Abstract
Rationale
Due to the relatively short existence of alternative tobacco products, gaps exist in our current understanding of their long-term respiratory health effects. We therefore undertook the first-ever side-by-side comparison of the impact of chronic inhalation of aerosols emitted from electronic cigarettes (EC) and heated tobacco products (HTP), and combustible cigarettes (CC) smoke.
Objectives
To evaluate the potential differential effects of alternative tobacco products on lung inflammatory responses and efficacy of vaccination in comparison to CC.
Methods
Mice were exposed to emissions from EC, HTP, CC, or air for 8 weeks. BAL and lung tissue were analyzed for markers of inflammation, lung damage, and oxidative stress. Another group was exposed for 12 weeks and vaccinated and challenged with a bacterial respiratory infection. Antibody titers in BAL and sera and pulmonary bacterial clearance were assessed.
Main results
EC- and HTP-aerosols significantly augmented lung immune cell infiltrates equivalent to that achieved following CC-exposure. HTP and CC significantly increased neutrophil numbers compared to EC. All products augmented numbers of B cells, T cells, and pro-inflammatory IL17A+ T cells in the lungs. Decreased lung antioxidant activity and lung epithelial and endothelial damage was induced by all products. EC and HTP differentially augmented inflammatory cytokines/chemokines in the BAL. Generation of immunity following vaccination was impaired by EC and HTP but to a lesser extent than CC, with a CC > HTP > EC hierarchy of suppression of pulmonary bacterial clearance.
Conclusions
HTP and EC-aerosols induced a proinflammatory pulmonary microenvironment, lung damage, and suppressed efficacy of vaccination.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-023-02568-2.
Keywords: Heat-not-burn, IQOS; heated tobacco products, Electronic cigarettes; e-cigarette, Combustible cigarettes; smoking, Vaping, Immunity, Lung damage
Background
Tobacco use, particularly smoking of combustible cigarettes (CCs), remains a global public health problem and a key risk factor for disorders including respiratory diseases [1–10]. The tobacco industry innovatively commercialized alternative products like electronic cigarettes (e-cigarettes, ECs) and Heated Tobacco Products (HTPs) to reduce the harmful effects of inhaling toxic byproducts of tobacco combustion. These distinct product classes share common characteristics: (a) they are designed for inhalation, (b) they utilize electronic devices to produce nicotine-containing aerosols, and (c) they do not rely on combustion. A critical difference between those two types of alternative tobacco products is the source of nicotine: ECs aerosolize a solution containing nicotine but no tobacco leaf, whereas HTPs heat sticks containing actual tobacco (Table 1).
Table 1.
Comparison of product performance characteristics and primary ingredients of combustible cigarettes (CC), Heated Tobacco Products (HTP), and e-cigarettes (EC)
| Combustible cigarettes (CC) | Heated tobacco products (HTP) | E-cigarettes (EC) | |
|---|---|---|---|
| Nicotine | Yes | Yes |
Yes (in most products*) |
| Tobacco | Yes | Yes |
No (nicotine in a form of liquid solution) |
| Combustion | Yes |
No (a potential risk of incomplete combustion) |
No (a potential risk of thermal degradation of nicotine solution ingredients) |
| Temperature |
Yes (very high during puffs) |
Yes (generally lower than in combustible cigarettes) |
Yes (generally lower than in combustible cigarettes) |
| Electronic system | No | Yes | Yes |
| Example of the product |

|

|

|
*Some brands of EC are available in a nicotine-free version
Manufacturers of both types of alternative tobacco products center their marketing strategies around purportedly lower health risks than CC. Those reduced health risk claims are primarily based on reductions in toxicant levels in emissions from ECs and HTPs compared to CCs. For example, industry-funded studies have shown the absence of numerous combustion by-products (including CO and 1,3-butadiene) in aerosols emitted by HTPs [11–14]. However, because HTP still contains tobacco, several tobacco-related toxicants (e.g. cancer causing tobacco-specific nitrosamines, TSNAs) have been found in emissions from those products. Numerous independently funded studies have shown that aerosols emitted from ECs also do not contain combustion by-products and additionally do not contain tobacco-related toxicants [11–14]. However, chronic use of EC or HTP still results in repeated inhalation of respiratory toxicants. HTPs emit levels of respiratory toxicants intermediate between those found in emissions form ECs and CCs [15–18]. Deliver concerns have been raised about the potential unique respiratory health risks of EC use, including the effects of inhaled flavorings, nicotine solvents, and their thermal breakdown products [19–21].
The respiratory system responds to foreign agents, including inhaled smoke by initiating a process of inflammation [22–24]. Amid this protective response, respiratory epithelial cells are activated to induce damage-associated molecular patterns and proinflammatory cytokines and chemokines, which serve as chemoattractants for various immune cells [23]. The overall milieu generated is very immunosuppressive and results in a diminution of immune response to vaccination and pulmonary infection [7–10]. This complex response has been well characterized for tobacco smoke, however little is known if similar responses are elicited by alternative tobacco products (e.g. infiltration and activation of the same immune cell subsets to the lung, release of similar cytokines). If the responses elicited are indeed similar, then the question remains if the magnitude of the changes is also equivalent to tobacco smoke. If the responses seen by exposures to EC and HTPs are weaker compared to tobacco smoke, this could suggest a potential reduction in health risk in smoker switching to ATPs.
Due to the relatively short existence of alternative tobacco products, data on their long-term respiratory health effects are currently unavailable. In the interim, evidence from animal studies may provide crucial information on the potential adverse risks of these emerging tobacco products. Recognizing the knowledge gap in the field, we undertook the first-ever side-by-side comparison of the impact of chronic inhalation of aerosols emitted from EC and HTP, and CC smoke to determine if a hierarchy exists in their potential to induce detrimental pulmonary effects and to suppress immunity.
Materials and methods
Overview of the study protocol
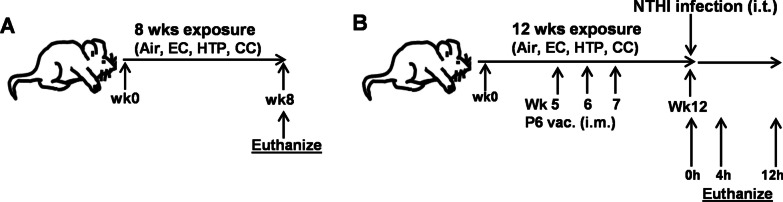
Using an animal exposure model, we compared the impact of chronic inhalation of EC, HTP, and CC emissions on lung inflammation and immunity. Mice were exposed to emissions from EC, HTP, CC, or air (control) (Fig. 1). At week 8 after exposures, one group of animals (Fig. 1A; n = 10 for air, n = 20 for EC, HTP, and CC/group) were sacrificed, and BAL and lung tissue were collected and analyzed for markers of immune response in lungs, lung damage, and oxidative stress. A second group of animals (Fig. 1B; n = 20 for air, EC, HTP, and CC/group) received intramuscular (i.m.) prophylactic vaccination against a respiratory pathogen at weeks 5, 6, and 7. Vaccination efficacy was measured by quantifying antigen-specific antibody titers in serum (weeks 5–12) and in BAL at euthanasia. Finally, all vaccinated animals were challenged at week 12 with a respiratory pathogen and bacterial clearance from the lungs and lung damage were assessed immediately after intratracheal challenge, 4 and 12 h later. Details of tobacco products, assessment of pulmonary inflammation, lung damage, markers of oxidative stress, quantification of myeloperoxidase activity (MPO) and neutrophil elastase (NE), and vaccination efficacy are provided in Additional file 1.
Fig. 1.
Schema of 8-week and 12-week animal exposures. Mice were exposed to aerosols from 3 products for 8 weeks (A) or 12 weeks (B). Air-exposed animals served as control for each group. Mice exposed for 12 weeks were vaccinated i.m. at wk5, wk6 and wk7 after the start of exposures, and were given acute intratracheal challenge with NTHI (106 cfus/mouse in 50uL PBS) at the end of the wk12 for 0, 4 and 12 h
Animal exposure conditions
Animal exposure conditions are provided in detail in Additional file 1. We decided to expose animals to an equivalent dose of nicotine delivered from all tested products. Nicotine equivalency was determined by quantifying serum cotinine levels (a nicotine metabolite) in blood samples collected 30 min post-exposure. Despite differences in the puffing protocols used in our experiments, we achieved equivalent exposure to nicotine from all tested products (Table 2).
Table 2.
Exposure conditions to emissions from EC, HTP, and CC and air (control)
| Air | EC | HTP | CC | |
|---|---|---|---|---|
| Puffing protocol | ||||
| Number of products used over 5 h | – | Approx. 0.5 ml | 20 tobacco sticks | 30 cigarettes |
| Number of puff clusters over 5 h | 13 | 13 | 20 | 30 |
| Number of puffs per cluster | 11 | 11 | 12 | 8 |
| Total number of puffs taken over 5 h | 143 | 143 | 240 | 240 |
| Interval between puff clusters | 20 min | 20 min | 9 min | 6 min |
| Airborne exposure | ||||
| Airborne nicotine (µg/m3) | 17.7 ± 13.6 | 336.7 ± 86.3 | 649.5 ± 262.2 | 1097.0 ± 361.7 |
| PM5.0 (mg/m3) | < LOQ | 39.0 ± 18.6 | 18.6 ± 5.7 | 34.8 ± 16.0 |
| TPM (mg/m3) | < LOQ | 302.0 ± 127.4 | 18.1 ± 7.5 | 292.4 ± 72.5 |
| Thirdhand exposure | ||||
| Nicotine deposited on surface (mg/m2/5 h) | < LOQ | < LOQ | < LOQ | < LOQ |
| Nicotine deposited on fur (mg/m2/8 wks) | < LOQ | < LOQ | < LOQ | < LOQ |
| Biomarker of nicotine exposure | ||||
| Serum cotinine in males (ng/ml) | < LOQ | 32.2 ± 10.0* | 34.8 ± 10.3* | 33.5 ± 10.0* |
| Serum cotinine in females (ng/ml) | < LOQ | 52.7 ± 30.1* | 53.6 ± 6.6* | 56.3 ± 28.5* |
< LOQ = below the limit of quantitation (LOQs were as follows: airborne nicotine 0.4 µg/m3; PM5.0 4.0 mg/m3; TPM 0.001 mg/m3; surface nicotine 1.25 mg/m2; nicotine deposited on fur 31.3 mg/m2; cotinine 2.0 ng/ml). * No significant changes were found between exposure conditions (p < 0.05; Kruskal–Wallis non-parametric test with Dunn’s multiple comparisons)
Statistical analysis
Statistical analyses performed were similar to those presented in our recently published paper [25]. Due to the relatively small samples size, statistically significant differences between the mean rank values of different exposure groups (EC, HTP, CC and air controls) were determined by performing Kruskal–Wallis’s non-parametric test. P values were corrected for multiple testing using the ‘two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli’ false discovery rate (FDR) method and the differences between two groups were considered statistically significant at p < 0.05 when FDR was set at Q < 0.1. We also evaluated if there were differences between male versus female mice in the responses to inhalation of CBD and nicotine aerosols in comparison with air. All statistical analyses were carried out using GraphPad Prism 9.5.1 software (GraphPad; La Jolla, California, USA). Data are shown as bar diagrams with mean ± SE. For Figs. 7 and 8, to assess differences between the mean values of different exposure groups (EC, HTP, CC, and air), we performed a 2-way ANOVA with Tukey's post-test comparisons using GraphPad Prism 9.5.1 software.
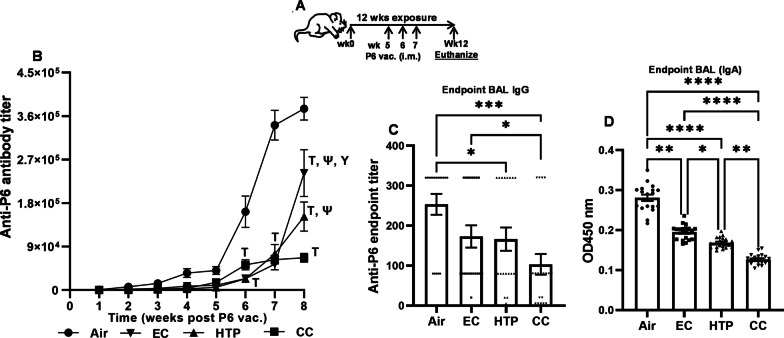
Fig. 7.
Chronic exposure to alternative tobacco product aerosols suppresses development of antibody responses to vaccination. Animals exposed to air, EC, HTP, and CC received prophylactic P6 Ag vaccination i.m. against a respiratory pathogen at weeks 5, 6, and 7 after exposures were started as shown in schema (A). Vaccination efficacy was measured by quantifying antigen-specific antibody titers in serum (weeks 5–12) and in BAL at euthanasia. Weekly serum was collected from aerosol-exposed, P6 immunized mice as described in Additional file 1 and total anti-P6 Ig levels were measured in weekly serum (B) and endpoint BAL (C) samples. To quantify mucosal IgA Ab levels (C) in the BAL of mice, the OD values at 450 nm were measured using BAL dilutions at 1:400 in P6-specific ELISA as described previously [10] and provided in detail in Additional file 1: supplemental methods. Data are shown as curve (B) or as bar diagram with individual data sets (C, D) and given as mean ± SE. Two-way ANOVA with Tukey’s post-test multiple comparisons (A) or non-parametric Kruskal–Wallis test with FDR correction for multiple comparison (C, D) was performed to determine statistically significant differences between two groups by GraphPad Prism 9.5.1 software (GraphPad; La Jolla, California, USA). Difference between two groups was considered significant at p < 0.05, and symbols ***p < 0.001; ****p < 0.0001 are used to denote significant difference between two groups. For, two-way ANOVA, symbols denote as follows: ┬p < 0.0001 vs Air; Ψp < 0.0001 vs CC; Υp < 0.001 vs IQOS. n = 20 for air, EC, HTP, and CC (10 M + 10F) per group for each exposure condition
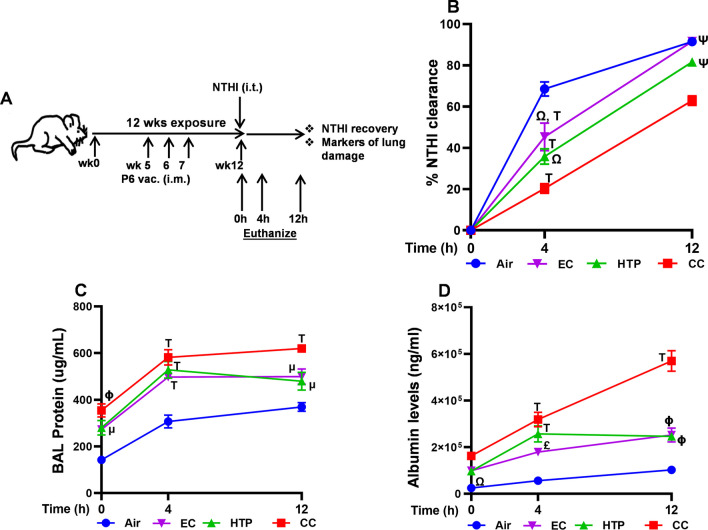
Fig. 8.
Chronic alternative tobacco product aerosol-suppressed vaccination efficacy translates to diminished bacterial clearance from the lungs. All exposed-mice were given an intratracheal instillation of 1 million NTHI cfu 8 weeks after P6 vaccination. Animals were euthanized 0, 4 and 12 h after bacterial challenge and BAL and lungs were harvested. Lung tissue homogenates were prepared to measure %NTHI clearance, and the BAL was processed to quantify the markers of lung damage as shown in schema (A) and described in detail in Additional file 1. Lung bacterial burden was calculated as NTHI clearance from the lungs of mice and measured by bacterial colony-plating assay. Data are represented as %NTHI clearance (B). Levels of total proteins (C) and albumin (D) in the BAL were quantified as described in Additional file 1: supplementary methods. Two-way ANOVA was performed to determine statistically significant differences between two groups and p-values were calculated using Tukey’s post-test multiple comparison by GraphPad Prism 9.5.1 software (GraphPad; La Jolla, California, USA). Difference between two groups was considered significant at p < 0.05, and symbols μp < 0.05 vs. Air; £p < 0.01 vs. Air; Φp < 0.001 vs. Air; ┬p < 0.0001 vs. Air; Ωp < 0.01 vs. CC; Ψp < 0.0001 vs. CC and Υp < 0.001 vs. IQOS are used to denote significant difference between two groups. Number of mice was n = 6 at 0 h, n = 8 at 4 h and n = 6 at 12 h per group; mean ± SEM
We excluded 5 animals that died during the study for reasons unrelated to the experimental exposures, considering them missing at random and did not include them in the statistical analysis. We evaluated differences between male and female mice using a similar approach.
Results
Chronic inhalation exposure to aerosols emitted from alternative tobacco products resulted in the accumulation of innate and adaptive immune cells in the lungs
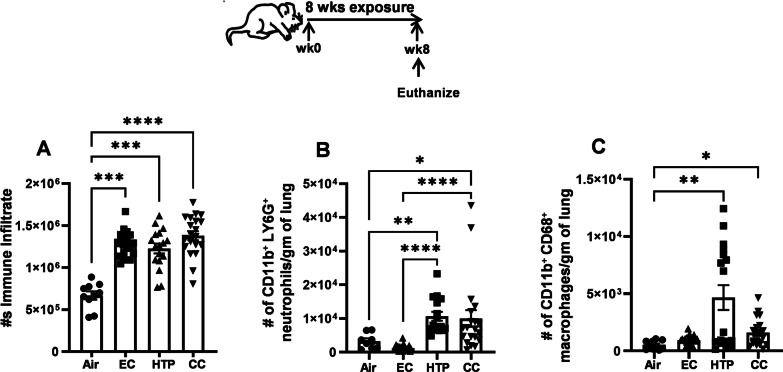
Chronic inhalation exposure to EC and HTP induced a significant augmentation of total immune cell infiltrates in the lungs compared to air (Fig. 2A), equivalent to that achieved following CC exposure. Following HTP exposure, male mice showed significantly more immune-cell infiltration than female mice (Additional file 1: Fig. S2A). HTP- and CC-induced augmentation in the number of neutrophils in the lungs was significantly more compared to EC (p < 0.0001) (Fig. 2B). The augmentation of neutrophils following HTP exposure was similar in magnitude to that found after CC exposure, with no sex-related differences (Additional file 1: Fig. S2B).
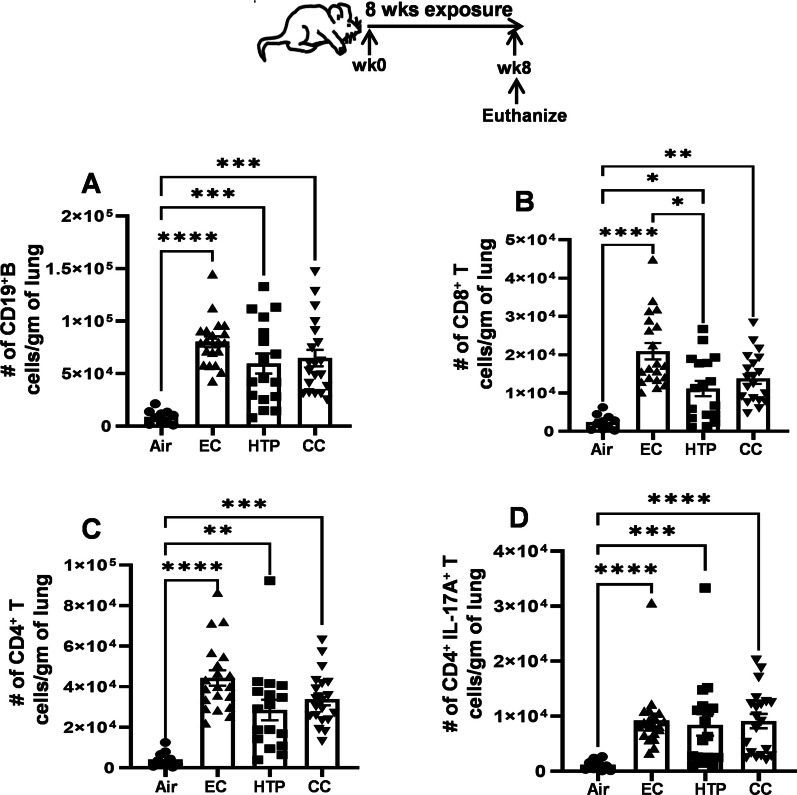
Fig. 2.
Chronic inhalatory exposure to aerosols emitted from alternative tobacco products modulates lung innate immune-cell accumulation. Total numbers of leukocytes (A), CD11b+Ly6G+ neutrophils (B), CD11b+ CD68+ macrophages in the lungs of mice exposed to air, EC, HTP or CC were determined by multicolor flow cytometry using specific antibody markers. Staining protocol and gating strategy are described previously [47] and depicted in Additional file 1: Fig. S1 and with details in online Additional file 1. Data are shown as bar diagrams with mean ± SE. Non-parametric Kruskal–Wallis test with FDR correction for multiple comparison was performed to see if statistically significant differences exist between two groups using GraphPad Prism V.9 software (GraphPad; La Jolla, California, USA). Difference between two groups is considered significant at p < 0.05 and are indicated with symbols **p < 0.01; ***p < 0.001; ****p < 0.0001 using a post-test comparison with Tukey’s correction. In each exposure condition, n = 10 for air (5M + 5F) and n = 20 for EC, HTP, and CC (10M + 10F) per group were used
HTP and CC but not EC significantly augmented the numbers of CD11b+ CD68+ macrophages in the lung, compared to air (p < 0.05) (Fig. 2C). We observed sex-related differences in the augmentation of CD11b+CD68+ macrophages in the lungs of male mice following HTP and CC exposures (Additional file 1: Fig. S2C).
The numbers of CD19+ B cells recruited to the lung were equivalent following EC, HTP, and CC exposures (Fig. 3A) and markedly augmented compared to air (p < 0.001). All three products significantly increased the numbers of CD8+ T cells in the lungs compared to air; however, this was greater after EC exposure compared to HTP (p < 0.05) (Fig. 3B). CD8+ T cell numbers following HTP and CC exposures were equivalent. Aerosol inhalation from EC and HTP products induced significant augmentation of CD4+ T cell numbers in the lungs compared to air(p < 0.01), and this augmentation was similar in magnitude as observed following CC exposure (Fig. 3C). The numbers of CD4+ T cells following EC and HTP exposures were not significantly different from those observed following CC exposure. While increased numbers of B cells were detected in the lungs of male compared to female mice following HTP and CC exposures, CD8+, and CD4+ T cells were augmented in male mice only after HTP-exposure (Additional file 1: Fig. S3A–C). Infiltration of pro-inflammatory CD4+IL17A+ T cells to the lungs was significantly greater following exposure of all three products compared to air (p < 0.001) (Fig. 3D). Male mice had significantly increased numbers of CD4+IL17A+ T cells after HTP and CC exposures (Additional file 1: Fig. S3D).
Fig. 3.
Chronic exposure to alternative tobacco product-aerosols induces adaptive and proinflammatory immune-cell accumulation in the lungs. Total numbers of CD19+ B cells (A), CD8+ T cells (B), CD4+ T cells (C) and CD4+IL17A+ inflammatory T cells in the lungs of mice exposed to air, EC, HTP or CC were calculated using multicolor flow cytometry after staining with specific antibody markers. Gating strategy was similar to that described previously [47] and depicted in Additional file 1: Fig. S1. Data are given as bar diagrams with mean ± SE. Non-parametric Kruskal–Wallis test with FDR correction for multiple comparison was performed to see if statistically significant differences exist between two groups using GraphPad Prism V.9 software (GraphPad; La Jolla, California, USA). Difference between two groups is considered significant at p < 0.05 and are indicated with symbols *p < 0.05, **p < 0.01; ***p < 0.001; ****p < 0.0001 using a post-test comparison with Tukey’s correction. In each exposure condition, we used n = 10 animals for air (5M + 5F) and n = 20 animals for EC, HTP, and CC (10M + 10F) per group
Chronic exposure to aerosols emitted from alternative tobacco products modulated proinflammatory cytokines and chemokines levels
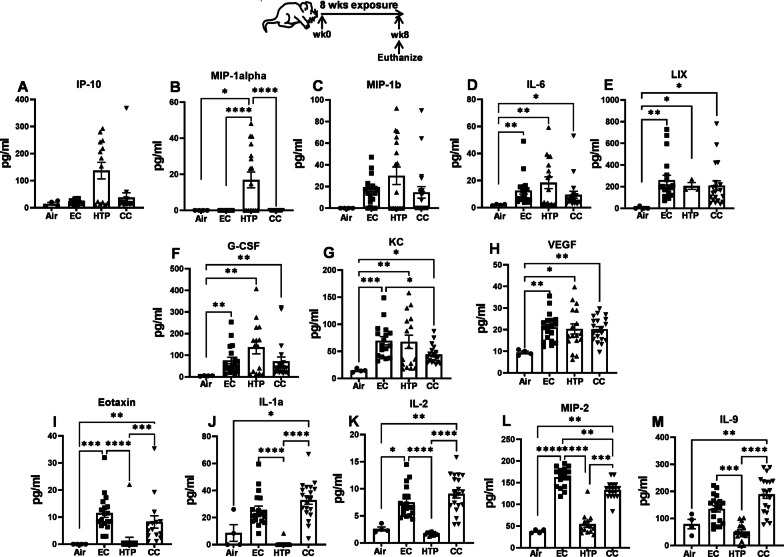
Overall, the levels of various inflammation-associated cytokines and chemokines in the BAL were modulated after exposure to each product. While increased IP10, and MIP-1β were detected in the BAL after HTP exposure compared to air, the differences were not statistically significant (Fig. 4A, C). Chronic exposure to HTP, but not to EC, elevated MIP-1α when compared to air (p < 0.05), and their levels after HTP exposure were markedly higher than following EC (p < 0.0001) and CC exposures (p < 0.0001) (Fig. 4B). IL6, LIX, G-CSF, KC and VEGF levels were augmented following exposure to all three products compared to air (p < 0.05) (Fig. 4D–H) with no differences when comparing EC, HTP, and CC, except for KC, where these levels following EC exposure were higher compared with CC exposure (p < 0.05). Compared to air, augmented levels of eotaxin, IL-1α, IL-2, and MIP-2 were found in BAL following exposure to EC but not HTP. Levels measured in the BAL of the EC group were significantly higher compared to HTP (p < 0.0001) (Fig. 4I–L). The levels of eotaxin, IL-1α, IL-2 and MIP-2 were significantly lower following HTP than CC exposure (p < 0.001). The levels of IL-9 following EC and HTP exposures were unchanged when compared to air but were significantly lower following HTP compared to EC and CC exposures (p < 0.001) (Fig. 4M).
Fig. 4.
Chronic exposure to alternative tobacco product aerosols modulated pulmonary proinflammatory cytokine and chemokine levels. Levels of inflammatory cytokines and chemokines in the BAL (A–M) of mice were measured at end of the 8-week exposure to air, EC, HTP or CC aerosols using MILLIPLEX MAP Kit as described previously [47] and in detail in Additional file 1: supplemental methods. Data are presented as bar diagrams with mean ± SE. Non-parametric Kruskal–Wallis test with FDR correction for multiple comparison was performed to see if significant differences exist between groups using GraphPad Prism V.9 (GraphPad; La Jolla, California, USA). Difference between two groups is considered significant at p < 0.05 and indicated with symbols *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. For each exposure condition, n = 10 animals for air (5M + 5F) and n = 20 animals for EC, HTP, and CC (10M + 10F) per group
Inhalation of HTP and EC aerosols resulted in lung damage
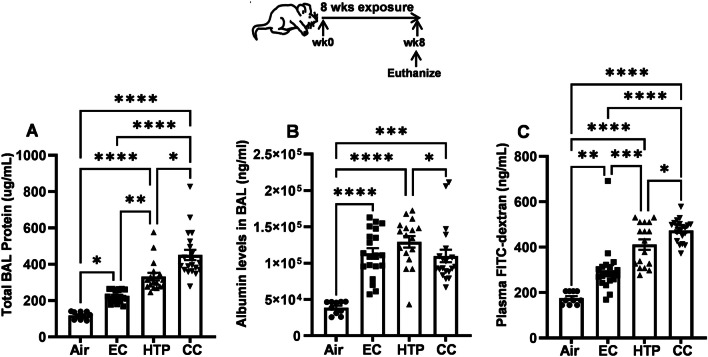
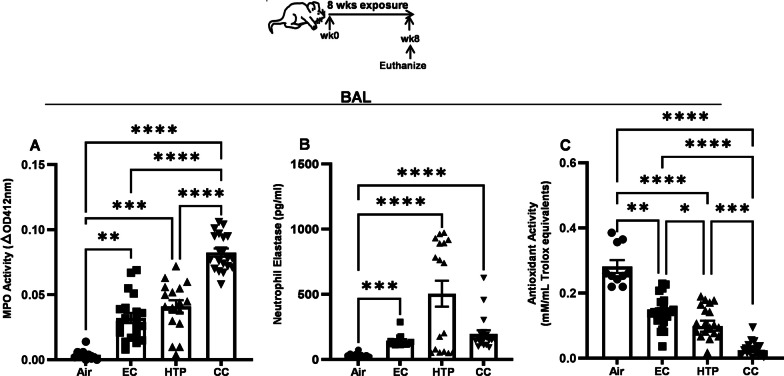
The levels of total proteins in the BAL were significantly increased following CC, HTP, and EC exposures compared to air, with CC > HTP > EC > air hierarchy (Fig. 5A). All products equivalently augmented the leak of albumin into the bronchoalveolar space of mice compared to air (p < 0.001), and this leak following HTP exposure was significantly more than after CC exposure (p < 0.05) (Fig. 5B). The levels of FITC-dextran in the plasma of mice exposed to EC, HTP, and CC were significantly higher than air (p < 0.01) (Fig. 5C). Additionally, while the plasma FITC-dextran levels were significantly greater following HTP and CC vs. EC exposure (p < 0.001), these levels were higher when comparing CC vs HTP groups (p < 0.05) (Fig. 5C). Only sex-based difference observed in these lung damage marker levels was higher levels of albumin in the BAL of female mice following EC exposure (Additional file 1: Fig. S4A–C). Chronic exposure to HTP and EC augmented MPO activity in the BAL compared to air (p < 0.01) (Fig. 6A). When compared to CC, the MPO activity induced following HTP (p < 0.001) or EC exposure (p < 0.0001) was lower (Fig. 6A), and no sex-based differences were noted (Additional file 1: Fig. S5A). NE levels in the BAL were markedly higher following exposure to all three products when compared to air exposure (p < 0.001) (Fig. 6B). Significantly elevated NE levels were noted in male compared with female mice following exposure to HTP (Additional file 1: Fig. S5B).
Fig. 5.
Chronic inhalatory exposure to aerosols from alternative tobacco products induces markers of lung damage. At the end of the 8-week exposures, mice were euthanized, BAL harvested and the levels of total proteins (A), albumin (B) in the BAL, and the levels of FITC-dextran (C) leaking into plasma were quantified as described previously [47] and given in detail in Additional file 1. Results are shown as bar diagrams with mean ± SE. Differences between groups is considered significant at p < 0.05 and are indicated as symbols *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001, calculated after performing non-parametric Kruskal–Wallis test with FDR correction for multiple comparisons by employing GraphPad Prism V.9 software (GraphPad; La Jolla, California, USA). n = 10 animals for air (5M + 5F) and n = 20 animals for EC, HTP, and CC (10M + 10F)/group were used for each exposure condition
Fig. 6.
Chronic inhalatory exposures to EC, HTP and CC aerosols induce lung damage. Mice exposed to aerosols from alternative tobacco products and CC for 8 weeks were euthanized and BAL harvested. MPO activity, levels of NE and antioxidant potential in the BAL were measured as described previously [47] and details provided in Additional file 1. Data are presented as bar diagrams with mean ± SE. Difference between two groups is considered significant at p < 0.05 and is indicated by symbols *p < 0.05, **p < 0.01, ***p < 0.001 ****p < 0.0001, calculated after performing a non-parametric Kruskal–Wallis rank test with FDR correction for multiple comparisons by employing GraphPad Prism V.9 software (GraphPad; La Jolla, California, USA). We used n = 10 animals for air (5 M + 5F) and n = 20 animals for EC, HTP, and CC (10M + 10F) per group for each exposure condition
Exposure to aerosols emitted from alternative tobacco prducts decreased lung antioxidant activity
The decreased antioxidant activity resulting from exposure to all products was significant compared to air (p < 0.01) (Fig. 6C); however, the suppression of antioxidant activity was maximal after exposure to CC compared to EC and HTP (p < 0.001) (Fig. 6C). Additionally, antioxidant activity following HTP exposure was significantly more suppressed than following EC exposure (p < 0.05). No sex-based differences were observed in antioxidant activity following any of these exposures (Additional file 1: Fig. S5C).
Chronic exposure to alternative tobacco product aerosols diminished the efficacy of vaccination
When aerosol-exposed mice were vaccinated with P6 Ag (Fig. 7A), we noted that the kinetics of the appearance of serum anti-P6 antibodies following exposures to all products were slower, and the magnitude of antibody titers accumulating in the sera of mice from wk4 onwards was substantially lower compared with air-exposed mice (Fig. 7B, p < 0.0001). While the titers of anti-P6 antibodies in the serum of vaccinated mice exposed to HTP or EC were equivalent from weeks 1 to 7, the end-point antibody titers at wk8 post-vaccination were significantly more suppressed in the serum of HTP-exposed compared with EC-exposed mice (p < 0.001) (Fig. 7B). Antibody titers in CC-exposed mice were suppressed at week 8 compared to both alternative tobacco products (p < 0.0001) (Fig. 7B).
End-point anti-P6 titers after vaccination were significantly lower in the BAL of mice exposed to only HTP and CC compared to air-exposed control (p < 0.05) (Fig. 7C). Additionally, all products significantly decreased P6-specific mucosal IgA antibody levels in the BAL of P6-vaccinated mice compared to air (p < 0.01) (Fig. 7D). The levels of anti-P6 IgA antibodies were lower following HTP vs. EC exposure (p < 0.05) but maximally suppressed following CC compared to both EC and HTP exposures (p < 0.01) (Fig. 7D).
To test whether this reduction in vaccine-induced systemic and mucosal immunity translates to a delay and reduction in the ability of these mice to clear an acute respiratory infection, we intratracheally challenged aerosol-exposed, P6-immunized mice with live Nontypeable Haemophilus influenzae (NTHI) bacteria 8 weeks after vaccination and measured pulmonary bacterial clearance at various time-points post-acute challenge (Fig. 8A). Kinetics of NTHI clearance from lungs was slower in HTP- and EC-exposed P6-vaccinated mice compared with the air-exposed P6-vaccinated controls (Fig. 8B, p < 0.05). CC-exposed mice had reduced NTHI clearance compared to HTP-and EC-exposed groups (p < 0.01 at 4 h and p < 0.0001 at 12 h, respectively) (Fig. 8B), with a CC > HTP > EC > air hierarchy of suppression of bacterial clearance in the lungs. Exposure to HTP and EC exacerbated the accumulation of infection-induced total proteins in the BAL at all time points (p < 0.05) and albumin levels at 4 and 12 h following acute challenge compared to air (p < 0.01) (Fig. 8C, D). A product hierarchy of CC > HTP = EC > air was observed in the augmentation of the levels of both these markers in the BAL.
Discussion
Our study provides evidence on how chronic inhalation of aerosols generated from two alternative tobacco products, HTP and EC, impacts the pulmonary inflammatory responses and efficacy of vaccine-induced antibacterial immunity. Importantly, to answer the question of whether those two emerging tobacco products can be considered as potentially harm-reducing alternatives for people who smoke CC, we have compared responses caused by HTP and EC to the effects of CC smoke. Since no reports have directly compared the pulmonary effects HTP vs. EC, our studies were also designed to address this critical gap in knowledge. Generally, the observed induction of detrimental effects associated with chronic exposure to three tobacco products tested in our study followed a CC > HTP > EC hierarchy. However, it is worth noting that in many cases, a proinflammatory pulmonary milieu induced by HTP was comparable to that induced by CC.
As observed in our study, the augmentation in the levels of inflammatory cytokines/chemokines likely mediates HTP-induced pulmonary immune-cell infiltration of leukocytes, neutrophils, and macrophages in a manner equivalent to or greater than EC. While CC overall inflicted more significant damage than both alternative tobacco products, the proinflammatory effects of chronic HTP inhalation were often comparable to that of CC. We are cognizant that the chemical composition of EC and HTP aerosols differs from that of CC smoke, which could play a critical role in the differential accumulation/recruitment of immune cells in the lungs [15–21]. Emissions from HTPs contain numerous chemicals that are also found in CC smoke, and which are classified as highly toxic, e.g., diacetyl, 2,3-pentanedione, hydroxymethylfurfural, and diethylhexyl phthalate [11]. Potentially toxic furans, which are byproducts of the thermal decomposition of sugars, and pyridines, which are produced following the thermal decomposition of nicotine, were detected in the emissions from HTPs and CCs, but are not commonly seen in emissions from ECs [12]. Despite generally lower emission of toxicants from ECs and HTPs compared to CCs, concerns have been raised about respiratory health effects resulting from exposure to toxic carbonyl compounds (incl. formaldehyde, acetaldehyde, and acrolein), which are thermal decomposition of humectants used in HTPs and ECs [13]. Aerosols from ECs often contain high concentrations of common flavoring chemicals (incl. benzaldehyde and cinnamaldehyde), which are known airway irritants and sensitizers and have been reported to cause occupational asthma [14].
The accumulation of neutrophils in the lungs in response to EC and HTP exposure can have significant consequences, as these cells are the source of MPO and NE [26, 27]. MPO is an important inflammatory and oxidative stress marker in several conditions, including lung injury [28, 29]. Augmentation in neutrophilic infiltration and increased NE activity with resulting disruption of lung epithelial barrier integrity is one of the mechanisms through which CC induces pulmonary injury [27, 30, 31]. In this context, the neutrophil and macrophage infiltration induced by emissions from alternative tobacco products could have accounted for the enhanced levels of MPO and NE we detected and likely resulted also in augmented pulmonary oxidative stress.
Previous studies have shown that CC increases susceptibility to respiratory infections by compromising antibacterial immunity [7–10]. Since vaccine-induced immunity is necessary to prevent infections, CC has been demonstrated to reduce the efficacy of prophylactic vaccines [8, 10, 32]. We have previously demonstrated that NTHI P6 protein as a vaccine candidate protects mice from an acute NTHI challenge [8, 10, 33]. Our results clearly show that chronic exposure to aerosols emitted from EC and HTP suppress the production of systemic and mucosal antigen-specific antibodies in a manner akin to CC. Mucosal IgA antibodies, a critical first line of defense against respiratory pathogens, were also significantly diminished following EC and HTP exposures. Suppressed efficacy of P6 vaccination translated into the reduced pulmonary clearance of NTHI bacteria after an acute challenge and led to augmented infection-induced infiltration of neutrophils or macrophages and exacerbated lung damage. These detrimental effects were similar to those seen after CC exposure and similar to previous findings [8, 10, 34]. Following these exposures, a hierarchy of suppression of the efficacy of P6 vaccination and subsequent reduction of bacterial clearance from the lungs of vaccinated mice was found as CC > HTP > EC.
Although the exact mechanisms contributing to EC- and HTP-induced pulmonary inflammation and increased bacterial burden in the lungs have not been established, they could be similar to CC-induced effects. As reported for CC and EC, a potential mechanism could include impaired phagocytic activity [34–36]. Those studies suggested that CC and EC impaired the phagocytic activity of granulocytes and monocytes, contributing to bacterial colonization and increased pathogen burden in the respiratory mucosa, factors that might associate with dysbiosis of the lung microbiome, poor airway health and increased infections in COPD patients [37–41]. While immunosuppressive agents in these products are largely unknown, an analysis of individual constituents in these products and their contribution to inflammatory changes, damage, and immune suppression would be informative. Chung et al. demonstrated that significant immunosuppressive effects could be mediated by nicotine [41]. Importantly, in our study, the exposure protocol for the three products was calibrated so that an equivalent dose of nicotine was delivered to all mice; thus, the differential effects observed are likely unrelated to the differential nicotine intake from tested products. Previous findings [7–10] and our current results support the conclusion that the increased bacterial burden in the lungs of mice exposed to EC or HTP aerosols could be due to impaired innate immune defenses and decreased efficacy of vaccine-induced immunity, including suppressed mucosal antigen-specific IgA production.
An essential strength of our study is the use of precisely adjusted experimental conditions that resulted in animals being exposed to equivalent doses of nicotine from three different tobacco products. This approach eliminated potential confounding effects of differences in physical properties of aerosols emitted from three products that may have affected nicotine delivery to lungs, incl. particulate concentration and aerodynamic particle size. Despite different puffing topographies and different airborne concentrations of nicotine achieved from the three products, our experiments led to similar nicotine exposure across all experimental conditions. This innovative approach allowed us to compare the effects of tested products in realistic conditions, reflecting observations from human studies showing that people who use alternative tobacco products have comparable exposure to nicotine to people who smoke CC [42–45]. Importantly, higher levels of cotinine, a major nicotine metabolite, were observed in female mice compared to male mice in our experiments. This is consistent with the sex-dependent pharmacokinetics of nicotine metabolism in mice, as several studies have shown a faster rate of nicotine elimination from the liver of female than male mice [46–48].
Our study has several limitations. Although we selected popular brands of EC and HTP, it remains to be determined whether our findings can be generalized to other types and brands of alternative tobacco products. Since we performed whole-body exposures, our findings may differ from studies following nose-only exposures. However, our routine sampling did not detect nicotine deposited on animal fur, thereby indicating that inhalation was a primary route of exposure. Since our study directly compared the effects of continued exposure to single products, we did not test the effects of switching between products (e.g., from CC to EC or from CC to HTP). This type of “switching studies” are essential since they are relevant to real-life scenarios when smokers switch to alternative products to reduce the harm associated with smoking CC. However, Husari et al. recently reported that substituting 50% of daily CC exposure with either EC or HTP exposure did not significantly attenuate acute lung injury in a mouse model [49]. Finally, studies are needed to evaluate the effects of concurrent exposures to two products (CC plus EC and CC plus HTP) since epidemiological studies have consistently shown high rates of concurrent use of multiple tobacco products [50–54].
Conclusions
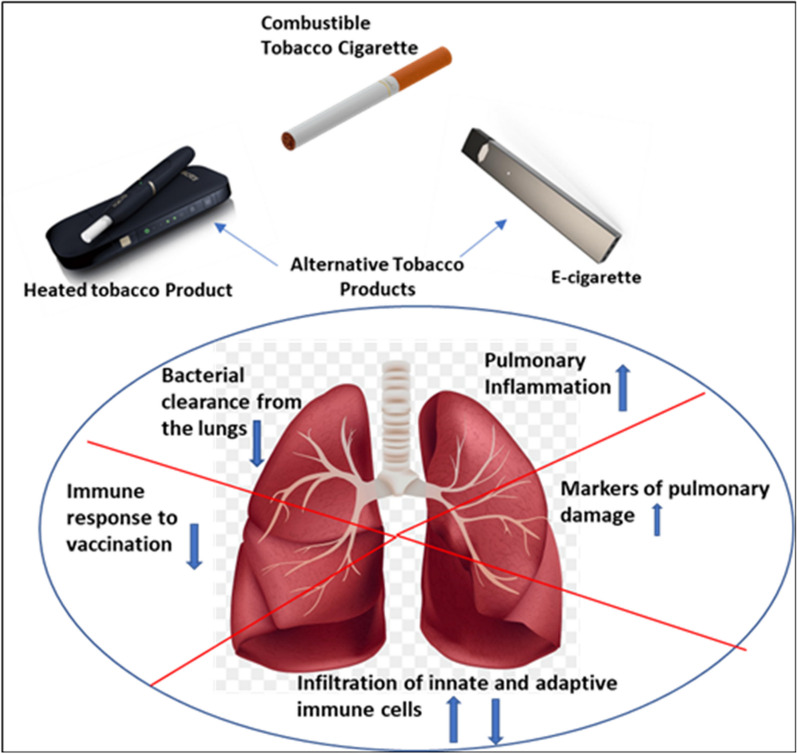
While the alternative tobacco product use has become increasingly popular, the pulmonary health effects resulting from the chronic inhalation of aerosols emitted from these products when compared to smoking combustible tobacco cigarettes are unknown. This study provides evidence on how chronic inhalation exposure to aerosols from two alternative tobacco products impacts pulmonary inflammatory responses, induces oxidative stress, lung endothelial and epithelial damage, and suppresses the efficacy of vaccine-induced antibacterial immunity leading to increased pathogen burden in the lungs of exposed mice in a manner like combustible cigarette smoke (as graphically depicted in Fig. 9). Our study has the potential to open up a broader dialog among clinicians and users, by providing key insights to the adverse respiratory health consequences and immunity suppressive effects resulting from the use of alternative tobacco products. While combustible cigarette smoke overall resulted in more damage than both alternative tobacco products, the proinflammatory effects of chronic HTP inhalation were often comparable to that of CC with the recognition that alternative tobacco product use is not risk-free.
Fig. 9.
Graphical depiction of alternative tobacco product aerosol-induced detrimental pulmonary effects. The cartoon shows the pulmonary effects induced after chronic exposure to aerosols from alternative tobacco products, HTP and EC, as compared to those induced by cigarette smoke. Upward arrows depict increase, while downward arrows show a decrease
Supplementary Information
Additional file 1. Do alternative tobacco products induce less adverse respiratory risk than cigarettes?
Acknowledgements
This study was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01 HL142511) to YT and MLG, the US Food and Drug Administration (FDA), and the National Cancer Institute Grant U54CA228110 to MLG, and P30CA016056 involving the use of Roswell Park Core facilities. The content is solely the authors' responsibility and does not necessarily represent the official views of the sponsors, the NIH, or the US FDA. The sponsors had no role in the writing of the manuscript or the decision to submit it for publication.
Abbreviations
- EC
Electronic cigarettes
- HTP
Heated tobacco products
- CC
Combustible cigarettes
- BAL
Broncho alveolar lavage
- MPO
Myeloperoxidase
- NE
Neutrophil elastase
- NTHI
Nontypeable Haemophilus influenzae
Author contributions
Concept and design: TAB, MLG and YT. Data collection: TAB, SGK, NL, MLG and YT. Data analysis: TAB, MLG and SGK. Data interpretation: TAB, MLG and YT. Statistical support: TAB, AH, MLG and YT. TAB wrote the initial draft of the manuscript. TAB, MLG and YT revised the manuscript. All authors approved the final draft of the manuscript. YT and MLG have full access to all study data and take responsibility for the integrity of the data and accuracy of the data analysis.
Funding
This study was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01 HL142511) to YT and MLG, the US Food and Drug Administration (FDA), and the National Cancer Institute Grant U54CA228110 to MLG, and P30CA016056 involving the use of Roswell Park Core facilities. The content is solely the authors' responsibility and does not necessarily represent the official views of the sponsors, the NIH, or the US FDA. The sponsors had no role in the writing of the manuscript or the decision to submit it for publication.
Availability of data and materials
All data relevant to the study are included in the article or uploaded as online Additional file 1.
Declarations
Ethics approval and consent to participate
All mice were maintained in a specific pathogen-free environment in barrier facilities at the Roswell Park Comprehensive Cancer Center in Buffalo, NY, with a light/dark cycle of 12/12 h. Animal procedures were approved by the Institutional Animal Care and Use Committee and complied with all state, federal, and NIH regulations.
Competing interests
MLG reports research grant from Pfizer and personal fees from Johnson & Johnson, outside of this work. Others report none.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vellappally S, Fiala Z, Smejkalová J, et al. Smoking related systemic and oral diseases. Acta Medica (Hradec Kralove) 2007;50(3):161–166. doi: 10.14712/18059694.2017.76. [DOI] [PubMed] [Google Scholar]
- 2.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368:351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Bookshelf ID: NBK179276.
- 4.Alberg AJ, Shopland DR, Cummings KM. The 2014 Surgeon General's report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. 2014;179(4):403–412. doi: 10.1093/aje/kwt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Facts Sheet- Tobacco. 2019; http://www.who.int/mediacentre/factsheets/fs339/en/. Date last updated May 2022; Accessed April 2023.
- 6.U.S. Food and Drug Administration FDA Authorizes Marketing of IQOS Tobacco Heating System with ‘Reduced Exposure’ Information. https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-iqos-tobacco-heating-system-reduced-exposure-information. Accessed March, 2023.
- 7.Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9(5):377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 8.Lugade AA, Bogner PN, Thatcher TH, et al. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J Immunol. 2014;192(11):5226–5235. doi: 10.4049/jimmunol.1302584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhat TA, Panzica L, Kalathil SG, et al. Immune dysfunction in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12:S169–175. doi: 10.1513/AnnalsATS.201503-126AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhat TA, Kalathil SG, Bogner PN, et al. Secondhand smoke induces inflammation and impairs immunity to respiratory infections. J Immunol. 2018;200(8):2927–2940. doi: 10.4049/jimmunol.1701417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilies BD, Moosakutty SP, Kharbatia NM, et al. Identification of volatile constituents released from IQOS heat-not-burn tobacco HeatSticks using a direct sampling method. Tob Control. 2020 doi: 10.1136/tobaccocontrol-2019-055521. [DOI] [PubMed] [Google Scholar]
- 12.Bekki K, Uchiyama S, Inaba Y, et al. Analysis of furans and pyridines from new generation heated tobacco product in Japan. Environ Health Prev Med. 2021;26(1):89. doi: 10.1186/s12199-021-01008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farsalinos KE, Gillman G. Carbonyl emissions in E-cigarette aerosol: a systematic review and methodological considerations. Front Physiol. 2018;11(8):1119. doi: 10.3389/fphys.2017.01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clapp PW, Jaspers I. Electronic cigarettes: their constituents and potential links to asthma. Curr Allergy Asthma Rep. 2017;17(11):79. doi: 10.1007/s11882-017-0747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uguna CN, Snape CE. Should IQOS emissions be considered as smoke and harmful to health? A review of the chemical evidence. ACS Omega. 2022;7(26):22111–22124. doi: 10.1021/acsomega.2c01527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Kaassamani M, Yen M, Talih S, et al. Analysis of mainstream emissions, secondhand emissions and the environmental impact of IQOS waste: a systematic review on IQOS that accounts for data source. Tob Control. 2022 doi: 10.1136/tobaccocontrol-2021-056986. [DOI] [PubMed] [Google Scholar]
- 17.Kärkelä T, Tapper U, Kajolinna T. Comparison of 3R4F cigarette smoke and IQOS heated tobacco product aerosol emissions. Environ Sci Pollut Res Int. 2022;29(18):27051–27069. doi: 10.1007/s11356-021-18032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Statement on Heated Tobacco Products and the US FDA Decision Regarding IQOS. https://www.who.int/news/item/27-07-2020-who-statement-on-heated-tobacco-products-and-the-us-fda-decision-regarding-iqos. July 2020. Accessed March 2023.
- 19.Pisinger C, Mackay J. New tobacco products do not protect public health. Ann Am Thorac Soc. 2019;16(11):1363–1365. doi: 10.1513/AnnalsATS.201905-411PS. [DOI] [PubMed] [Google Scholar]
- 20.Traboulsi H, Cherian M, Abou Rjeili M, et al. Inhalation toxicology of vaping products and implications for pulmonary health. Int J Mol Sci. 2020;21(10):3495. doi: 10.3390/ijms21103495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon T, Karey E, Rebuli ME, et al. E-cigarette toxicology. Annu Rev Pharmacol Toxicol. 2022;62:301–322. doi: 10.1146/annurev-pharmtox-042921-084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartl D, Tirouvanziam R, Laval J, et al. Innate Immunity of the lung: from basic mechanisms to translational medicine. J Innate Immun. 2018;10(5–6):487–501. doi: 10.1159/000487057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2015;16(1):27–35. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strzelak A, Ratajczak A, Adamiec A, et al. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. 2018;15(5):1033. doi: 10.3390/ijerph15051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhat TA, Kalathil SG, Goniewicz ML, et al. Not all vaping is the same: differential pulmonary effects of vaping cannabidiol versus nicotine. Thorax. 2023;78:922–932. doi: 10.1136/thorax-2022-218743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoshino H, Laan M, Sjöstrand M, et al. Increased elastase and myeloperoxidase activity associated with neutrophil recruitment by IL-17 in airways in vivo. J Allergy Clin Immunol. 2000;105(1 Pt 1):143–149. doi: 10.1016/S0091-6749(00)90189-1. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh A, Coakley RD, Ghio AJ, et al. Chronic e-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung. Am J Respir Crit Care Med. 2019;200:1392–1401. doi: 10.1164/rccm.201903-0615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan AA, Alsahli MA, Rahmani AH. Myeloperoxidase as an active disease biomarker: recent biochemical and pathological perspectives. Med Sci (Basel) 2018;6:33. doi: 10.3390/medsci6020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faith M, Sukumaran A, Pulimood AB, et al. How reliable an indicator of inflammation is myeloperoxidase activity? Clin Chim Acta. 2008;396:23–25. doi: 10.1016/j.cca.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro SD, Goldstein NM, Houghton AM, et al. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol. 2003;163(6):2329–2335. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antunes MA, Rocco PRM. Elastase-induced pulmonary emphysema: insights from experimental models. An Acad Bras Cienc. 2011;83:1385–1396. doi: 10.1590/S0001-37652011005000039. [DOI] [PubMed] [Google Scholar]
- 32.Ferrara P, Ponticelli D, Agüero F, et al. Does smoking have an impact on the immunological response to COVID-19 vaccines? Evidence from the VASCO study and need for further studies. Public Health. 2022;203:97–99. doi: 10.1016/j.puhe.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMahon M, Murphy TF, Kyd J, et al. Role of an immunodominant T cell epitope of the P6 protein of nontypeable Haemophilus influenzae in murine protective immunity. Vaccine. 2005;23(27):3590–3596. doi: 10.1016/j.vaccine.2005.01.151. [DOI] [PubMed] [Google Scholar]
- 34.Voss M, Wonnenberg B, Honecker A, et al. Cigarette smoke-promoted acquisition of bacterial pathogens in the upper respiratory tract leads to enhanced inflammation in mice. Respir Res. 2015;16(1):41. doi: 10.1186/s12931-015-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ween MP, Whittall JJ, Hamon R, et al. Phagocytosis and Inflammation: exploring the effects of the components of E-cigarette vapor on macrophages. Physiol Rep. 2017;5(16):e13370. doi: 10.14814/phy2.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corriden R, Moshensky A, Bojanowski CM, et al. E-cigarette use increases susceptibility to bacterial infection by impairment of human neutrophil chemotaxis, phagocytosis, and NET formation. Am J Physiol Cell Physiol. 2020;318(1):C205–C214. doi: 10.1152/ajpcell.00045.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baginski TK, Dabbagh K, Satjawatcharaphong C, et al. Cigarette smoke synergistically enhances respiratory mucin induction by proinflammatory stimuli. Am J Respir Cell Mol Biol. 2006;35(2):165–174. doi: 10.1165/rcmb.2005-0259OC. [DOI] [PubMed] [Google Scholar]
- 38.Berenson CS, Kruzel RL, Eberhardt E, et al. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J Infect Dis. 2013;208(12):2036–2045. doi: 10.1093/infdis/jit400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Arcos I, Geraghty P, Baumlin N, et al. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax. 2016;71(12):1119–1129. doi: 10.1136/thoraxjnl-2015-208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jubrail J, Kurian N, Niedergang F. Macrophage phagocytosis cracking the defect code in COPD. Biomed J. 2017;40(6):305–312. doi: 10.1016/j.bj.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung S, Baumlin N, Dennis JS, et al. Electronic cigarette vapor with nicotine causes airway mucociliary dysfunction preferentially via TRPA1 receptors. Am J Respir Crit Care Med. 2019;200(9):1134–1145. doi: 10.1164/rccm.201811-2087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahab L, Goniewicz ML, Blount BC, et al. Nicotine, carcinogen, and toxin exposure in long-term E-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann Intern Med. 2017;166(6):390–400. doi: 10.7326/M16-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goniewicz ML, Smith DM, Edwards KC, et al. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Netw Open. 2018;1(8):e185937. doi: 10.1001/jamanetworkopen.2018.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudasingwa G, Kim Y, Lee C, et al. Comparison of nicotine dependence and biomarker levels among traditional cigarette, heat-not-burn cigarette, and liquid E-cigarette users: results from the think study. Int J Environ Res Public Health. 2021;18(9):4777. doi: 10.3390/ijerph18094777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akiyama Y, Sherwood N. Systematic review of biomarker findings from clinical studies of electronic cigarettes and heated tobacco products. Toxicol Rep. 2021;8:282–294. doi: 10.1016/j.toxrep.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hatchell PC, Collins AC. The influence of genotype and sex on behavioral sensitivity to nicotine in mice. Psychopharmacology. 1980;71(1):45–49. doi: 10.1007/BF00433251. [DOI] [PubMed] [Google Scholar]
- 47.Siu EC, Wildenauer DB, Tyndale RF. Nicotine self-administration in mice is associated with rates of nicotine inactivation by CYP2A5. Psychopharmacology. 2006;184(3–4):401–408. doi: 10.1007/s00213-006-0306-6. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen K, Kanamori K, Shin CS, et al. The impact of sex on changes in plasma corticosterone and cotinine levels induced by nicotine in C57BL/6J mice. Brain Sci. 2020;10(10):705. doi: 10.3390/brainsci10100705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Husari A, El-Harakeh M, Shihadeh A, et al. The substitution of fifty percent of combustible tobacco smoke exposure with either electronic cigarettes or heated tobacco products did not attenuate acute lung injury in an animal model. Nicotine Tob Res. 2023 doi: 10.1093/ntr/ntad045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasza KA, Ambrose BK, Conway KP, et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N Engl J Med. 2017;376(4):342–353. doi: 10.1056/NEJMsa1607538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutanto E, Miller C, Smith DM, et al. Concurrent daily and non-daily use of heated tobacco products with combustible cigarettes: findings from the 2018 ITC Japan Survey. Int J Environ Res Public Health. 2020;17(6):2098. doi: 10.3390/ijerph17062098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pokhrel P, Herzog TA, Kawamoto CT, et al. Heat-not-burn tobacco products and the increased risk for poly-tobacco use. Am J Health Behav. 2021;45(1):195–204. doi: 10.5993/AJHB.45.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen DT. Dual and poly-use of novel and conventional nicotine and tobacco product use in Europe: challenges for population health, regulatory policies, and the ways ahead. Front Public Health. 2023;11:1093771. doi: 10.3389/fpubh.2023.1093771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanton CA, Halenar MJ. Patterns and correlates of multiple tobacco product use in the United States. Nicotine Tob Res. 2018;20:S1–S4. doi: 10.1093/ntr/nty081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Do alternative tobacco products induce less adverse respiratory risk than cigarettes?
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online Additional file 1.