, and secondly, it is easy to combine with negatively charged proteins to produce cytotoxicity. Therefore, structural modification of PEIs is highly desirable. Zhang et al. constructed the R11-SSPEI/FAM-miR-145 complex using disulfide-bonded and polyarginine (R11)-modified PEIs loaded with FAM-tagged miR-145. The disulfide bond enhances the biocompatibility and degradability of PEIs, thereby reducing the toxic effects on cells.R11, a peptide that is specifically ingested in prostate cancer, confers targeting ability to PEIs. And FAM is a fluorescent dye that is capable of tracing the labeled nucleic acids. Injecting the complex into PCa-transplanted tumor-bearing mice was able to preferentially accumulate in tumor tissues, increase miR-145 levels, and effectively suppress tumors [182].
Chitosan can be obtained by partial deacetylation of chitin. Chitin is a natural polymer polysaccharide widely found in the shells of shrimps, crabs, insects and the cell walls of fungi, with good biocompatibility, biodegradability and non-toxicity [183]. However, the transfection efficiency of chitosan is relatively low, which can be improved by changing the molecular weight, degree of deacetylation, nitrogen-phosphorus ratio of chitosan, and by performing suitable chemical modifications [184]. Santos-Carballal et al. found that when chitosan had a molecular weight of approximately 40 kDa, a degree of acetylation of 12%, and a ( ±) charge ratio of 1.5, its transfection efficiency approached that of the harmaFECT and Novafect O 25 commercial reagents. Employing this chitosan complex loaded with miR-145 to transfect MCF-7 cells was able to significantly alter the levels of the corresponding target mRNAs without significant cytotoxicity [185].
There are over 200 types of dendritic macromolecules, including common ones such as polyamide amine (PAMAM), polylysine (PLL), and polypropylene imide (PPI). Dendritic macromolecules have a large cavity structure and a large number of positively charged amino groups, which are able to electrostatically bind to a large number of miRNAs, and have the advantages of large loading capacity, high transfection efficiency, good water solubility, and modifiability. Elfiky et al. constructed the LA-PAMAM/pmiR-218 complex using LA-modified hyperbranched PAMAM loaded with a plasmid expressing miR-128. The complex was able to effectively inhibit tumor development in HCC mice, where LA was able to specifically bind to the asialoglycoprotein receptor, which is highly expressed on HCC cells, thus conferring the complex targeting ability [186]. However, dendritic macromolecules also have some disadvantages, such as being difficult to biodegrade and having a large positive charge, which may cause some cytotoxicity if they accumulate in cells. Therefore, dendritic macromolecules modified with pegylation, glycosylation, acetylation, and peptide to neutralize some of the positive charges or to increase biodegradability may be effective measures to address the shortcomings.
Polylactic acid hydroxyacetic acid copolymer (PLGA) is a nano material with good biocompatibility and biodegradability. It can be decomposed into lactic acid and glycolic acid in the body and absorbed by the human body, so it has no cytotoxicity. PLGA is slowly degraded intracellularly and its degradation time is related to the ratio of lactic acid to hydroxyacetic acid, so it also has a controlled slow-release capability [187]. However, PLGA has a relatively low sample load and encapsulation rate [188, 189]. In addition, it is negatively charged under physiological conditions, which is unfavorable for cellular uptake. Wang et al. employed HA and PEI-modified PLGA to construct a HA-PEI-PLGA complex, which showed improved encapsulation rate and transfection efficiency. Treatment of triple-negative breast cancer cells MDA-MB-231 using the complex loaded with DOX and miR-542-3p significantly increased the content of both and promoted apoptosis [190].
In addition, many materials such as MOF and hydrogels can also be used as carriers for delivering miRNAs. In conclusion, all these vectors have their advantages and disadvantages (Table 3). In the future, more carriers will be developed and improved, which will provide technical support for miRNA delivery and clinical translation.
Table 3.
Advantages and disadvantages of vectors
| Type of vector | Advantages | Disadvantages |
|---|---|---|
| Viral vectors | High transfection efficiency | Biosafety risk, immune response, complex preparation, and small loading volume |
| Lipid nanoparticle | Simple preparation and good biocompatibility | Positively charged LNP may directly damage cell membranes and cause cytotoxicity |
| Gold nanoparticles | Easy control of shape and size, good biocompatibility, low cytotoxicity, and capability of photothermal effect | / |
| Superparamagnetic iron oxide nanoparticles | Good biocompatibility, modifiability, low cytotoxicity, degradability, Magnetic targeting, magneto-thermal effect, and magnetic resonance capability | / |
| Mesoporous silica nanoparticles | Good modifiability, biocompatibility, thermal stability, biodegradability | / |
| Polyethylenimine | High lysosomal escape ability and high transfection efficiency | Difficult to degrade and cytotoxic |
| Chitosan | Good biocompatibility, biodegradability and non-toxicity | Transfection efficiency is relatively low |
| Dendritic macromolecules | High transfection efficiency, good water solubility, and modifiability | Difficult to degrade and cytotoxic |
| Polylactic acid hydroxyacetic acid copolymer | Good biocompatibility biodegradability, and non-toxicity | A relatively low sample load, encapsulation rate, and transfection efficiency |
Prospects and conclusions
Cancer is a problem that mankind needs to address urgently, and although treatments have advanced over the years, its mortality rate remains high. This is mainly related to the complex mechanism of tumor development, the emergence of drug resistance, tumor recurrence, and other factors. Studying tumor development at the molecular level can help provide new ideas for cancer treatment. miRNAs are a class of non-coding RNAs that regulate a series of physiological processes by degrading mRNAs or inhibiting their translation through binding to the 3′-UTR of target mRNAs. miR-1-3p is an extremely important member of the miRNA family and was first found to be abundantly expressed in the cardiac and skeletal muscles and involved in their development. In recent years, miR-1-3p has been found to be significantly down-regulated in a variety of tumors and has an important role in tumor development, diagnosis, prognosis, and drug resistance, and is considered a tumor suppressor with great potential.
MiR-1-3p is encoded by the miR-1–2 gene located on chromosome 18q11.2 and is produced by shearing through a series of enzymes, which is similar to the production process of other miRNAs. Its level is regulated by a variety of factors, such as lncRNA, circRNA, DNA methylation, SNP, histone acetylation, and transcription factors. The study of the role of miR-1-3p in tumorigenesis and development is the theoretical basis for miRNA gene therapy. miR-1-3p expression levels are significantly down-regulated in a wide range of tumors, and its overexpression can effectively inhibit the malignant phenotype of tumors and promote their apoptosis. In terms of drug resistance, miR-1-3p can increase the sensitivity of some anti-tumor drugs. This may be related to its inhibition of cell survival-related signaling pathways, multidrug resistance genes, and reduction-related protein genes. In addition, miR-1-3p plays an important role in tumor diagnosis, prognosis, and drug toxicity assessment. The miR-1-3p in serum has good diagnostic potential in CRC, and its diagnostic value is superior to CEA and CA211. MiR-1-3p also has certain diagnostic capabilities in STAD, and its combined use with miR-125b-5p, miR-196a-5p, and miR-149-5p increases diagnostic accuracy. At present, there is limited research on the role of miR-1-3p in tumor diagnosis, and there is great research space. The combination of miR-1-3p with other miRNAs to construct a set of miRNA diagnostic panels and develop them into diagnostic kits is a promising direction. MiR-1-3p also plays an important indicator role in the prognosis of tumor patients, especially as the only independent factor for recurrence in patients after radical prostatectomy. The detection of miR-1-3p levels helps to understand the prognosis of patients so that relevant measures can be taken for intervention and improve their survival rate. In terms of drug toxicity assessment, due to the high expression of miR-1-3p in myocardial and skeletal muscles, when myocardial or skeletal muscle damage occurs due to drug use, intracellular miR-1-3p will be released into the bloodstream. Therefore, detecting the expression level of miR-1-3p in serum can evaluate the degree of drug-induced cardiotoxicity or skeletal muscle toxicity. It is not difficult to see that the long-term use of cardiotoxic or skeletal muscle toxic drugs will interfere with the diagnosis and prognosis of patients, so this point needs special attention.
At present, the clinical translation of miR-1-3p is full of opportunities and challenges, especially in targeted therapy. Currently, two siRNA gene drugs, patisiran and givosiran, have been approved by the FDA for clinical use, but miRNA drugs are still in the clinical trial stage. Among them, two studies are in Phase I, three studies are in Phase II, and five studies have been suspended or discontinued. For example, MRX34 (a mimetic of miR-34) was used in a clinical trial to treat melanoma, primary liver cancer, and hematological malignancies, but was forced to discontinue due to severe immune reactions in patients (NCT01829971). This may be related to the different functional characteristics of miRNA and siRNA. SiRNA is an exogenous RNA that binds to the translation region of mRNA and exerts silencing effects. The degree of sequence complementarity can reach 100%, and its target genes are generally 1–3. And miRNA is endogenous RNA that exerts silencing effects by binding to the untranslated region of mRNA, with a complementary degree of 20–90%, and its target genes ranging from dozens to even hundreds [191]. This means that there are too many targets for miRNA, which may lead to unknown side effects. Through functional enrichment analysis of the target genes of miR-34, it was found that there are two immune-related pathways (immune system and cytokine signaling in the immune system), including 28 approved drug target genes and 41 unapproved drug target genes [191]. Therefore, this provides a reasonable explanation for the serious immune-related adverse events caused by MRX34 treatment in the Phase I clinical trial.
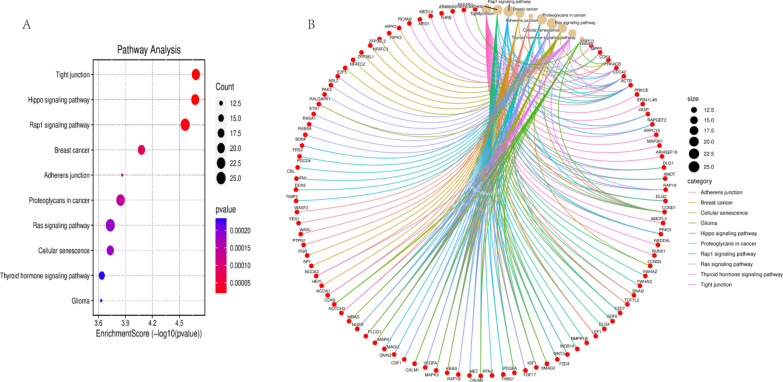
MiR-1-3p similarly requires attention to the problems posed by multiple targets. Research has shown that overexpression of miR-1-3p increases the degree of injury in ischemia–reperfusion (I/R) mice, manifested as myocardial cell apoptosis and an increase in myocardial infarction area [192]. On the other hand, overexpression of miR-1-3p increases the risk of arrhythmia in normal or myocardial infarction rats [193]. In addition, in the diabetes rat model, high glucose induces the upregulation of miR-1-3p in cardiomyocytes through the MEK1/2 pathway and serum response factor (SRF) and then promotes cardiomyocyte apoptosis by targeting HSP60 [194]. Therefore, miR-1-3p has the potential to increase myocardial damage in patients with diabetes. Collect experimentally validated miR-1-3p targets through the TargetScan database (https://www.targetscan.org/vert_80/) for pathway enrichment analysis. The top 10 pathways with enrichment scores include tight junctions, Hippo signaling pathways, Rap1 signaling pathways, etc. (Fig. 5). These signaling pathways can become therapeutic targets in tumor cells, while they can also produce other different effects in other cells. According to reports, tight junction signaling pathways regulate cardiac conduction and intercellular communication, and Hippo and Rap1 signaling pathways are involved in the occurrence and development of I/R and arrhythmia [195–198]. This indicates that miR-1-3p plays an important role in cardiovascular disease, which is consistent with previous studies. If miR-1-3p enters other tissues, such as liver, spleen, lung, kidney, brain, etc., some unpredictable effects will occur. Therefore, too many targets are one of the main reasons for the slow development of miRNA drugs. With the rapid development of active targeting vectors, it brings new hope to the drug development of miRNA. Nanoparticles with active targeting ability can effectively deliver miRNAs to tumor sites, thereby avoiding or weakening its impact on other healthy tissues. With the continuous development of miRNA theory research and carrier research, miR-1-3p is likely to be able to achieve clinical translation in the near future, bringing benefits to cancer patients.
Fig. 5.
Pathway enrichment map of miR-1-3p targets. A The enrichment analysis of the miR-1-3p target pathway showed that the enrichment scores of tight junction, Hippo signaling pathway, Rap1 signaling pathway, breast cancer, adherens junction, proteoglycans in cancer, Ras signaling pathway, cellular senescence, thyroid hormone signaling pathway, and glioma ranked in the top ten. B Relevant targets in the pathways of the top ten enriched scores
Acknowledgements
Not applicable.
Abbreviations
- miRNA
MicroRNA
- 3′-UTR
3′-Untranslated region
- MyoD
Myogenic differentiation antigen
- Mef2
Myocyte enhancer factor 2
- Hand2
Heart and neural crest derivatives-expressed transcript 2
- MIB1
Mindcomb homolog 1
- pri-miRNA
Primary miRNA
- pre-miRNA
Precursor miRNA
- RISC
RNA-induced silencing complex
- lncRNA
Long non-coding RNA
- ceRNA
Competitive endogenous RNA
- circRNA
Circular RNA
- C1GALT1
Core 1 beta1,3-galactosyltransferase 1
- GOLPH3
Golgiphosphoprotein 3
- JUP
Junction plakoglobin
- SNP
Single nucleotide polymorphism
- AAA
Abdominal aortic aneurysm
- SND1
Staphylococcal nuclease and tudor domain containing 1
- TLR4
Toll like receptor 4
- GC
Gastric cancer
- STC2
Stanniocalcin 2
- CENPF
Centromere protein F
- G6PD
Glucose-6-phosphate dehydrogenase
- PPP
Pentose phosphate pathway
- NADPH
Nicotinamide adenine dinucleotide phosphate
- GPX4
Glutathione peroxidase 4
- GSH
Glutathione
- FSP1
Ferroptosis suppressor protein 1
- CoQH2
Reduced Coenzyme Q
- GCH1
GTP cyclohydrolase 1
- BH4
Tetrahydrobiopterin
- DHODH
Dihydroorotate dehydrogenase
- ROS
Reactive oxygen species
- NOX
NADPH oxidase
- CRC
Colorectal cancer
- YWHAZ
Tyrosine 3/tryptophan 5 monooxygenase activation protein zeta
- EMT
Epithelial mesenchymal transition
- IGF1
Insulin-like growth factor 1
- mTOR
Mammalian target of rapamycin
- IGF1R
Insulin-like growth factor 1 receptor
- TGF-β
Transforming growth factor-β
- TGF-β1
Transforming growth factor-β1
- NAMPT
Nicotinamide phosphoribosyl transferase
- NSCLC
Non-small cell lung cancer
- LUAD
Lung adenocarcinoma
- LUSC
Lung squamous cell carcinoma
- FAM83A
Family with sequence similarity 83 member A
- MAPK
Mitogen activated protein kinase
- CHKA
Choline kinase α
- CELSR3
Cadherin EGF LAG seven-pass G-type receptor 3
- PAICS
Phosphoribosylaminoimidazole carboxylase
- BLCA
Bladder cancer
- CCL2
C–C motif chemokine ligand 2
- HSP47
Heat shock protein 47
- ERK
Extracellular regulated protein kinases
- H3K4
Histone H3 lysine 4
- TAM
Tumor-associated macrophages
- VEGF-C
Vascular endothelial growth factor C
- C1GALT1
Core 1 beta1,3-galactosyltransferase 1
- MUC16
Mucin16
- GLS
Glutaminase
- Gln
Glutamine
- Glu
Glutamic acid
- α-KG
α-Ketoglutarate
- GOT1
Glutamic acid transaminase 1
- TCA
The tricarboxylic acid
- BDNF
Brain-derived neurotrophic factor
- TrkB
Tyrosine kinase receptor B
- HCC
Hepatocellular carcinoma
- ICC
Intrahepatic cholangiocarcinoma
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- NAFLD
Non-alcoholic fatty liver disease
- SOX9
Sex-determining region Y-box 9
- CXCL5
C-X-C motif chemokine 5
- PI3K
Phosphatidylinositol-3-kinase
- ERK1
Extracellular regulated protein kinases 1
- ERK2
Extracellular regulated protein kinases 2
- ORC6
Origin recognition complex bundle 6
- OS
Overall survival
- RFS
Relapse-free survival
- TQ
Thymoquinone
- DEN
Diethylnitrosamine
- PCa
Prostate cancer
- CDK2
Cyclin-dependent kinase 2
- CDK4
Cyclin-dependent kinase 4
- E2F5
E2F transcription factor 5
- PFTK1
PFTAIRE protein kinase 1
- CDK
Cyclin-dependent kinase
- CORO1C
Coronin 1C
- LASP1
LIM and SH3 protein 1
- EC
Esophageal cancer
- ESCC
Esophageal squamous cell carcinoma
- EAC
Esophageal adenocarcinoma
- TAGLN2
Transgelin2
- PIK3CA
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
- TPM3
Tropomyosin 3
- OSCC
Oral squamous cell carcinoma
- HPV
Human papilloma virus
- DKK1
Dickkopf homolog 1
- RCC
Renal cell carcinoma
- OA
Ovarian cancer
- BC
Breast cancer
- VASP
Vasodilator-stimulated phosphoprotein
- Rap1
RAS-associated protein 1
- STAD
Stomach adenocarcinoma
- CEA
Carcinoembryonic antigen
- CA211
Carcinoembryonic antigen 211
- EV
Extracellular vesicle
- LM
Leptomeningeal metastases
- CSF
Cerebrospinal fluid exosomes
- PR
Partial response
- PD
Progressive disease
- TKI
Tyrosine kinase inhibitor
- EGFR
Epidermal growth factor receptor
- HGF
Hepatocyte growth factor
- c-Met
Cellular-mesenchymal epithelial transition factor
- AML
Acute myeloid leukemia
- VCR
Vincristine
- ADR
Adriamycin
- BAG4
BAG family molecular chaperone regulator 4
- EDN1
Endothelin 1
- ABCB1
ATP-binding cassette sub-family B member 1
- AXL
Axl receptor tyrosine kinase
- NF-κB
Nuclear transcription factor-κB
- Bcl-2
B-cell lymphoma 2
- EVI-1
Ecotropic viral integration site-1
- MINOS1
Mitochondrial inner membrane organizing system 1
- GPD2
Glycerol-3-phosphate dehydrogenase 2
- LRPPRC
Leucine-rich pentatricopeptide-repeat containing
- CDK14
Cyclin-dependent kinase 4
- YY1
Yin Yang 1
- TNKS2
Tankyrase 2
- LNP
Lipid nanoparticle
- DOX
Doxorubicin
- YAP1
Yes-associated protein 1
- ATG7
Autophagy-related protein 7
- AEG-1
Astrocyte elevated gene 1
- MDR1
Multidrug resistance gene 1
- PEI
Polyethylenimine
- PEG
Polyethylene glycol
- MSNs
Mesoporous silica nanoparticles
- FA
Folic acid
- MRI
Magnetic resonance imaging
- HA
Hyaluronic acid
- ZEB1
Zinc finger E-box binding homeobox 1
- ZEB2
Zinc finger E-box binding homeobox 2
- PAMAM
Polyamide amine
- PLL
Polylysine
- PPI
Polypropylene imide
- PLGA
Polylactic acid hydroxyacetic acid copolymer
- SRF
Serum response factor
Author contributions
This manuscript was completed by SD through literature search, organization, and writing. FL, SX, and JH have read this manuscript and proposed revisions. LG has made revisions to the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Changsha Central Hospital Affiliated to University of South China Foundation of Key Program (YNKY202205), Scientific Research Project of Education Department of Hunan Province (22A0320), Natural Science Foundation of Hunan Province (2021JJ30753) and Hunan Health High-level Talents Support Program.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Zheng RS, Zhang SW, Sun KX, Chen R, Wang SM, Li L, Zeng HM, Wei WW, He J. Cancer statistics in China, 2016. Zhonghua Zhong Liu Za Zhi. 2023;45:212–220. doi: 10.3760/cma.j.cn112152-20220922-00647. [DOI] [PubMed] [Google Scholar]
- 3.Bradley JA, Mendenhall NP. Novel radiotherapy techniques for breast cancer. Annu Rev Med. 2018;69:277–288. doi: 10.1146/annurev-med-042716-103422. [DOI] [PubMed] [Google Scholar]
- 4.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davern M, Lysaght J. Cooperation between chemotherapy and immunotherapy in gastroesophageal cancers. Cancer Lett. 2020;495:89–99. doi: 10.1016/j.canlet.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335–3337. doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi SJ, Jung SW, Huh S, Chung YS, Cho H, Kang H. Alteration of DNA methylation in gastric cancer with chemotherapy. J Microbiol Biotechnol. 2017;27:1367–1378. doi: 10.4014/jmb.1704.04035. [DOI] [PubMed] [Google Scholar]
- 8.Goldie JH. Drug resistance and cancer chemotherapy strategy in breast cancer. Breast Cancer Res Treat. 1983;3:129–136. doi: 10.1007/BF01803555. [DOI] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 11.Mishima Y, Stahlhut C, Giraldez AJ. miR-1-2 gets to the heart of the matter. Cell. 2007;129:247–249. doi: 10.1016/j.cell.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townley-Tilson WH, Callis TE, Wang D. MicroRNAs 1, 133, and 206: critical factors of skeletal and cardiac muscle development, function, and disease. Int J Biochem Cell Biol. 2010;42:1252–1255. doi: 10.1016/j.biocel.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohler U, Yekta S, Lim LP, Bartel DP, Burge CB. Patterns of flanking sequence conservation and a characteristic upstream motif for microRNA gene identification. RNA. 2004;10:1309–1322. doi: 10.1261/rna.5206304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117:2369–2376. doi: 10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 18.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 19.Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 20.Hutvágner G, McLachlan J, Pasquinelli AE, Bálint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 21.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 22.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 24.Xing C, Sun SG, Yue ZQ, Bai F. Role of lncRNA LUCAT1 in cancer. Biomed Pharmacother. 2021;134:111158. doi: 10.1016/j.biopha.2020.111158. [DOI] [PubMed] [Google Scholar]
- 25.Tang K, Lv D, Miao L, Mao Y, Yu X. LncRNA TUG1 functions as a ceRNA for miR-1-3p to promote cell proliferation in hepatic carcinogenesis. J Clin Lab Anal. 2022;36:e24415. doi: 10.1002/jcla.24415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai X, Liang Z, Liu L, Guo K, Xu S, Wang H. Silencing of MALAT1 inhibits migration and invasion by sponging miR-1-3p in prostate cancer cells. Mol Med Rep. 2019;20:3499–3508. doi: 10.3892/mmr.2019.10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Dai Z, Xia C, Jin L, Chen X. Suppression of long non-coding RNA MALAT1 inhibits survival and metastasis of esophagus cancer cells by sponging miR-1-3p/CORO1C/TPM3 axis. Mol Cell Biochem. 2020;470:165–174. doi: 10.1007/s11010-020-03759-x. [DOI] [PubMed] [Google Scholar]
- 28.Tao S, Bai Z, Liu Y, Gao Y, Zhou J, Zhang Y, Li J. Exosomes derived from tumor cells initiate breast cancer cell metastasis and chemoresistance through a MALAT1-dependent mechanism. J Oncol. 2022;2022:5483523. doi: 10.1155/2022/5483523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Luo X, Liu Y, Han G, Sun D. Long noncoding RNA RMRP promotes proliferation and invasion via targeting miR-1-3p in non-small-cell lung cancer. J Cell Biochem. 2019;120:15170–15181. doi: 10.1002/jcb.28779. [DOI] [PubMed] [Google Scholar]
- 30.Deng P, Li K, Gu F, Zhang T, Zhao W, Sun M, Hou B. LINC00242/miR-1-3p/G6PD axis regulates Warburg effect and affects gastric cancer proliferation and apoptosis. Mol Med. 2021;27:9. doi: 10.1186/s10020-020-00259-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang D, Zhang H, Wang X, Hu B, Zhang F, Wei H, Li L. LINC01518 knockdown inhibits tumorigenicity by suppression of PIK3CA/Akt pathway in oesophageal squamous cell carcinoma. Artif Cells Nanomed Biotechnol. 2019;47:4284–4292. doi: 10.1080/21691401.2019.1699815. [DOI] [PubMed] [Google Scholar]
- 32.Ma Y, Zhou G, Li M, Hu D, Zhang L, Liu P, Lin K. Long noncoding RNA DANCR mediates cisplatin resistance in glioma cells via activating AXL/PI3K/Akt/NF-κB signaling pathway. Neurochem Int. 2018;118:233–241. doi: 10.1016/j.neuint.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Shan G. CircRNA in cancer: fundamental mechanism and clinical potential. Cancer Lett. 2021;505:49–57. doi: 10.1016/j.canlet.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu T, Wang Y, Fan Y, Fang N, Wang T, Xu T, Shu Y. CircRNAs in cancer metabolism: a review. J Hematol Oncol. 2019;12:90. doi: 10.1186/s13045-019-0776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu X, Luo X, Long X, Jiang S, Xie X, Zhang Q, Wang H. CircAGO2 promotes colorectal cancer progression by inhibiting heat shock protein family B (small) member 8 via miR-1-3p/retinoblastoma binding protein 4 axis. Funct Integr Genomics. 2023;23:78. doi: 10.1007/s10142-023-00990-9. [DOI] [PubMed] [Google Scholar]
- 38.Tan Z, Jiang Y, Liang L, Wu J, Cao L, Zhou X, Song Z, Ye Z, Zhao Z, Feng H, et al. Dysregulation and prometastatic function of glycosyltransferase C1GALT1 modulated by cHP1BP3/ miR-1-3p axis in bladder cancer. J Exp Clin Cancer Res. 2022;41:228. doi: 10.1186/s13046-022-02438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antequera F, Bird A. Number of CpG islands and genes in human and mouse. Proc Natl Acad Sci USA. 1993;90:11995–11999. doi: 10.1073/pnas.90.24.11995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsen F, Gundersen G, Lopez R, Prydz H. CpG islands as gene markers in the human genome. Genomics. 1992;13:1095–1107. doi: 10.1016/0888-7543(92)90024-M. [DOI] [PubMed] [Google Scholar]
- 41.Saxonov S, Berg P, Brutlag DL. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc Natl Acad Sci USA. 2006;103:1412–1417. doi: 10.1073/pnas.0510310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan AE, Davies TJ, Mc Auley MT. The role of DNA methylation in ageing and cancer. Proc Nutr Soc. 2018;77:412–422. doi: 10.1017/S0029665118000150. [DOI] [PubMed] [Google Scholar]
- 43.Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell. 2014;26:577–590. doi: 10.1016/j.ccr.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendizabal I, Yi SV. Whole-genome bisulfite sequencing maps from multiple human tissues reveal novel CpG islands associated with tissue-specific regulation. Hum Mol Genet. 2016;25:69–82. doi: 10.1093/hmg/ddv449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duca RB, Massillo C, Farré PL, Graña KD, Moro J, Gardner K, Lacunza E, De Siervi A. Hsa-miR-133a-3p, miR-1-3p, GOLPH3 and JUP combination results in a good biomarker to distinguish between prostate cancer and non-prostate cancer patients. Front Oncol. 2022;12:997457. doi: 10.3389/fonc.2022.997457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou M, Li H, Chen K, Ding W, Yang C, Wang X. CircSKA3 downregulates miR-1 through methylation in glioblastoma to promote cancer cell proliferation. Cancer Manag Res. 2021;13:509–514. doi: 10.2147/CMAR.S279097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li T, Jiang B, Wu Y, Yang J, Ma C, Yuan Y. Association of genetic polymorphisms and serum levels of miR-1–3p with postoperative mortality following abdominal aortic aneurysm repair. J Clin Med. 2023;12:946. doi: 10.3390/jcm12030946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li T, Jing J, Sun L, Gong Y, Yang J, Ma C, Yuan Y. The SNP rs4591246 in pri-miR-1-3p is associated with abdominal aortic aneurysm risk by regulating cell phenotypic transformation via the miR-1-3p/TLR4 axis. Int Immunopharmacol. 2023;118:110016. doi: 10.1016/j.intimp.2023.110016. [DOI] [PubMed] [Google Scholar]
- 49.Scadden AD. The RISC subunit Tudor-SN binds to hyper-edited double-stranded RNA and promotes its cleavage. Nat Struct Mol Biol. 2005;12:489–496. doi: 10.1038/nsmb936. [DOI] [PubMed] [Google Scholar]
- 50.Caudy AA, Ketting RF, Hammond SM, Denli AM, Bathoorn AM, Tops BB, Silva JM, Myers MM, Hannon GJ, Plasterk RH. A micrococcal nuclease homologue in RNAi effector complexes. Nature. 2003;425:411–414. doi: 10.1038/nature01956. [DOI] [PubMed] [Google Scholar]
- 51.Elbarbary RA, Miyoshi K, Myers JR, Du P, Ashton JM, Tian B, Maquat LE. Tudor-SN-mediated endonucleolytic decay of human cell microRNAs promotes G(1)/S phase transition. Science. 2017;356:859–862. doi: 10.1126/science.aai9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehmusvaara S, Haikarainen T, Saarikettu J, Martinez Nieto G, Silvennoinen O. Inhibition of RNA binding in SND1 increases the levels of miR-1–3p and sensitizes cancer cells to navitoclax. Cancers (Basel) 2022;14:3100. doi: 10.3390/cancers14133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 54.Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40:250–260. doi: 10.1111/apt.12814. [DOI] [PubMed] [Google Scholar]
- 55.Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. 2023;20:338–349. doi: 10.1038/s41571-023-00747-0. [DOI] [PubMed] [Google Scholar]
- 56.Ke J, Zhang BH, Li YY, Zhong M, Ma W, Xue H, Wen YD, Cai YD. MiR-1-3p suppresses cell proliferation and invasion and targets STC2 in gastric cancer. Eur Rev Med Pharmacol Sci. 2019;23:8870–8877. doi: 10.26355/eurrev_201910_19282. [DOI] [PubMed] [Google Scholar]
- 57.Zhou S, Han H, Yang L, Lin H. MiR-1-3p targets CENPF to repress tumor-relevant functions of gastric cancer cells. BMC Gastroenterol. 2022;22:145. doi: 10.1186/s12876-022-02203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stanton RC. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life. 2012;64:362–369. doi: 10.1002/iub.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, Koppula P, Wu S, Zhuang L, Fang B, et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–590. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silic-Benussi M, Sharova E, Ciccarese F, Cavallari I, Raimondi V, Urso L, Corradin A, Kotler H, Scattolin G, Buldini B, et al. mTOR inhibition downregulates glucose-6-phosphate dehydrogenase and induces ROS-dependent death in T-cell acute lymphoblastic leukemia cells. Redox Biol. 2022;51:102268. doi: 10.1016/j.redox.2022.102268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang BL. Neuroprotection by glucose-6-phosphate dehydrogenase and the pentose phosphate pathway. J Cell Biochem. 2019;120:14285–14295. doi: 10.1002/jcb.29004. [DOI] [PubMed] [Google Scholar]
- 62.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 64.Moreno EC, Pascual A, Prieto-Cuadra D, Laza VF, Molina-Cerrillo J, Ramos-Muñoz ME, Rodríguez-Serrano EM, Soto JL, Carrato A, García-Bermejo ML, Guillén-Ponce C. Novel molecular characterization of colorectal primary tumors based on miRNAs. Cancers (Basel) 2019;11:346. doi: 10.3390/cancers11030346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Du G, Yu X, Chen Y, Cai W. MiR-1-3p suppresses colorectal cancer cell proliferation and metastasis by inhibiting YWHAZ-mediated epithelial-mesenchymal transition. Front Oncol. 2021;11:634596. doi: 10.3389/fonc.2021.634596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye LL, Cheng ZG, Cheng XE, Huang YL. Propofol regulates miR-1-3p/IGF1 axis to inhibit the proliferation and accelerates apoptosis of colorectal cancer cells. Toxicol Res (Camb) 2021;10:696–705. doi: 10.1093/toxres/tfab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang XW, Zhang YJ. Targeting mTOR network in colorectal cancer therapy. World J Gastroenterol. 2014;20:4178–4188. doi: 10.3748/wjg.v20.i15.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang W, Feng G, Wang L, Teng F, Wang L, Li W, Zhang Y, Zhou Q. MeCP2 deficiency promotes cell reprogramming by stimulating IGF1/AKT/mTOR signaling and activating ribosomal protein-mediated cell cycle gene translation. J Mol Cell Biol. 2018;10:515–526. doi: 10.1093/jmcb/mjy018. [DOI] [PubMed] [Google Scholar]
- 69.Zhou Y, Liang X, Chang H, Shu F, Wu Y, Zhang T, Fu Y, Zhang Q, Zhu JD, Mi M. Ampelopsin-induced autophagy protects breast cancer cells from apoptosis through Akt-mTOR pathway via endoplasmic reticulum stress. Cancer Sci. 2014;105:1279–1287. doi: 10.1111/cas.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lv X, Zhang J, Zhang J, Guan W, Ren W, Liu Y, Xu G. A negative feedback loop between NAMPT and TGF-β signaling pathway in colorectal cancer cells. Onco Targets Ther. 2021;14:187–198. doi: 10.2147/OTT.S282367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 72.Campbell JD, Alexandrov A, Kim J, Wala J, Berger AH, Pedamallu CS, Shukla SA, Guo G, Brooks AN, Murray BA, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48:607–616. doi: 10.1038/ng.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fan X, Zou X, Liu C, Peng S, Zhang S, Zhou X, Zhu J, Zhu W. Identify miRNA-mRNA regulation pairs to explore potential pathogenesis of lung adenocarcinoma. Aging (Albany NY) 2022;14:8357–8373. doi: 10.18632/aging.204341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu PJ, Chen YH, Tsai KW, Yeah HY, Yeh CY, Tu YT, Yang CY. Involvement of microRNA-1-FAM83A axis dysfunction in the growth and motility of lung cancer cells. Int J Mol Sci. 2020;21:8833. doi: 10.3390/ijms21228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miao H, Zeng Q, Xu S, Chen Z. miR-1-3p/CELSR3 participates in regulating malignant phenotypes of lung adenocarcinoma cells. Curr Gene Ther. 2021;21:304–312. doi: 10.2174/1566523221666210617160611. [DOI] [PubMed] [Google Scholar]
- 76.Song Y, Wang Z, He L, Sun F, Zhang B, Wang F. Dysregulation of pseudogenes/lncRNA-Hsa-miR-1-3p-PAICS pathway promotes the development of NSCLC. J Oncol. 2022;2022:4714931. doi: 10.1155/2022/4714931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.von Hardenberg J, Martini T, Knauer A, Ströbel P, Becker A, Herrmann E, Schubert C, Steidler A, Bolenz C. Expression and predictive value of lymph-specific markers in urothelial carcinoma of the bladder. Urol Oncol. 2014;32(54):e59–e17. doi: 10.1016/j.urolonc.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 78.Nakashima M, Matsui Y, Kobayashi T, Saito R, Hatahira S, Kawakami K, Nakamura E, Nishiyama H, Ogawa O. Urine CXCL1 as a biomarker for tumor detection and outcome prediction in bladder cancer. Cancer Biomark. 2015;15:357–364. doi: 10.3233/CBM-150472. [DOI] [PubMed] [Google Scholar]
- 79.Zhang M, Zhuang Q, Cui L. MiR-194 inhibits cell proliferation and invasion via repression of RAP2B in bladder cancer. Biomed Pharmacother. 2016;80:268–275. doi: 10.1016/j.biopha.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 80.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 81.Zeegers MP, Tan FE, Dorant E, van Den Brandt PA. The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer. 2000;89:630–639. doi: 10.1002/1097-0142(20000801)89:3<630::AID-CNCR19>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 82.Zeegers MP, Goldbohm RA, van den Brandt PA. A prospective study on active and environmental tobacco smoking and bladder cancer risk (The Netherlands) Cancer Causes Control. 2002;13:83–90. doi: 10.1023/A:1013954932343. [DOI] [PubMed] [Google Scholar]
- 83.Wang W, Shen F, Wang C, Lu W, Wei J, Shang A, Wang C. MiR-1-3p inhibits the proliferation and invasion of bladder cancer cells by suppressing CCL2 expression. Tumour Biol. 2017;39:1010428317698383. doi: 10.1177/1010428317698383. [DOI] [PubMed] [Google Scholar]
- 84.Eckstein M, Epple E, Jung R, Weigelt K, Lieb V, Sikic D, Stöhr R, Geppert C, Weyerer V, Bertz S, et al. CCL2 expression in tumor cells and tumor-infiltrating immune cells shows divergent prognostic potential for bladder cancer patients depending on lymph node stage. Cancers (Basel) 2020;12:1253. doi: 10.3390/cancers12051253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma W, Ou T, Cui X, Wu K, Li H, Li Y, Peng G, Xia W, Wu S. HSP47 contributes to angiogenesis by induction of CCL2 in bladder cancer. Cell Signal. 2021;85:110044. doi: 10.1016/j.cellsig.2021.110044. [DOI] [PubMed] [Google Scholar]
- 86.Chen C, He W, Huang J, Wang B, Li H, Cai Q, Su F, Bi J, Liu H, Zhang B, et al. LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nat Commun. 2018;9:3826. doi: 10.1038/s41467-018-06152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wan Y, Yu LG. Expression and impact of C1GalT1 in cancer development and progression. Cancers (Basel) 2021;13:6305. doi: 10.3390/cancers13246305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang J, Wang L, Mao S, Liu M, Zhang W, Zhang Z, Guo Y, Huang B, Yan Y, Huang Y, Yao X. miR-1-3p contributes to cell proliferation and invasion by targeting glutaminase in bladder cancer cells. Cell Physiol Biochem. 2018;51:513–527. doi: 10.1159/000495273. [DOI] [PubMed] [Google Scholar]
- 89.Luo M, Wu L, Zhang K, Wang H, Zhang T, Gutierrez L, O'Connell D, Zhang P, Li Y, Gao T, et al. miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. 2018;25:1457–1472. doi: 10.1038/s41418-017-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dai SM, Li FJ, Long HZ, Zhou ZW, Luo HY, Xu SG, Gao LC. Relationship between miRNA and ferroptosis in tumors. Front Pharmacol. 2022;13:977062. doi: 10.3389/fphar.2022.977062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou Q, Zhan H, Lin F, Liu Y, Yang K, Gao Q, Ding M, Liu Y, Huang W, Cai Z. LincRNA-p21 suppresses glutamine catabolism and bladder cancer cell growth through inhibiting glutaminase expression. Biosci Rep. 2019;39:BSR20182372. doi: 10.1042/BSR20182372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao L, Yan P, Guo FF, Liu HJ, Zhao ZF. MiR-1-3p inhibits cell proliferation and invasion by regulating BDNF-TrkB signaling pathway in bladder cancer. Neoplasma. 2018;65:89–96. doi: 10.4149/neo_2018_161128N594. [DOI] [PubMed] [Google Scholar]
- 95.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 96.Wong MC, Jiang JY, Goggins WB, Liang M, Fang Y, Fung FD, Leung C, Wang HH, Wong GL, Wong VW, Chan HL. International incidence and mortality trends of liver cancer: a global profile. Sci Rep. 2017;7:45846. doi: 10.1038/srep45846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. doi: 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47:S2–6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang H, Zhang Z, Gao L, Qiao Z, Yu M, Yu B, Yang T. miR-1-3p suppresses proliferation of hepatocellular carcinoma through targeting SOX9. Onco Targets Ther. 2019;12:2149–2157. doi: 10.2147/OTT.S197326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ren Z, Chen Y, Shi L, Shao F, Sun Y, Ge J, Zhang J, Zang Y. Sox9/CXCL5 axis facilitates tumour cell growth and invasion in hepatocellular carcinoma. Febs j. 2022;289:3535–3549. doi: 10.1111/febs.16357. [DOI] [PubMed] [Google Scholar]
- 101.Guo C, Zhou S, Yi W, Yang P, Li O, Liu J, Peng C. SOX9/MKLN1-AS axis induces hepatocellular carcinoma proliferation and epithelial–mesenchymal transition. Biochem Genet. 2022;60:1914–1933. doi: 10.1007/s10528-022-10196-6. [DOI] [PubMed] [Google Scholar]
- 102.Ma XL, Hu B, Tang WG, Xie SH, Ren N, Guo L, Lu RQ. CD73 sustained cancer-stem-cell traits by promoting SOX9 expression and stability in hepatocellular carcinoma. J Hematol Oncol. 2020;13:11. doi: 10.1186/s13045-020-0845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen H, Bao L, Hu J, Wu D, Tong X. ORC6, negatively regulated by miR-1-3p, promotes proliferation, migration, and invasion of hepatocellular carcinoma cells. Front Cell Dev Biol. 2021;9:652292. doi: 10.3389/fcell.2021.652292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Balasov M, Huijbregts RP, Chesnokov I. Functional analysis of an Orc6 mutant in Drosophila. Proc Natl Acad Sci USA. 2009;106:10672–10677. doi: 10.1073/pnas.0902670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang XK, Wang QQ, Huang JL, Zhang LB, Zhou X, Liu JQ, Chen ZJ, Liao XW, Huang R, Yang CK, et al. Novel candidate biomarkers of origin recognition complex 1, 5 and 6 for survival surveillance in patients with hepatocellular carcinoma. J Cancer. 2020;11:1869–1882. doi: 10.7150/jca.39163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moawad AW, Szklaruk J, Lall C, Blair KJ, Kaseb AO, Kamath A, Rohren SA, Elsayes KM. Angiogenesis in hepatocellular carcinoma; pathophysiology, targeted therapy, and role of imaging. J Hepatocell Carcinoma. 2020;7:77–89. doi: 10.2147/JHC.S224471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 108.Tadros SA, Attia YM, Maurice NW, Fahim SA, Abdelwahed FM, Ibrahim S, Badary OA. Thymoquinone suppresses angiogenesis in DEN-induced hepatocellular carcinoma by targeting miR-1–3p. Int J Mol Sci. 2022;23:15904. doi: 10.3390/ijms232415904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Attard G, Parker C, Eeles RA, Schröder F, Tomlins SA, Tannock I, Drake CG, de Bono JS. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 110.Narita S, Nara T, Sato H, Koizumi A, Huang M, Inoue T, Habuchi T. Research evidence on high-fat diet-induced prostate cancer development and progression. J Clin Med. 2019;8:597. doi: 10.3390/jcm8050597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li SM, Wu HL, Yu X, Tang K, Wang SG, Ye ZQ, Hu J. The putative tumour suppressor miR-1-3p modulates prostate cancer cell aggressiveness by repressing E2F5 and PFTK1. J Exp Clin Cancer Res. 2018;37:219. doi: 10.1186/s13046-018-0895-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hazar-Rethinam M, Endo-Munoz L, Gannon O, Saunders N. The role of the E2F transcription factor family in UV-induced apoptosis. Int J Mol Sci. 2011;12:8947–8960. doi: 10.3390/ijms12128947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Malumbres M, Harlow E, Hunt T, Hunter T, Lahti JM, Manning G, Morgan DO, Tsai LH, Wolgemuth DJ. Cyclin-dependent kinases: a family portrait. Nat Cell Biol. 2009;11:1275–1276. doi: 10.1038/ncb1109-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guo H, Zhao J, Li X, Sun F, Qin Y, Yang X, Xiong X, Yin Q, Wang X, Gao L, et al. Identification of miR-1-3p, miR-143-3p and miR-145-5p association with bone metastasis of Gleason 3+4 prostate cancer and involvement of LASP1 regulation. Mol Cell Probes. 2023;68:101901. doi: 10.1016/j.mcp.2023.101901. [DOI] [PubMed] [Google Scholar]
- 115.McCormack VA, Menya D, Munishi MO, Dzamalala C, Gasmelseed N, Leon Roux M, Assefa M, Osano O, Watts M, Mwasamwaja AO, et al. Informing etiologic research priorities for squamous cell esophageal cancer in Africa: a review of setting-specific exposures to known and putative risk factors. Int J Cancer. 2017;140:259–271. doi: 10.1002/ijc.30292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F. Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. Am J Gastroenterol. 2017;112:1247–1255. doi: 10.1038/ajg.2017.155. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y, Chen X, Li J, Xia C. Quercetin antagonizes esophagus cancer by modulating miR-1-3p/TAGLN2 pathway-dependent growth and metastasis. Nutr Cancer. 2022;74:1872–1881. doi: 10.1080/01635581.2021.1972125. [DOI] [PubMed] [Google Scholar]
- 118.Jerjes W, Upile T, Petrie A, Riskalla A, Hamdoon Z, Vourvachis M, Karavidas K, Jay A, Sandison A, Thomas GJ, et al. Clinicopathological parameters, recurrence, locoregional and distant metastasis in 115 T1–T2 oral squamous cell carcinoma patients. Head Neck Oncol. 2010;2:9. doi: 10.1186/1758-3284-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Warnakulasuriya S, Chen THH. Areca nut and oral cancer: evidence from studies conducted in humans. J Dent Res. 2022;101:1139–1146. doi: 10.1177/00220345221092751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Z, Wang J, Chen Z, Wang K, Shi L. MicroRNA-1-3p inhibits the proliferation and migration of oral squamous cell carcinoma cells by targeting DKK1. Biochem Cell Biol. 2018;96:355–364. doi: 10.1139/bcb-2017-0015. [DOI] [PubMed] [Google Scholar]
- 121.Fu Y, Liu H, Long M, Song L, Meng Z, Lin S, Zhang Y, Qin J. Icariin attenuates the tumor growth by targeting miR-1-3p/TNKS2/Wnt/β-catenin signaling axis in ovarian cancer. Front Oncol. 2022;12:940926. doi: 10.3389/fonc.2022.940926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qu W, Chen X, Wang J, Lv J, Yan D. MicroRNA-1 inhibits ovarian cancer cell proliferation and migration through c-Met pathway. Clin Chim Acta. 2017;473:237–244. doi: 10.1016/j.cca.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 123.Zhang D, Qu B, Hu B, Cao K, Shen H. MiR-1-3p enhances the sensitivity of ovarian cancer cells to ferroptosis by targeting FZD7. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022;47:1512–1521. doi: 10.11817/j.issn.1672-7347.2022.210800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fu D, Wang C, Yu L, Yu R. Induction of ferroptosis by ATF3 elevation alleviates cisplatin resistance in gastric cancer by restraining Nrf2/Keap1/xCT signaling. Cell Mol Biol Lett. 2021;26:26. doi: 10.1186/s11658-021-00271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stope MB, Hettenbach D, Kaul A, Paditz M, Diesing K, Burchardt M, Zygmunt M, Mustea A, Koensgen D. The tumor suppressor microRNA-1 exhibits restricted inhibition of proliferation of ovarian cancer cells. Anticancer Res. 2016;36:3329–3334. [PubMed] [Google Scholar]
- 126.Barzaman K, Karami J, Zarei Z, Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, Safari E, Farahmand L. Breast cancer: biology, biomarkers, and treatments. Int Immunopharmacol. 2020;84:106535. doi: 10.1016/j.intimp.2020.106535. [DOI] [PubMed] [Google Scholar]
- 127.Peng J, Yuan C, Wu Z, Wang Y, Yin W, Lin Y, Zhou L, Lu J. Upregulation of microRNA-1 inhibits proliferation and metastasis of breast cancer. Mol Med Rep. 2020;22:454–464. doi: 10.3892/mmr.2020.11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu R, Li J, Lai Y, Liao Y, Liu R, Qiu W. Hsa-miR-1 suppresses breast cancer development by down-regulating K-ras and long non-coding RNA MALAT1. Int J Biol Macromol. 2015;81:491–497. doi: 10.1016/j.ijbiomac.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 129.Yang L, Cai N, Zhao L. MicroRNA-1 regulates the growth and chemosensitivity of breast cancer cells by targeting MEK/ERK pathway. J buon. 2020;25:2215–2220. [PubMed] [Google Scholar]
- 130.Wu L, Wang T, He D, Li X, Jiang Y. miR-1 inhibits the proliferation of breast cancer stem cells by targeting EVI-1. Onco Targets Ther. 2018;11:8773–8781. doi: 10.2147/OTT.S188836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang S, Liu C, Zhang X. Mitochondrial damage mediated by miR-1 overexpression in cancer stem cells. Mol Ther Nucleic Acids. 2019;18:938–953. doi: 10.1016/j.omtn.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bukowski RM. Metastatic clear cell carcinoma of the kidney: therapeutic role of bevacizumab. Cancer Manag Res. 2010;2:83–96. doi: 10.2147/CMAR.S7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu J, Huang Y, Cheng Q, Wang J, Zuo J, Liang Y, Yuan G. miR-1-3p suppresses the epithelial–mesenchymal transition property in renal cell cancer by downregulating Fibronectin 1. Cancer Manag Res. 2019;11:5573–5587. doi: 10.2147/CMAR.S200707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sponziello M, Rosignolo F, Celano M, Maggisano V, Pecce V, De Rose RF, Lombardo GE, Durante C, Filetti S, Damante G, et al. Fibronectin-1 expression is increased in aggressive thyroid cancer and favors the migration and invasion of cancer cells. Mol Cell Endocrinol. 2016;431:123–132. doi: 10.1016/j.mce.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 135.Corre I, Verrecchia F, Crenn V, Redini F, Trichet V. The osteosarcoma microenvironment: a complex but targetable ecosystem. Cells. 2020;9:976. doi: 10.3390/cells9040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen C, Xie L, Ren T, Huang Y, Xu J, Guo W. Immunotherapy for osteosarcoma: fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021;500:1–10. doi: 10.1016/j.canlet.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 137.Zhang G, Guan Q, Zhao Y, Wang S, Li H. miR-1–3p inhibits osteosarcoma cell proliferation and cell cycle progression while promoting cell apoptosis by targeting CDK14 to inactivate Wnt/Beta-catenin signaling. Mol Biotechnol. 2023 doi: 10.1007/s12033-023-00811-1. [DOI] [PubMed] [Google Scholar]
- 138.Yadav V, Chen SH, Yue YG, Buchanan S, Beckmann RP, Peng SB. Co-targeting BRAF and cyclin dependent kinases 4/6 for BRAF mutant cancers. Pharmacol Ther. 2015;149:139–149. doi: 10.1016/j.pharmthera.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 139.O'Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417–430. doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 140.Pitts TM, Davis SL, Eckhardt SG, Bradshaw-Pierce EL. Targeting nuclear kinases in cancer: development of cell cycle kinase inhibitors. Pharmacol Ther. 2014;142:258–269. doi: 10.1016/j.pharmthera.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 141.Zhang G, Zhu Y, Jin C, Shi Q, An X, Song L, Gao F, Li S. CircRNA_0078767 promotes osteosarcoma progression by increasing CDK14 expression through sponging microRNA-330-3p. Chem Biol Interact. 2022;360:109903. doi: 10.1016/j.cbi.2022.109903. [DOI] [PubMed] [Google Scholar]
- 142.Gu Z, Hou Z, Zheng L, Wang X, Wu L, Zhang C. Long noncoding RNA LINC00858 promotes osteosarcoma through regulating miR-139-CDK14 axis. Biochem Biophys Res Commun. 2018;503:1134–1140. doi: 10.1016/j.bbrc.2018.06.131. [DOI] [PubMed] [Google Scholar]
- 143.Ji Q, Xu X, Li L, Goodman SB, Bi W, Xu M, Xu Y, Fan Z, Maloney WJ, Ye Q, Wang Y. miR-216a inhibits osteosarcoma cell proliferation, invasion and metastasis by targeting CDK14. Cell Death Dis. 2017;8:e3103. doi: 10.1038/cddis.2017.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zheng L, Hu N, Zhou X. TCF3-activated LINC00152 exerts oncogenic role in osteosarcoma through regulating miR-1182/CDK14 axis. Pathol Res Pract. 2019;215:373–380. doi: 10.1016/j.prp.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 145.Dai J, Xu L, Hu X, Han G, Jiang H, Sun H, Zhu G, Tang X. Long noncoding RNA OIP5-AS1 accelerates CDK14 expression to promote osteosarcoma tumorigenesis via targeting miR-223. Biomed Pharmacother. 2018;106:1441–1447. doi: 10.1016/j.biopha.2018.07.109. [DOI] [PubMed] [Google Scholar]
- 146.Hosseini F, Alemi F, Malakoti F, Mahmoodpoor A, Younesi S, Yousefi B, Asemi Z. Targeting Wnt/β-catenin signaling by microRNAs as a therapeutic approach in chemoresistant osteosarcoma. Biochem Pharmacol. 2021;193:114758. doi: 10.1016/j.bcp.2021.114758. [DOI] [PubMed] [Google Scholar]
- 147.Yang CH, Wang Y, Sims M, Cai C, Pfeffer LM. MicroRNA-1 suppresses glioblastoma in preclinical models by targeting fibronectin. Cancer Lett. 2019;465:59–67. doi: 10.1016/j.canlet.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 148.Zhang J, Li N, Fu J, Zhou W. Long noncoding RNA HOTAIR promotes medulloblastoma growth, migration and invasion by sponging miR-1/miR-206 and targeting YY1. Biomed Pharmacother. 2020;124:109887. doi: 10.1016/j.biopha.2020.109887. [DOI] [PubMed] [Google Scholar]
- 149.He C, Yang J, Ding J, Li S, Wu H, Xiong Y, Zhou F, Jiang Y, Teng L, Yang J. Downregulation of glucose-6-phosphate dehydrogenase by microRNA-1 inhibits the growth of pituitary tumor cells. Oncol Rep. 2018;40:3533–3542. doi: 10.3892/or.2018.6755. [DOI] [PubMed] [Google Scholar]
- 150.Ralser DJ, Condic M, Egger E, Koensgen D, Mustea A, Stope MB. Evaluation of the diagnostic potential of circulating microRNAs miR-1 and miR-21 in patients with ovarian cancer. Anticancer Res. 2022;42:5839–5845. doi: 10.21873/anticanres.16092. [DOI] [PubMed] [Google Scholar]
- 151.Chen X, Li X, Peng X, Zhang C, Liu K, Huang G, Lai Y. Use of a four-miRNA panel as a biomarker for the diagnosis of stomach adenocarcinoma. Dis Markers. 2020;2020:8880937. doi: 10.1155/2020/8880937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu X, Li S, Xu X, Wu S, Chen R, Jiang Q, Li Y, Xu Y. The potential value of miR-1 and miR-374b as biomarkers for colorectal cancer. Int J Clin Exp Pathol. 2015;8:2840–2851. [PMC free article] [PubMed] [Google Scholar]
- 153.Xu B, Shen X, Yang Z, Zhao T, Liu B, Gao S, Shi Y, Bao C. Plasma miR-1, but not extracellular vesicle miR-1, functions as a potential biomarker for colorectal cancer diagnosis. Clin Lab. 2021;67:65. doi: 10.7754/Clin.Lab.2020.200344. [DOI] [PubMed] [Google Scholar]
- 154.Wei W, Leng J, Shao H, Wang W. MiR-1, a potential predictive biomarker for recurrence in prostate cancer after radical prostatectomy. Am J Med Sci. 2017;353:315–319. doi: 10.1016/j.amjms.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 155.Karatas OF, Guzel E, Suer I, Ekici ID, Caskurlu T, Creighton CJ, Ittmann M, Ozen M. miR-1 and miR-133b are differentially expressed in patients with recurrent prostate cancer. PLoS ONE. 2014;9:e98675. doi: 10.1371/journal.pone.0098675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li H, Xia M, Zheng S, Lin Y, Yu T, Xie Y, Shen Y, Liu X, Qian X, Yin Z. Cerebrospinal fluid exosomal microRNAs as biomarkers for diagnosing or monitoring the progression of non-small cell lung cancer with leptomeningeal metastases. Biotechnol Genet Eng Rev. 2023 doi: 10.1080/02648725.2023.2183613. [DOI] [PubMed] [Google Scholar]
- 157.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 158.Rigaud VO, Ferreira LR, Ayub-Ferreira SM, Ávila MS, Brandão SM, Cruz FD, Santos MH, Cruz CB, Alves MS, Issa VS, et al. Circulating miR-1 as a potential biomarker of doxorubicin-induced cardiotoxicity in breast cancer patients. Oncotarget. 2017;8:6994–7002. doi: 10.18632/oncotarget.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F, Wang Q, Wang S, Rong D, Reiter FP, et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020;5:87. doi: 10.1038/s41392-020-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang H, Tang Y, Yang D, Zheng L. MicroRNA-591 functions as a tumor suppressor in hepatocellular carcinoma by lowering drug resistance through inhibition of far-upstream element-binding protein 2-mediated phosphoinositide 3-kinase/Akt/mammalian target of rapamycin axis. Pharmacology. 2019;104:173–186. doi: 10.1159/000501162. [DOI] [PubMed] [Google Scholar]
- 161.Dietrich P, Koch A, Fritz V, Hartmann A, Bosserhoff AK, Hellerbrand C. Wild type Kirsten rat sarcoma is a novel microRNA-622-regulated therapeutic target for hepatocellular carcinoma and contributes to sorafenib resistance. Gut. 2018;67:1328–1341. doi: 10.1136/gutjnl-2017-315402. [DOI] [PubMed] [Google Scholar]
- 162.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Suda K, Mizuuchi H, Maehara Y, Mitsudomi T. Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation–diversity, ductility, and destiny. Cancer Metastasis Rev. 2012;31:807–814. doi: 10.1007/s10555-012-9391-7. [DOI] [PubMed] [Google Scholar]
- 164.Jiao D, Chen J, Li Y, Tang X, Wang J, Xu W, Song J, Li Y, Tao H, Chen Q. miR-1-3p and miR-206 sensitizes HGF-induced gefitinib-resistant human lung cancer cells through inhibition of c-Met signalling and EMT. J Cell Mol Med. 2018;22:3526–3536. doi: 10.1111/jcmm.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Deng L, Jiang L, Lin XH, Tseng KF, Liu Y, Zhang X, Dong RH, Lu ZG, Wang XJ. The PI3K/mTOR dual inhibitor BEZ235 suppresses proliferation and migration and reverses multidrug resistance in acute myeloid leukemia. Acta Pharmacol Sin. 2017;38:382–391. doi: 10.1038/aps.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ramakrishnan V, Xu B, Akers J, Nguyen T, Ma J, Dhawan S, Ning J, Mao Y, Hua W, Kokkoli E, et al. Radiation-induced extracellular vesicle (EV) release of miR-603 promotes IGF1-mediated stem cell state in glioblastomas. EBioMedicine. 2020;55:102736. doi: 10.1016/j.ebiom.2020.102736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guo W, Wu Z, Chen J, Guo S, You W, Wang S, Ma J, Wang H, Wang X, Wang H, et al. Nanoparticle delivery of miR-21–3p sensitizes melanoma to anti-PD-1 immunotherapy by promoting ferroptosis. J Immunother Cancer. 2022;10:e004381. doi: 10.1136/jitc-2021-004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Huang S, Duan S, Wang J, Bao S, Qiu X, Li C, Liu Y, Yan L, Zhang Z, Yurong Hu. Folic-acid-mediated functionalized gold nanocages for targeted delivery of anti-miR-181b in combination of gene therapy and photothermal therapy against hepatocellular carcinoma. Adv Func Mater. 2016;26:2532–2544. doi: 10.1002/adfm.201504912. [DOI] [Google Scholar]
- 169.Mahmoudi M, Simchi A, Milani AS, Stroeve P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J Colloid Interface Sci. 2009;336:510–518. doi: 10.1016/j.jcis.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 170.Cheong SJ, Lee CM, Kim SL, Jeong HJ, Kim EM, Park EH, Kim DW, Lim ST, Sohn MH. Superparamagnetic iron oxide nanoparticles-loaded chitosan-linoleic acid nanoparticles as an effective hepatocyte-targeted gene delivery system. Int J Pharm. 2009;372:169–176. doi: 10.1016/j.ijpharm.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 171.Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Krüger A, Gänsbacher B, Plank C. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 172.Pan Z, Shi Z, Wei H, Sun F, Song J, Huang Y, Liu T, Mao Y. Magnetofection based on superparamagnetic iron oxide nanoparticles weakens glioma stem cell proliferation and invasion by mediating high expression of microRNA-374a. J Cancer. 2016;7:1487–1496. doi: 10.7150/jca.15515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gao Y, Qian H, Tang X, Du X, Wang G, Zhang H, Ye F, Liu T. Superparamagnetic iron oxide nanoparticle-mediated expression of miR-326 inhibits human endometrial carcinoma stem cell growth. Int J Nanomedicine. 2019;14:2719–2731. doi: 10.2147/IJN.S200480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhi D, Yang T, Yang J, Fu S, Zhang S. Targeting strategies for superparamagnetic iron oxide nanoparticles in cancer therapy. Acta Biomater. 2020;102:13–34. doi: 10.1016/j.actbio.2019.11.027. [DOI] [PubMed] [Google Scholar]
- 175.Dadfar SM, Camozzi D, Darguzyte M, Roemhild K, Varvarà P, Metselaar J, Banala S, Straub M, Güvener N, Engelmann U, et al. Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance. J Nanobiotechnology. 2020;18:22. doi: 10.1186/s12951-020-0580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Thorat ND, Lemine OM, Bohara RA, Omri K, El Mir L, Tofail SA. Superparamagnetic iron oxide nanocargoes for combined cancer thermotherapy and MRI applications. Phys Chem Chem Phys. 2016;18:21331–21339. doi: 10.1039/C6CP03430F. [DOI] [PubMed] [Google Scholar]
- 177.Asefa T, Tao Z. Biocompatibility of mesoporous silica nanoparticles. Chem Res Toxicol. 2012;25:2265–2284. doi: 10.1021/tx300166u. [DOI] [PubMed] [Google Scholar]
- 178.Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24:1504–1534. doi: 10.1002/adma.201104763. [DOI] [PubMed] [Google Scholar]
- 179.Suib SL. A review of recent developments of mesoporous materials. Chem Rec. 2017;17:1169–1183. doi: 10.1002/tcr.201700025. [DOI] [PubMed] [Google Scholar]
- 180.Garrido-Cano I, Adam-Artigues A, Lameirinhas A, Blandez JF, Candela-Noguera V, Lluch A, Bermejo B, Sancenón F, Cejalvo JM, Martínez-Máñez R, Eroles P. Delivery of miR-200c-3p using tumor-targeted mesoporous silica nanoparticles for breast cancer therapy. ACS Appl Mater Interfaces. 2023;15:38323–38334. doi: 10.1021/acsami.3c07541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang T, Xue X, He D, Hsieh JT. A prostate cancer-targeted polyarginine-disulfide linked PEI nanocarrier for delivery of microRNA. Cancer Lett. 2015;365:156–165. doi: 10.1016/j.canlet.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 183.Huang G, Liu Y, Chen L. Chitosan and its derivatives as vehicles for drug delivery. Drug Deliv. 2017;24:108–113. doi: 10.1080/10717544.2017.1399305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cao Y, Tan YF, Wong YS, Liew MWJ, Venkatraman S. Recent advances in chitosan-based carriers for gene delivery. Mar Drugs. 2019;17:381. doi: 10.3390/md17060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Santos-Carballal B, Aaldering LJ, Ritzefeld M, Pereira S, Sewald N, Moerschbacher BM, Götte M, Goycoolea FM. Physicochemical and biological characterization of chitosan-microRNA nanocomplexes for gene delivery to MCF-7 breast cancer cells. Sci Rep. 2015;5:13567. doi: 10.1038/srep13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Elfiky AM, Mohamed RH, Abd El-Hakam FE, Yassin MA, ElHefnawi M. Targeted delivery of miR-218 via decorated hyperbranched polyamidoamine for liver cancer regression. Int J Pharm. 2021;610:121256. doi: 10.1016/j.ijpharm.2021.121256. [DOI] [PubMed] [Google Scholar]
- 187.Gabler F, Frauenschuh S, Ringe J, Brochhausen C, Götz P, Kirkpatrick CJ, Sittinger M, Schubert H, Zehbe R. Emulsion-based synthesis of PLGA-microspheres for the in vitro expansion of porcine chondrocytes. Biomol Eng. 2007;24:515–520. doi: 10.1016/j.bioeng.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 188.Patil Y, Panyam J. Polymeric nanoparticles for siRNA delivery and gene silencing. Int J Pharm. 2009;367:195–203. doi: 10.1016/j.ijpharm.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Barichello JM, Morishita M, Takayama K, Nagai T. Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Dev Ind Pharm. 1999;25:471–476. doi: 10.1081/DDC-100102197. [DOI] [PubMed] [Google Scholar]
- 190.Wang S, Zhang J, Wang Y, Chen M. Hyaluronic acid-coated PEI-PLGA nanoparticles mediated co-delivery of doxorubicin and miR-542-3p for triple negative breast cancer therapy. Nanomedicine. 2016;12:411–420. doi: 10.1016/j.nano.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 191.Zhang S, Cheng Z, Wang Y, Han T. The risks of miRNA therapeutics: in a drug target perspective. Drug Des Devel Ther. 2021;15:721–733. doi: 10.2147/DDDT.S288859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pan Z, Sun X, Ren J, Li X, Gao X, Lu C, Zhang Y, Sun H, Wang Y, Wang H, et al. miR-1 exacerbates cardiac ischemia-reperfusion injury in mouse models. PLoS ONE. 2012;7:e50515. doi: 10.1371/journal.pone.0050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 194.Shan ZX, Lin QX, Deng CY, Zhu JN, Mai LP, Liu JL, Fu YH, Liu XY, Li YX, Zhang YY, et al. miR-1/miR-206 regulate Hsp60 expression contributing to glucose-mediated apoptosis in cardiomyocytes. FEBS Lett. 2010;584:3592–3600. doi: 10.1016/j.febslet.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 195.Lisewski U, Shi Y, Wrackmeyer U, Fischer R, Chen C, Schirdewan A, Jüttner R, Rathjen F, Poller W, Radke MH, Gotthardt M. The tight junction protein CAR regulates cardiac conduction and cell-cell communication. J Exp Med. 2008;205:2369–2379. doi: 10.1084/jem.20080897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Matsuda T, Zhai P, Sciarretta S, Zhang Y, Jeong JI, Ikeda S, Park J, Hsu CP, Tian B, Pan D, et al. NF2 activates hippo signaling and promotes ischemia/reperfusion injury in the heart. Circ Res. 2016;119:596–606. doi: 10.1161/CIRCRESAHA.116.308586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zheng M, Li RG, Song J, Zhao X, Tang L, Erhardt S, Chen W, Nguyen BH, Li X, Li M, et al. Hippo-yap signaling maintains sinoatrial node homeostasis. Circulation. 2022;146:1694–1711. doi: 10.1161/CIRCULATIONAHA.121.058777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang H, Xue W, Ding C, Wang C, Xu B, Chen S, Zha B, Sun Y, Zhu H, Zhang J, Dong L. Vitexin mitigates myocardial ischemia/reperfusion injury in rats by regulating mitochondrial dysfunction via Epac1-Rap1 signaling. Oxid Med Cell Longev. 2021;2021:9921982. doi: 10.1155/2021/9921982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.