Abstract
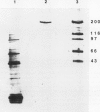
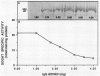
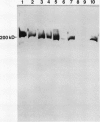
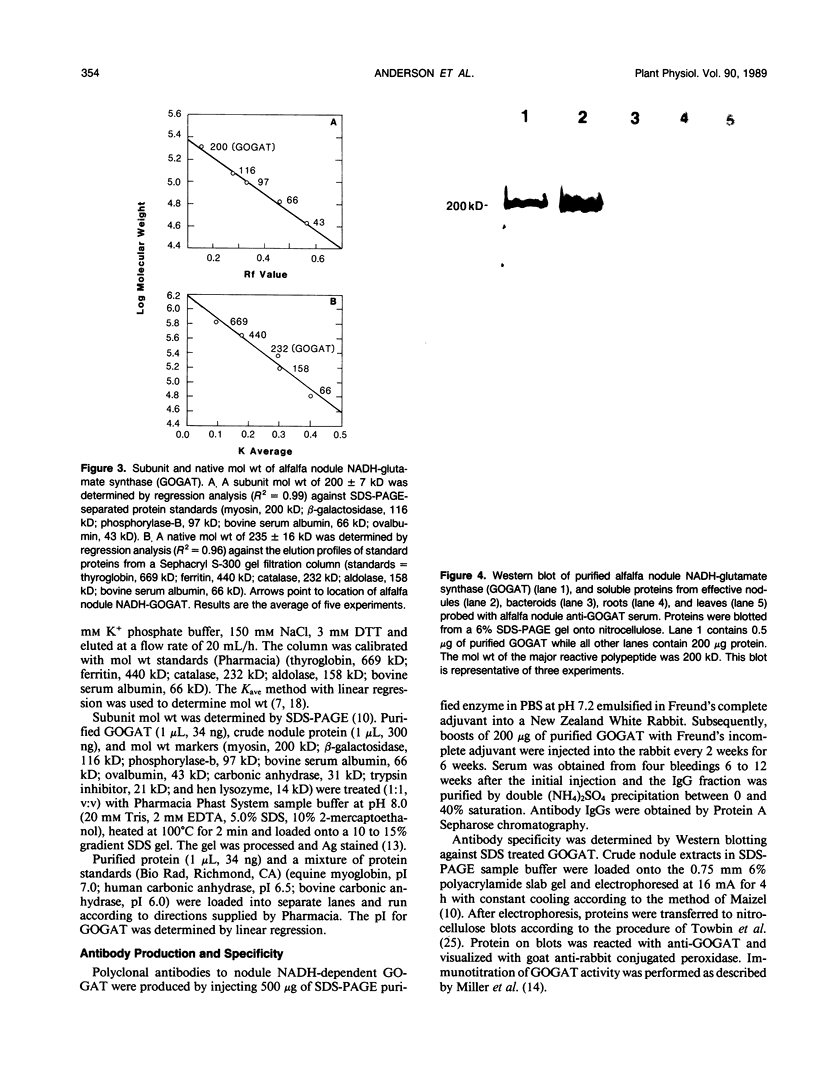
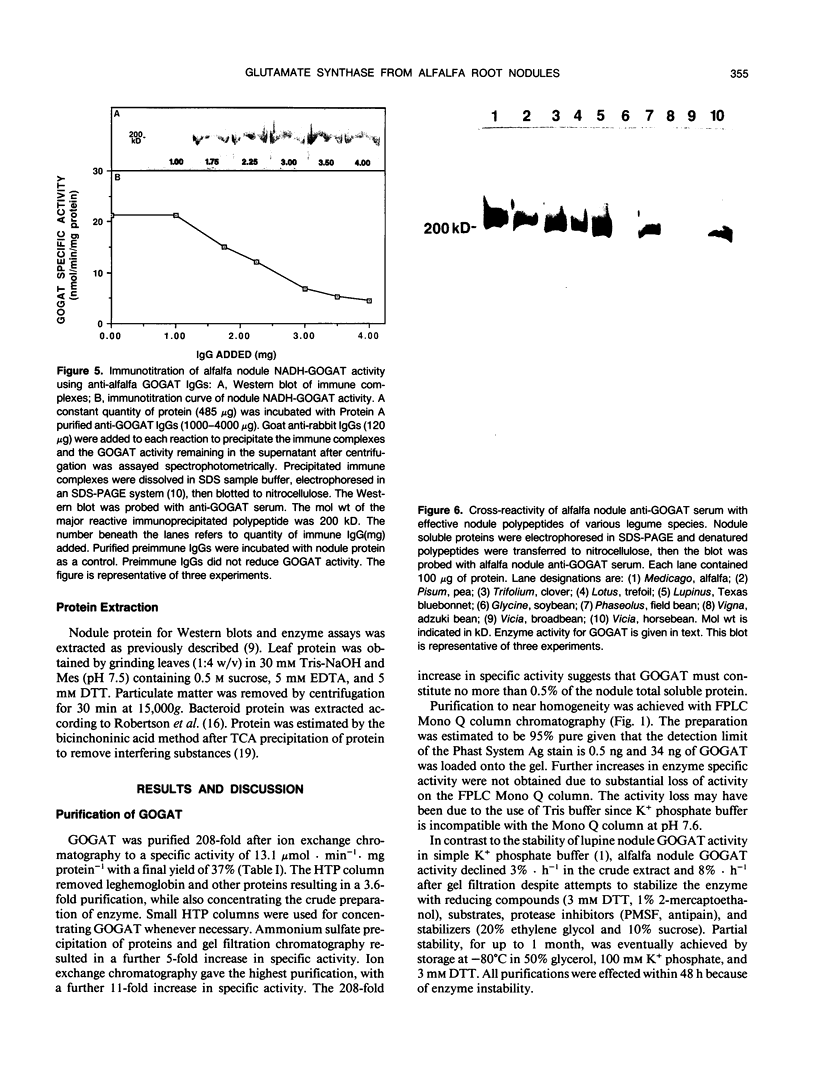
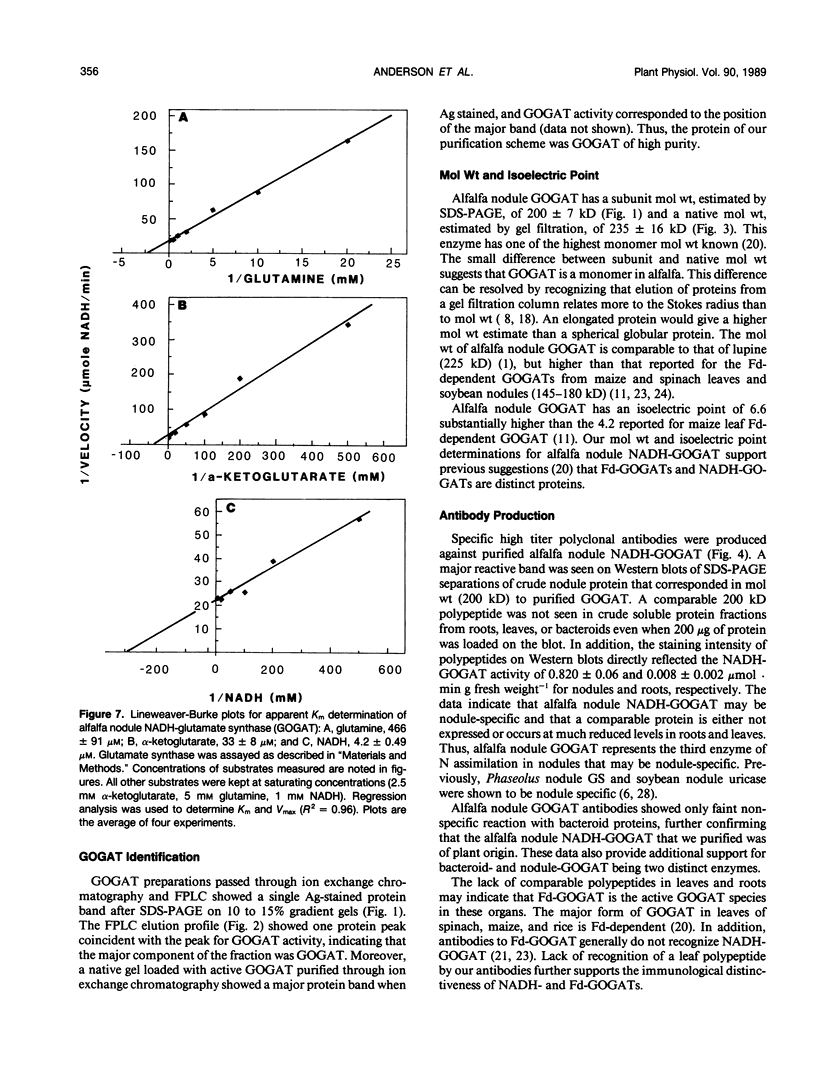
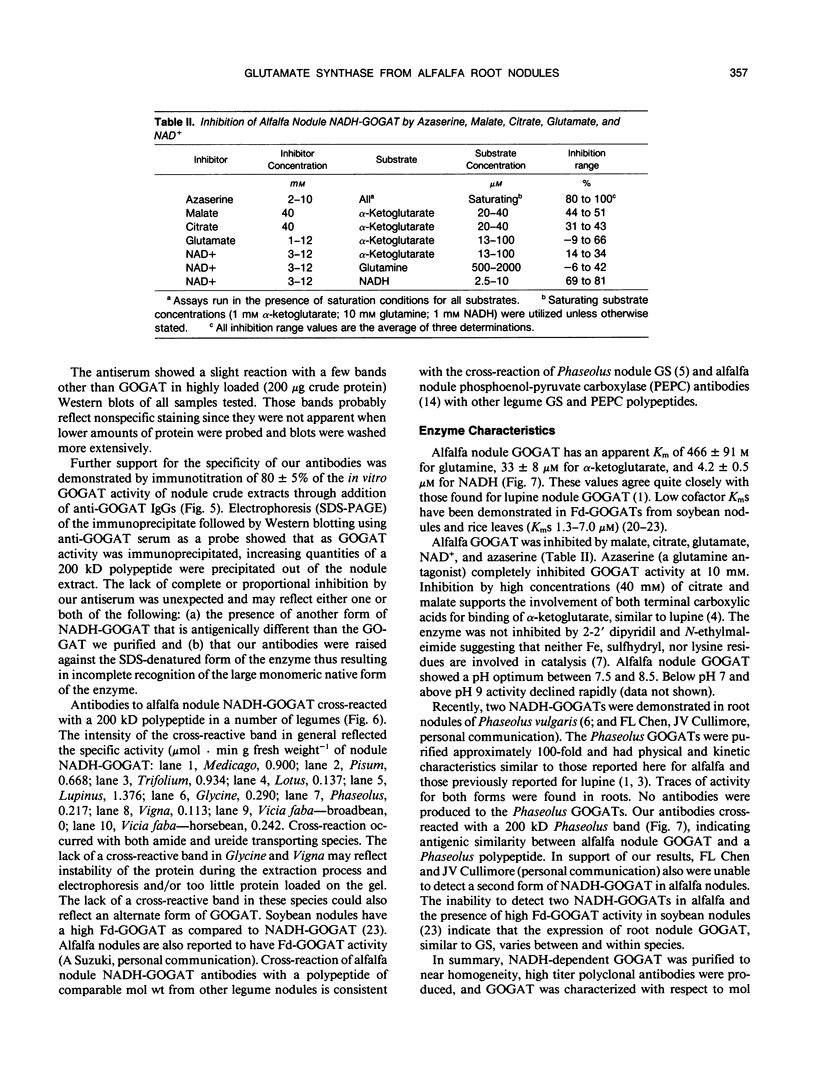
Glutamate synthase (GOGAT), a key enzyme in the pathway for the assimilation of symbiotically fixed dinitrogen (N2) into amino acids in alfalfa (Medicago sativa L.) root nodules, was purified and used to produce high titer polyclonal antibodies. Purification resulted in a 208-fold increase in specific activity to 13 micromole per minute per milligram of protein and an activity yield of 37%. Further purification to near homogeneity was achieved by fast protein liquid chromatography, but with substantial loss of activity. Enzymic activity was highly labile, losing 3% per hour even when substrates, stabilizers, and reducing agents were included in buffers. However, activity could be partially stabilized for up to 1 month by storing GOGAT at −80°C in 50% glycerol. The subunit molecular weight of GOGAT was estimated at 200 ± 7 kilodaltons with a native molecular weight of 235 ± 16 kilodaltons, which suggested that GOGAT is a monomer of unusually high molecular weight. The pl was estimated to be 6.6. The Km values for glutamine, α-ketoglutarate, and NADH were 466, 33, and 4.2 micromolar, respectively. Antibodies were produced to NADH-GOGAT. Specificity of the antibodies was shown by immunotitration of GOGAT activity. Alfalfa nodule NADH-GOGAT antibodies cross-reacted with polypeptides of a similar molecular weight in a number of legume species. Western blots probed with anti-GOGAT showed that the high GOGAT activity of nodules as compared to roots was associated with increased levels of GOGAT polypeptides. Nodule NADH-GOGAT appeared to be highly expressed in effective nodules and little if any in other organs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boland M. J., Benny A. G. Enzymes of nitrogen metabolism in legume nodules. Purification and properties of NADH-dependent glutamate synthase from lupin nodules. Eur J Biochem. 1977 Oct 3;79(2):355–362. doi: 10.1111/j.1432-1033.1977.tb11816.x. [DOI] [PubMed] [Google Scholar]
- Boland M. J., Court C. B. Glutamate synthase (NADH) from lupin nodules. Specificity of the 2-oxoglutarate site. Biochim Biophys Acta. 1981 Feb 13;657(2):539–542. doi: 10.1016/0005-2744(81)90339-9. [DOI] [PubMed] [Google Scholar]
- Boland M. J. Kinetic mechanism of NADH-dependent glutamate synthase from lupin nodules. Eur J Biochem. 1979 Sep;99(3):531–539. doi: 10.1111/j.1432-1033.1979.tb13285.x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Groat R. G., Vance C. P. Root Nodule Enzymes of Ammonia Assimilation in Alfalfa (Medicago sativa L.) : DEVELOPMENTAL PATTERNS AND RESPONSE TO APPLIED NITROGEN. Plant Physiol. 1981 Jun;67(6):1198–1203. doi: 10.1104/pp.67.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Miller S. S., Boylan K. L., Vance C. P. Alfalfa Root Nodule Carbon Dioxide Fixation : III. Immunological Studies of Nodule Phosphoenolpyruvate Carboxylase. Plant Physiol. 1987 Jun;84(2):501–508. doi: 10.1104/pp.84.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. G., Warburton M. P., Lyttleton P., Fordyce A. M., Bullivant S. Membranes in lupin root nodules. II. Preparation and properties of peribacteroid membranes and bacteroid envelope inner membranes from developing lupin nodules. J Cell Sci. 1978 Apr;30:151–174. doi: 10.1242/jcs.30.1.151. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Oaks A., Jacquot J. P., Vidal J., Gadal P. An electron transport system in maize roots for reactions of glutamate synthase and nitrite reductase : physiological and immunochemical properties of the electron carrier and pyridine nucleotide reductase. Plant Physiol. 1985 Jun;78(2):374–378. doi: 10.1104/pp.78.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Vidal J., Gadal P. Glutamate synthase isoforms in rice: immunological studies of enzymes in green leaf, etiolated leaf, and root tissues. Plant Physiol. 1982 Sep;70(3):827–832. doi: 10.1104/pp.70.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta T. C., Faris M. A., Macdowall F. D. Pathways of Nitrogen Metabolism in Nodules of Alfalfa (Medicago sativa L.). Plant Physiol. 1986 Apr;80(4):1002–1005. doi: 10.1104/pp.80.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]