Abstract
Cocoa fermentations were performed in wooden boxes under the following four experimental regimens: beans naturally fermented with wild microflora; aseptically prepared beans with no inoculum; and beans inoculated with a defined cocktail containing microorganisms at a suitable concentration either at zero time or by using phased additions at appropriate times. The cocktail used consisted of a yeast, Saccharomyces cerevisiae var. chevalieri, two lactic acid bacterial species, Lactobacillus lactis and Lactobacillus plantarum, and two acetic acid bacterial species, Acetobacter aceti and Gluconobacter oxydans subsp. suboxydans. The parameters measured were cell counts (for yeasts, filamentous fungi, lactic acid bacteria, acetic acid bacteria, and spore formers, including reisolation and identification of all residual cell types), sugar, ethanol, acetic acid, and lactic acid contents (and contents of other organic acids), pH, and temperature. A cut test for bean quality and a sensorial analysis of chocolate made from the beans were also performed. The natural fermentation mimicked exactly the conditions in 800-kg boxes on farms. The aseptic box remained largely free of microflora throughout the study, and no significant biochemical changes occurred. With the zero-time inoculum the fermentation was almost identical to the natural fermentation. The fermentation with the phased-addition inoculum was similar, but many changes in parameters were slower and less pronounced, which led to a slightly poorer end product. The data show that the nearly 50 common species of microorganisms found in natural fermentations can be replaced by a judicious selection and concentration of members of each physiological group. This is the first report of successful use of a defined, mixed starter culture in such a complex fermentation, and it should lead to chocolate of more reliable and better quality.
Raw cocoa, from the tree Theobroma cacao, has to be processed or cured before it can be marketed as chocolate (6). Curing involves fermentation, followed by drying and roasting. The white mucilaginous pulp which covers the beans contains about 14% sugar and 1.5% pectin at pH 3.5. In Brazil, the beans are put in large wooden boxes, and a vigorous natural fermentation starts; this fermentation lasts for up to 7 days, during which time the beans are turned daily to increase aeration (20). During the fermentation, microbial activity in the mucilaginous pulp produces alcohols and acids and liberates heat, and complex biochemical reactions occur within the bean cotyledons due to the diffusion of metabolites from the microorganisms.
The microbial succession in the fermentation process has been clearly established (6, 10, 14, 21). Yeasts dominate the fermentation for the first 24 h. They are then briefly eclipsed by lactic acid bacteria, but as the pulp disappears, oxygen penetrates the box and acetic acid bacteria start to dominate, producing acetic acid. During this time the temperature rises to about 50°C, and the heat and acid result in chemical reactions in the beans known as curing. Finally, spore formers appear, and some of these organisms, together with filamentous fungi which may appear on the cooler surface, can produce taints. The concentrations of the three key metabolites in the pulp, ethanol, lactic acid, and acetic acid, sequentially increase and decrease during fermentation, but an excess of only acetic acid is still present at the end. The physiological roles of the microorganisms have not been established with complete certainty, but it is possible to clearly attribute the required biochemical changes in the pulp to the yeasts, lactic acid, and acetic acid bacteria (17). The most important roles of the yeasts seem to be (i) breakdown of citric acid in the pulp, leading to an increase in the pH from 3.5 to 4.2, which allows growth of bacteria; (ii) production of ethanol under low-oxygen and high-sugar conditions, which is eventually consumed oxidatively; (iii) production of organic acids (oxalic, phosphoric, succinic, malic, and acetic acids), which permeabilize and kill the bean cotyledons; (iv) production of some volatile organic compounds which may contribute either to chocolate flavor or, more likely, to precursors of chocolate flavor; and (v) secretion of pectinases which reduce the viscosity of the pulp, allowing aeration of the pulp mass. Twelve different species of yeast are commonly found, and Saccharomyces cerevisiae is the most abundant species (21).
More than 30 different species of bacteria have been isolated from fermentations (1, 10, 12, 13, 19). The great majority of lactic acid bacteria utilize glucose via the Embden-Meyerhof-Parnas pathway, yielding more than 85% lactic acid. However, some species utilize glucose via the hexose monophosphate shunt, forming 50% lactic acid, as well as combinations of ethanol, acetic acid, glycerol, mannitol, and carbon dioxide. Acetic acid bacteria are responsible for the oxidation of ethanol to acetic acid and the oxidation of acetic acid to carbon dioxide and water. The exothermic reactions of the acetic acid bacteria raise the temperature of the fermenting mass (4). The acidity of cocoa bean preparations, the high temperatures in the fermenting masses, and the diffusion and hydrolysis of proteins in the cotyledons have been attributed to the metabolism of these microorganisms (4). Aerobic spore-forming bacteria, such as Bacillus strains, produce a variety of chemical compounds, including 2,3-butanediol, pyrazines, acetic acid, and lactic acid, under fermentative conditions, which may contribute to the acidity and perhaps, at times, to off-flavors of fermented cocoa beans (19).
Fermentations are done on the farm, and the results are very variable in quality. There are problems of acidity or lack of cocoa flavor (due to incomplete fermentation) and off-flavors (due to overfermented beans and spoilage), all of which lead to low crop value for the farmer. It would be desirable to change the fermentation from a wholly natural and unpredictable process to a process initiated with an appropriate starter culture in which fermentation occurred more quickly. Since we have started to understand the basis of the fermentation process, it is now possible to consider ways in which it can be manipulated and improved to give more reliable results. More than 42 species of microorganisms involved in the fermentation process have been identified so far, but not all are likely to be truly essential. This paper describes attempts to replace natural fermentations with fermentations conducted with a small, defined cocktail containing several species.
MATERIALS AND METHODS
Fermentation protocol.
The cocoa pods used were pods of Comum hybrids obtained from the fields of Comissáo Executiva do Plano da Lavoura Cacaveira (CEPLAC), the Cocoa Research Centre, Itabuna, Brazil. All damaged and diseased pods were discarded. Only yellow (mature) pods were selected; these pods were identical to those required for fresh cocoa juice production. The pods were harvested and broken open the same day, and sugar, citrate, and water contents were measured to confirm maturity. Washed pods were submersed in good-quality hypochlorite-treated water, which was followed by spray washing. The pods were broken open manually either with sterilized machetes (for natural fermentation) or with sterilized stainless steel knives at a surface-sterilized stainless steel table by workers wearing protective clothing and sterilized rubber gloves and boots (aseptic conditions). After being deposited in sterilized 50-kg-capacity containers, the beans were immediately transferred to 200-kg-capacity box fermentors housed in a room separate from other natural fermentation preparations. When the defined cocktail was inoculated at time zero, it was added in four aliquots as the 50-kg-capacity containers were emptied into the fermentation boxes. The sterilized boxes were either new boxes or old boxes which had been cleaned, scraped to eliminate old contamination, and then sterilized with ethanol and checked for the absence of microbial contamination. All boxes were covered with sterilized jute sacks and incubated for 168 h. Mixing and aeration of the cocoa mass were achieved by transferring the contents into a similar box each day.
Sampling.
Samples were taken every 6 h, but for clarity, only every other data point is usually given below. Each sample consisted of 40 beans removed with sterile tongs from different points approximately 30 cm from the walls and 45 cm from the upper surface. The beans were transferred to Erlenmeyer flasks containing 400 ml of 0.1% peptone water and some sand to remove the mucilaginous pulp.
Enumeration and identification of microorganisms.
Inocula and reisolated species were treated similarly for isolation and identification. Yeasts and filamentous fungi were isolated on malt extract agar (pH 3.5) (5) and TYGKCC medium (10), which consisted of tryptone (0.5%), yeast extract (0.5%), d-glucose (0.1%), K2HPO4 (0.1%), CaCO3 (0.1%), and cocoa bean pulp (1.0%). Yeasts were identified by standard methods (5). Lactic acid bacteria were isolated on TJA medium (Difco) supplemented with d-glucose (1%, wt/vol) and cycloheximide (150 μg ml−1). Cells were incubated in an anaerobic jar (GasPak). Gram-positive, catalase-negative colonies were streaked onto TJA medium, stored at 4°C, and identified by standard methods (13). Acetic acid bacteria were isolated on MCT medium containing d-glucose (10 g), Casamino Acids (5 g), yeast extract (5 g), tomato juice (10 ml), penicillin (125 μg ml−1), and cycloheximide (150 μg ml−1). The cultures were incubated aerobically at approximately the same temperature as the temperature of the fermentation at the moment of sampling. Gram-negative, catalase-positive cells that could grow on ethanol and produce acetic acid were isolated for identification by standard methods (11, 12). Spore-forming bacteria were isolated on TYGKCC medium after the sample was first heated to 80°C for 20 min to destroy vegetative bacteria. They were identified by standard methods (19). For all of the species identified reference strains from culture collections were used for comparison.
Microorganisms used in the defined cocktail.
All of the microorganisms in the defined cocktail used had been isolated previously from fermenting cocoa beans from farms in the same area and been identified as described above. The yeast strain used has been deposited in the culture collection of the Fundação de Pesquisas e Tecnologia Tropical André Tosello, Campinas, São Paulo, Brazil. The microorganisms used were Saccharomyces cerevisiae var. chevalieri, the lactic acid bacteria Lactobacillus lactis, and Lactobacillus plantarum, and the acetic acid bacteria Acetobacter aceti and Gluconobacter oxydans subsp. suboxydans. To inoculate the boxes, the three types of organisms were first grown in 250-ml Erlenmeyer flasks in standard media (yeast cells were grown in YEPD medium [5], lactic acid bacteria were grown in TJA medium, and acetic acid bacteria were grown in MCT medium); the cells were centrifuged, resuspended in 1 liter of TYGKCC medium containing 2% cocoa pulp, incubated for 48 h, and then sprayed over the cocoa mass.
Substrates and metabolites.
Ethanol, acetic acid, lactic acid, malic acid, succinic acid, oxalic acid, d-glucose, sucrose, and fructose were obtained from pulp extracts and analyzed. Analyses were carried out with a high-performance liquid chromatography system (Gilson) equipped with a dual detection system consisting of a UV detector and an refractive index (RI) detector (18). An Aminex ion exclusion type HPX-87H column fitted with a Cation H+ Micro-Guard cartridge (Bio-Rad Laboratories, Richmond, Calif.) operated at a temperature of 25°C was used to achieve chromatographic separation. Water-soluble acids, sugars (fructose, glucose, and sucrose), and ethanol were eluted with 0.26 N sulfuric acid at a flow rate of 0.5 ml min−1. The acids were detected both by UV absorbance (215 μm) and by RI, while sugars and ethanol were detected only by RI. Individual sugars, acids, and ethanol were identified and their concentrations were determined by comparison with retention times and amounts of authentic standards. The total reducing sugar contents were determined by the dinitrosalicylic acid method (9). All samples were examined in triplicate. The coefficient of variation was less than 5% in each case.
Quality of fermented beans.
The cut test (index of fermentation), which relies on changes in color, is the standard test used to assess the suitability of cocoa beans for making chocolate (3, 23). The cut test was carried out with separate samples at different times after the beans were dried in sunshine. A total of 300 beans were cut lengthwise through the middle in order to expose the maximum cut surface of the cotyledons. Both halves were examined in full daylight and placed in one of the following categories: fully brown (fermented); partly brown, partly purple; purple; slaty; insect damaged; moldy; and germinated.
Organoleptic evaluation.
Samples of beans were removed from the fermentation boxes at different times, dried, roasted, and made into chocolate at CEPLAC (7). An organoleptic evaluation was performed by a panel of six specially trained individuals, who used standard organoleptic descriptions (2, 8).
RESULTS
Design of the experiments.
Four different, parallel fermentations were performed at the same time (Table 1), and this was repeated six times. In the first of the six experiments, contamination developed in box B due to cross-infection from the tools used to turn the pulp, and the fourth experiment was performed with overripe beans in which the sugar content was much lower, which led to a slower and less vigorous fermentation. The other four experiments gave essentially identical results; however, since the amounts and times varied slightly, averaging tended to obscure peaks and troughs, and therefore the data from one representative set of fermentations are shown below.
TABLE 1.
Experimental protocol for the four fermentation boxes
| Expt | Cocoa pods | Box and utensils | Inoculum |
|---|---|---|---|
| Box A | Unwashed | Untreated | Natural microflora |
| Box B | Washed | Surface sterilized | None |
| Box C | Washed | Surface sterilized | Defined cocktail, inoculum added at time zero |
| Box D | Washed | Surface sterilized | Phased addition of defined cocktail, with yeast added at time zero, lactic acid bacteria added at 24 h, and acetic acid bacteria added at 48 h |
Choice of species and inoculum.
Previous work on microbial succession suggested that the cocktail inoculum had to include one or more representatives of each of three groups, the yeasts, the lactic acid bacteria, and the acetic acid bacteria. The yeast species had to have high fermentative ability and contain pectinolytic enzymes. The yeast S. cerevisiae var. chevalieri was chosen since S. cerevisiae species were the predominant yeasts and this particular variety produces pectinases, can ferment all pulp sugars at pH 3.5 to 4.2, is ethanol tolerant, and was present at the beginning of natural fermentations. The normal yeast density at time zero in natural fermentations is about 106 cells g of pulp−1. Preliminary experiments were done to determine an inoculum size with which ethanol production was similar to the ethanol production in normal fermentations (box A), and an initial inoculum containing 1010 cells g−1 was used. L. lactis and L. plantarum, both members of the homolactic group, were chosen as representatives of the lactic acid bacteria. L. plantarum was present in all previous fermentations studied and was the most common Lactobacillus species. It can ferment a large number of sugars found in plant material and is tolerant to acidity (pH 3.5). The Lactobacillus inoculation rate used, 107 cells g of pulp−1, was equal to the size of the population found after 24 h in natural fermentations and proved to be successful in preliminary trials (data not shown). The acetic acid bacteria used, A. aceti and G. oxydans subsp. suboxydans, were found in all previous cocoa fermentations, could grow in the presence of 6.0% ethanol, which is not typical of culture collection isolates, and could tolerate a temperature of 45°C at pH 3.5. Acetobacter isolates were also added since they are able to oxidize ethanol to acetate and then to CO2 and water. The acetic acid bacterial inoculation rate used, 108 cells g of pulp−1, was equal to the size of the population found after 48 h in natural fermentations and proved to be successful in preliminary trials (data not shown).
Reisolated species.
Reisolated species were identified every 12 h, but this was discontinued for a group once no isolates could be found due to the catastrophic decline in numbers that normally occurs (Fig. 1). The natural fermentation in box A yielded the same kinds of species at approximately the same frequencies that have been observed previously in 800-kg boxes (21; data not shown). Box B remained relatively free of microbial activity throughout the experimental period except for some surface growth. Box C, which was inoculated with the defined cocktail, had a pattern of development (Table 2) that was also observed in box D and in the three other experiments (data not shown). With box D the changes in frequencies of bacterial species exhibited lags compared with box C in direct proportion to the delays in inoculation.
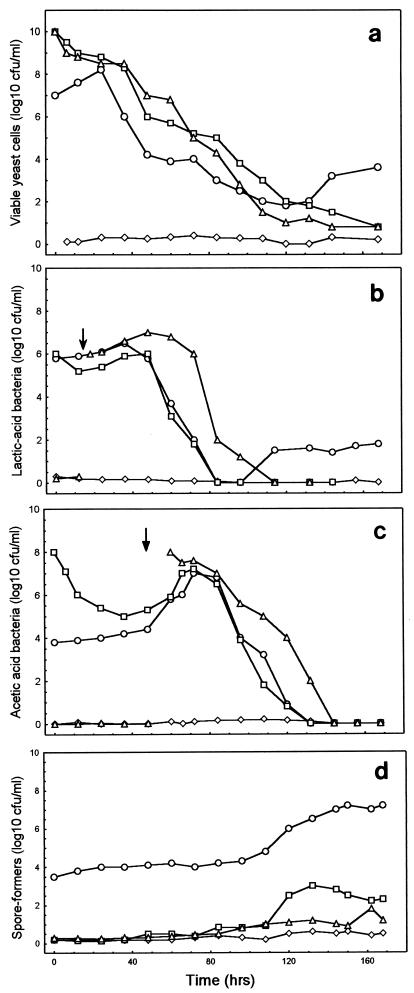
FIG. 1.
Cell densities in the fermentation boxes. Symbols: ○, box A; ◊, box B; □, box C; ▵, box D. (a) Yeasts. (b) Lactic acid bacteria. (c) Acetic acid bacteria. (d) Spore formers. The arrows indicate the times of inoculation of bacteria into box D.
TABLE 2.
Species isolated from box C
| Species | Frequency of reisolated species (%) at:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | 120 h | 144 h | 168 h | |
| S. cerevisiae var. chevalieria | 100 | 80 | 62 | 76 | 0 | 0 | 0 | 0 |
| S. cerevisiae | 0 | 20 | 33 | 16 | 38 | 40 | 40 | 10 |
| Candida rugopelliculosa | 0 | 0 | 5 | 8 | 18 | 27 | 40 | 40 |
| Candida bombi | 0 | 0 | 0 | 0 | 30 | 20 | 6 | 30 |
| Pichia fermentans | 0 | 0 | 0 | 0 | 14 | 13 | 14 | 20 |
| Total no. of yeasts reisolated | 35 | 35 | 21 | 25 | 16 | 15 | 15 | 10 |
| Lactobacillus plantaruma | 60 | 65 | 60 | 27 | 0 | 0 | 0 | 0 |
| Lactobacillus lactisa | 40 | 35 | 40 | 0 | 0 | 0 | 0 | 0 |
| Lactobacillus acidophilus | 0 | 0 | 0 | 73 | 0 | 0 | 0 | 0 |
| Total no. of lactobacilli reisolated | 35 | 33 | 27 | 15 | 0 | 0 | 0 | 0 |
| Acetobacter acetia | 50 | 50 | 40 | 40 | 0 | 0 | 0 | 0 |
| Gluconobacter oxydans subsp. suboxydansa | 50 | 50 | 60 | 20 | 0 | 0 | 0 | 0 |
| Gluconobacter oxydans-like | 0 | 0 | 0 | 40 | 100 | 0 | 0 | 0 |
| Total no. of acetic acid bacteria reisolated | 28 | 23 | 20 | 20 | 15 | 0 | 0 | 0 |
| Bacillus stearothermophilus | 0 | 0 | 0 | 0 | 0 | 47 | 56 | 77 |
| Bacillus subtilis | 0 | 0 | 0 | 0 | 0 | 53 | 44 | 23 |
| Total no. of bacilli isolated | 0 | 0 | 0 | 0 | 0 | 15 | 18 | 18 |
Organism included in the inoculum.
Some wild isolates made a significant impact on the final flora. The naturally predominant fermentative yeast S. cerevisiae accounted for one-third of the yeast population after 2 days but replaced Kluyveromyces marxianus only after the yeast population had declined by 6 orders of magnitude (Fig. 1a). Two Candida species and Pichia fermentans appeared, but only at the end of the fermentation. Kloeckera japonica similarly appeared in box D but was not among the 12 most common species found in natural fermentations (21). Lactobacillus acidophilus was the only different lactic acid bacterium that appeared in significant numbers after its period of physiological significance. In addition to the two inoculated acetic acid bacteria, a third type was identified. This organism had 80% of the characteristics of G. oxydans subsp. suboxydans but also had the ability to grow on and oxidize ethanol, which is a characteristic of A. aceti. Such a hybrid has been found previously in cider fermentations (11) and in cocoa fermentations (12), which perhaps represent special niches for this variety. This organism dominated the acetic acid bacterial population after 96 h.
Aseptic fermentation.
The uninoculated, low-contamination conditions used for box B were extremely successful, and microbial contamination and metabolic activity remained insignificant throughout the experiment. There was an increase in temperature of about 4°C and an increase in pH to 4.0 after 36 h, perhaps reflecting endogenous biochemical activity, but no further changes occurred. A total of 80% of the pulp was present at the end of the fermentation, and the beans showed no change in pigment content either inside or outside the seeds. The sugar concentration remained high throughout, and there were never more than 0.2 g of ethanol per liter, trace amounts of lactic acid, and 0.5 g of acetic acid per liter (data not shown). However, other organic acids, such as malic acid, succinic acid, and oxalic acid, were each produced at a level of about 1 g liter of pulp−1.
Yeasts.
In natural fermentations the yeast concentration starts at about 107 cells g of pulp−1 and peaks within 24 h after a 10-fold increase. The inoculated boxes were inoculated with 1010 cells g of pulp−1, but the concentration rapidly decreased 50-fold over a 24-h period until it reached a value similar to that in natural fermentations (Fig. 1a). Subsequent to this, the cell counts decreased in similar ways as usual, but only in the natural fermentation did the concentration increase at the end; this increase coincided with an increase in aeration. In natural fermentations, the increase is usually due to Candida and Kluyveromyces species, which were not present in the defined cocktail.
Filamentous fungi.
Small numbers of filamentous fungi were found in the cooler aerated parts of the surfaces in of boxes C and D, but none were present in the middle of the fermenting mass. No filamentous fungi were found in box B.
Lactic acid bacteria.
When lactic acid bacteria were present at the start of natural or inoculated fermentations, the results were identical; there was a small increase in concentration and then a dramatic decrease until the organisms were not detectable (Fig. 1b). When box D was inoculated with lactic acid bacteria at 24 h, essentially the same profile was obtained, but with a 24-h delay. The small, expected increases in the numbers of lactic acid bacteria that occur at the end of natural fermentations (21 data not shown) did not occur in the inoculated boxes, probably because the species found at the end of natural fermentations (Lactococcus spp.) were not present (not inoculated) in boxes B, C, and D.
Acetic acid bacteria.
The acetic acid bacterial culture inoculated at time zero exhibited a dramatic 3-order-of-magnitude decrease in viability over the first 36 h, probably due to low oxygen levels (anaerobic conditions) which eliminated the aerobic acetic acid bacteria (Fig. 2c). However, the cell densities were the same in all cultures (about 107 cells g of pulp−1) after 72 h, and the cell densities all decreased to zero over the next 72 h, although there was a 24-h delay in the disappearance of acetic acid bacteria from the culture inoculated with acetic acid bacteria at 48 h due to the excess acetic acid and high temperature.
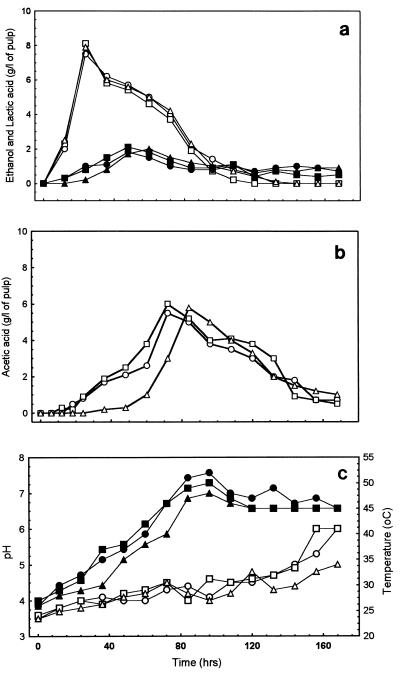
FIG. 2.
Concentrations of metabolites, pH, and temperature in the fermentation boxes. Symbols: ○ and •, box A; □ and ▪, box C; ▵ and ▴, box D. (a) Ethanol (open symbols) and lactic acid (solid symbols). (b) Acetic acid. (c) pH (open symbols) and temperature (solid symbols).
Spore formers.
The number of spore-forming bacteria steadily increased in box A in a typical manner (Fig. 1d). The numbers of spore-forming bacteria were very low in the other boxes, and the appearance of these organisms in boxes C and D was probably due to eventual contamination during the fermentation process. The Bacillus species found were the same as those found in natural fermentations (19).
Sugars, ethanol, lactic acid, and acetic acid.
As expected, in all three fermentations with yeasts present from time zero, all sugars disappeared rapidly, and there were no significant differences between boxes (data not shown). As a corollary, the ethanol profiles were identical in all respects when fermentation occurred (Fig. 2a). The concentration of lactic acid changed with the same profile in all three boxes, except that there was an approximately 12-h delay in the appearance and disappearance of lactic acid in the culture inoculated with lactic acid bacteria at 24 h (Fig. 2a). The acetic acid profiles for boxes A and C were almost identical throughout (Fig. 2b). Adding acetic acid bacteria after 48 h resulted in a delay in the appearance of acetic acid, but the peak amount and the kinetics of disappearance were similar to those observed under the other conditions studied.
Temperature and pH.
There were no major differences in either the temperature or pH profiles between boxes A and C, particularly during the crucial early stages of the fermentation (Fig. 2c). Box D was similar, except that the increase in temperature was both delayed and reduced in magnitude and the pH remained significantly lower at the end.
Quality of fermented beans.
The cut test revealed significant differences in the outcomes of the fermentations (Table 3). Natural fermentations (box A) take 6 to 7 days until the changes in the beans are complete. The fermentation in box C did not differ significantly in speed or efficiency. However, the fermentation obtained after phased inoculation (box D) was significantly inferior since only 90% of the beans were fully fermented after 7 days. Samples from box B revealed a high percentage of slaty beans, which indicated that the beans were dried without undergoing fermentation. No suitable changes occurred in box B.
TABLE 3.
Cut test performed with 300 beans
| Box | Category | % after fermentation for:
|
||
|---|---|---|---|---|
| 120 h | 144 h | 168 h | ||
| A | Fully fermented | 74 | 95 | 98 |
| Others | 26 | 5 | 2 | |
| B | Fully fermented | 0 | 0 | 0 |
| Slaty | 30 | 45 | 50 | |
| Germinated | 70 | 55 | 40 | |
| Purple | 0 | 0 | 10 | |
| C | Fully fermented | 70 | 93 | 97 |
| Others | 30 | 7 | 3 | |
| D | Fully fermented | 65 | 80 | 90 |
| Brown and purple | 29 | 16 | 10 | |
| Purple | 6 | 4 | 0 | |
Sensorial analysis.
The odor of the pulp during the fermentation changed from yeastlike (at 24 h) to alcoholic to vinegary to maltlike at the end. Sensorial analysis (Table 4) is complex since some of the characteristics should be strong (cocoa flavor), some should be weakly present (such as acidity, bitterness, and fruitiness), and some should be absent (astringency and off-flavors). The results obtained showed that the chocolates prepared from boxes A, C, and D differed little in flavor and were all satisfactory. However, in order of preference, the tasters preferred cocoa fermented for 7 days in box A, for 6 days in box C, for 7 days in box D, and for 7 days in box C.
TABLE 4.
Quality of chocolatea
| Box | Time (h) | Scores for the following characteristics:
|
Overall score | |||||
|---|---|---|---|---|---|---|---|---|
| Cocoa flavor | Fruitiness | Desired semibitter flavor | Acidity | Astringency | Off-flavors | |||
| A | 120 | 5 | 2 | 5 | 2 | 5 | 9 | 28 |
| 144 | 7 | 3 | 5 | 5 | 7 | 9 | 36 | |
| 168 | 8 | 4 | 7 | 6 | 9 | 8 | 43 | |
| B | 120 | 1 | 1 | 1 | 9 | 1 | 2 | 15 |
| 144 | 1 | 1 | 1 | 9 | 1 | 2 | 15 | |
| 168 | 1 | 1 | 1 | 9 | 1 | 2 | 15 | |
| C | 120 | 5 | 2 | 3 | 6 | 8 | 8 | 32 |
| 144 | 8 | 4 | 7 | 8 | 9 | 6 | 43 | |
| 168 | 8 | 4 | 7 | 8 | 9 | 5 | 41 | |
| D | 120 | 5 | 5 | 5 | 5 | 5 | 7 | 33 |
| 144 | 7 | 5 | 6 | 7 | 9 | 5 | 41 | |
| 168 | 7 | 5 | 7 | 8 | 9 | 5 | 41 | |
The positive characteristics are cocoa flavor, fruitiness, and desired semibitter flavor, which are measured on a scale from 9 (very desirable) to 1 (undesirable). In the case of desired semibitter flavor a value of 7 is optimal. The negative characteristics are acidity, astringency, and off-flavors, which are measured on a scale from 9 (not detectable) to 1 (very pronounced). Higher scores indicate higher overall quality.
DISCUSSION
Extrapolating the conclusions drawn from the experiments performed in this study to the commercial situation is justifiable since there are three pieces of evidence that support the contention that the events in 200-kg boxes mimic the events in full-size, 800-kg fermentation boxes. First, the fermentation profiles were similar with respect to speed, microbial succession, and yield of metabolites. Second, the species isolated were similar. Third, the qualities of the final products were identical. The sensorial analysis (Table 4) also showed that not only is a full 7-day fermentation desirable, but also toward the end of the fermentation the flavor qualities are either stable or improving.
With simple hygiene precautions it was clearly possible to create effectively sterile conditions (box B). The microbiological and physical conditions precluded entry of contaminants at the onset of the fermentation. This shows that the microbial activity in the inoculated boxes was initiated by the inoculum and, furthermore, that the new species that appeared in box C (and in box D) were an indirect result of this microbial activity. With boxes C and D the two most important observations were (i) that the inoculated species remained the dominant flora during the period when they were playing their major physiological roles and (ii) that clearly identifiable contaminant species present at the end of the fermentation were not detectable or were present in very low amounts immediately after the stage at which they were physiologically important. Since no molecular characterization was attempted, it was not possible to rule out the possibility that the isolates of the inoculated species present at the end were in fact not clonal derivatives of the initial inoculum. However, this seems unlikely since the same inoculated species were found at the same frequency at the end of all four successful experiments (data not shown) and for different strains to be the dominant organisms wild strains would have to outcompete sibling resident strains. Since wild isolates (rather than attenuated culture collection strains) were the source of the inoculum, there seems to be no obvious reason why this should occur. Notwithstanding this argument, even if intraspecific replacement did occur, it does not alter the conclusion of the experiment, namely, that the selected species are both necessary and sufficient to mimic natural fermentations. This suggests that the choice of microorganisms was soundly based. One clear advantage of a defined inoculum is that there is not natural contamination with spoilage organisms, such as spore formers, which become abundant in natural fermentations but which remain insignificant in defined inocula. The product is therefore more stable at the end of the fermentation.
All of the changes in box C were very similar to those in box A, which shows that it is possible to mimic the natural fermentation with a defined cocktail. This result implies that the principal features of the fermentation are understood and that only a few members of each physiological group are needed. However, there were some small but significant differences in the results of the organoleptic evaluation (Table 4). In box C the collection of attributes was best after only 144 h, which is 1 day less than the amount of time that it took for a natural fermentation and could be beneficial in increasing throughput. Higher productivity can be achieved provided that the harvest time is accurate since when the fermentations were allowed to continue for an additional 24 h, the quality deteriorated because of a slight increase in off-flavors. This is perhaps surprising since the number of spore formers was far less than the number of spore formers in box A. It was apparent that the phased inoculation of microorganisms in box D led to a slower and less robust fermentation with a lower pH at the end. However, the net effect on organoleptic qualities was much less marked, and there was little difference among boxes A, C, and D. The best aggregate scores were all 43 or 41, but this is misleading since all parameters are not equally prized, for example, fruitiness and lack of off-flavors. The results suggested that the phased inoculation of box D did not improve or accelerate the fermentation process compared to box C and the appearance of the beans (a commercially sensitive attribute) was not as good. Phased additions are neither necessary nor beneficial.
There may still be room for improvement in the choice of species when this technology is used commercially. Independent studies on the pectinolytic enzyme endopolygalacturonase (22), which is produced by Kluyveromyces marxianus, a yeast isolated from spontaneous cocoa fermentations (21), suggest that this yeast would be a better source of pectinase. A combination of Kluyveromyces marxianus and the naturally vigorous organism S. cerevisiae might be a better choice of yeasts for future defined inocula for use on the farm. It is also not clear why the yeast inoculum lost viability during the first 36 h. This also occurred at lower concentrations and in the presence of the natural microflora (data not shown). The loss of viability could have been due to how the cells were propagated, but this seems unlikely since cocoa pulp itself was one of the components of the medium. The amounts of lactic acid bacteria and acetic acid bacteria could perhaps be lower than the amounts used since lower amounts might reduce the amount of acidity and also speed up the fermentation, and only a single lactic acid bacterial species might be necessary.
How easily can such technology be delivered to farmers? First, there is the question of removing the natural flora. Pod washing is now done as a matter of routine by farmers in Bahia (Brazil) in order to reduce contamination of fresh cocoa juice, which, economically, is now more valuable to them than chocolate. Decontamination of utensils is relatively straightforward, but cleaning fermentation boxes would require extra effort and cost and this may not be practicable; therefore, the natural contamination would be greater under such production conditions. Second, there is the question of the inoculum. Two strategies are possible. One involves producing the consortium of yeasts and bacteria as a stable, dried product similar to the product available for baking bread. A large inoculum would be needed, and this could represent a significant expense for a farmer. However, this cost would be greatly reduced if after initial inoculation, the same species were used to reinfect subsequent fermentations without additional starter cultures. The alternative strategy would be to use a smaller inoculum to initiate a starter culture, which could then be used in turn to inoculate the main fermentation. The medium for this would have to be TYKCCC medium.
There have been two previous attempts to use starter cultures for cocoa fermentations (15, 16). However, in both studies no attempt was made to remove the natural microflora, nonfermentative yeasts or yeasts from culture collections were tried (with no success), no bacteria were added or studied, and there was no evidence that the fermentation could be improved or accelerated with these approaches. Not surprisingly, only when additional supplies of the naturally predominant yeast were added did good fermentation occur.
The alcoholic beverage industry has replaced traditional natural fermentations with defined inocula, high-quality raw materials, strict control of the fermentation, better treatment of the final product, and diversification of the market. Cocoa fermentations have a long way to go before they reach that stage of development, but this is the first report of the use of a successful defined starter culture in such a complex fermentation. By setting the initial conditions, defined inocula can now be considered one of the ways which should lead to chocolate of more reliable and better quality.
ACKNOWLEDGMENTS
I thank the technical staff of SETEA at CEPLAC/CEPEC. I also thank Alan Wheals (University of Bath, Bath, United Kingdom) for help with the manuscript.
REFERENCES
- 1.Carr J G, Davies P A, Dougan J. Report by University of Bristol Research Station, Long Ashton, Bristol, and Tropical Products Institute, Gray’s Inn Road, London. United Kingdom: University of Bristol; 1979. Cocoa fermentation in Ghana and Malaysia. II. [Google Scholar]
- 2.Clapperton J F, Lockwood G, Yow S T K, Lim D H K. Effects of planting materials on flavour. Cocoa Grower’s Bull. 1994;48:47–63. [Google Scholar]
- 3.del Boca C. Coca beans: quality requirements and methods of assessment. Rev Int Chocolaterie. 1962;17:218–221. [Google Scholar]
- 4.Forsyth W G C, Quesnel V C. Mechanisms of cocoa curing. Adv Enzymol. 1963;25:457–492. doi: 10.1002/9780470122709.ch10. [DOI] [PubMed] [Google Scholar]
- 5.Kreger-van Rij N J W, editor. The yeasts: a taxonomic study. 3rd ed. Amsterdam, The Netherlands: Elsevier; 1984. [Google Scholar]
- 6.Lehrian D W, Patterson G R. Cocoa fermentation. In: Reed G, editor. Biotechnology, a comprehensive treatise. Vol. 5. Basel, Switzerland: Verlag Chemie; 1983. pp. 529–575. [Google Scholar]
- 7.Lopez A. Fermentation and organoleptic quality of cacao as affected by partial removal of pulp juices from the beans prior to curing. Rev Theobroma. 1979;9:25–37. [Google Scholar]
- 8.Lopez A, McDonald C R. A definition of descriptors to be used for the qualification of chocolate flavour in organoleptic testing. Rev Theobroma. 1981;11:209–212. [Google Scholar]
- 9.Miller G L. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Biochem. 1959;31:426–428. [Google Scholar]
- 10.Ostovar K, Keeney P G. Isolation and characterization of microorganisms involved in the fermentation of Trinidad’s cacao beans. J Food Sci. 1973;38:611–617. [Google Scholar]
- 11.Passmore S M, Carr J G. The ecology of acetic acid bacteria with particular reference to cider manufacture. J Appl Bacteriol. 1975;38:151–158. [Google Scholar]
- 12.Passos F M L, Passos F J V. Descrição e classificação de bactérias isoladas da fermentação do cacau, com base em um análise numérica. Rev Microbiol. 1985;16:290–298. [Google Scholar]
- 13.Passos F M L, Silva D O, Lopez A, Ferreira C L L F, Guimarães W V. Characterization and distribution of lactic acid bacteria from traditional cocoa bean fermentations in Bahia. J Food Sci. 1984;49:205–208. [Google Scholar]
- 14.Roelofsen P A. Fermentation, drying, and storage of cocoa beans. Adv Food Res. 1958;8:225–296. [Google Scholar]
- 15.Samah O A, Ptih M F, Selamat J. Biochemical changes during fermentation of cocoa beans inoculated with Saccharomyces cerevisiae (wild strain) J Food Sci Technol. 1992;29:341–343. [Google Scholar]
- 16.Sanchez J, Daguenet G, Guiraud J P, Vincent J C, Galzy P. A study of the yeast flora and the effect of pure culture seeding during the fermentation of cocoa beans. Lebensm Wiss Technol. 1985;18:69–76. [Google Scholar]
- 17.Schwan, R. F. Microbiology of cocoa fermentation: a study to improve quality. In Proceedings of the 12th International Cocoa-Research Conference, Salvador, Bahia, November 1996. Cocoa Producers’ Alliance, Lagos, Nigeria.
- 18.Schwan R F, Souza S M M. Informe de pesquisas. Itabuna, Bahia, Brazil: Comissáo Executiva do Plano da Lavoura Cacavieira/Cocoa Research Centre; 1986. Quantificação para cromatografia liquida (HPLC) de álcool etilico, ácido lactico e acético produzido na fermentação tradicional de cacau; pp. 65–68. [Google Scholar]
- 19.Schwan R F, Vanetti M C D, Silva D O, Lopez A, de Moraes C A. Characterization and distribution of aerobic, spore-forming bacteria from cacao fermentations in Bahia. J Food Sci. 1986;51:1583–1584. [Google Scholar]
- 20.Schwan R F, Lopez A, Silva D O, Vanetti M C D. Influência da frequência e intervalos de revolvimentos sobre a fermentaçao de cacau e qualidade do chocolate. Rev Agrotrop. 1990;2:22–31. [Google Scholar]
- 21.Schwan R F, Rose A H, Board R G. Microbial fermentation of cocoa beans, with emphasis on enzymatic degradation of the pulp. J Appl Bacteriol Symp Suppl. 1995;79:96S–107S. [Google Scholar]
- 22.Schwan R F, Cooper R M, Wheals A E. Endopolygalacturonase secretion by Kluyveromyces marxianus and other cocoa pulp-degrading yeasts. Enzyme Microb Technol. 1997;21:234–244. [Google Scholar]
- 23.Shamsuddin S B, Dimick P S. Qualitative and quantitative measurements of cacao bean fermentation. In: Dimick P S, editor. Proceedings of cocoa biotechnology. University Park, Pa: Department of Food Science, Penn State University; 1986. pp. 55–78. [Google Scholar]