ABSTRACT
Mucosal vaccines and vaccines that block pathogen transmission are under-appreciated in vaccine development. However, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has shown that blocking viral transmission is an important attribute of efficient vaccines. Here, we investigated if recombinant influenza virus neuraminidase (NA) vaccines delivered at a mucosal site could protect from onward transmission of influenza B viruses in the guinea pig model. We tested four different scenarios in which sequential transmission was investigated in chains of three to four guinea pigs. The variables tested included a low and a high viral inoculum (104 vs 105 plaque-forming units) in the initial donor guinea pig and variation of exposure/cohousing time (1 day vs 6 days). In three out of four scenarios—low inoculum-long exposure, low inoculum-short exposure, and high inoculum-short exposure—transmission chains were efficiently blocked. Based on this data, we believe an intranasal recombinant NA vaccine could be used to efficiently curtail influenza virus spread in the human population during influenza epidemics.
IMPORTANCE
Vaccines that can slow respiratory virus transmission in the population are urgently needed for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza virus. Here, we describe how a recombinant neuraminidase-based influenza virus vaccine reduces transmission in vaccinated guinea pigs in an exposure intensity-based manner.
KEYWORDS: influenza virus, neuraminidase, transmission, mucosal immunity
INTRODUCTION
The current severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has highlighted how important it is that vaccines not only protect from disease but also limit onward transmission of pathogens. Similar to injected SARS-CoV-2 vaccines, influenza virus vaccines—even if well matched—often allow onward transmission of virus in vaccinated populations (1). The intramuscular administration of current SARS-CoV-2 vaccines as well as inactivated influenza virus vaccines contributes to this problem since this route of administration does not lead to robust mucosal antibody titers which could block infection or limit transmission (2 – 4).
Influenza virus vaccines typically induce an immune response focused on the viral hemagglutinin (HA), the receptor binding protein of influenza viruses which binds to terminal sialic acid on N-linked glycans on host cells. Antibodies to HA can neutralize virus efficiently and block infection. However, the location of the vaccine-induced antibodies in combination with the constant changes of the HA through antigenic drift often leads to suboptimal immunity after vaccination. Besides HA, influenza viruses express a second surface glycoprotein, the neuraminidase (NA), which is a receptor-destroying enzyme that cleaves terminal sialic acids from N-linked glycans (5 – 7). This activity is important for migration of incoming virus through mucosal fluids (8, 9). Mucosal fluids have high concentrations of glycosylated natural defense proteins, which can act as a virus trap and this is counteracted by NA. In addition, newly formed viral particles stick to the surface of infected cells and aggregate. The presence of NA enzymatic activity releases cell surface-bound virus and counters virus aggregation (10).
While both HA and NA proteins undergo antigenic drift, their drift is usually discordant and NA potentially evolves more slowly (11, 12). This, combined with its important function in the viral life cycle, makes it an attractive vaccine target. We and others have shown that vaccination with recombinant, stabilized NA protein can induce protective immunity in different animal models, especially when the antigen is given mucosally (13 – 21). Of note, this protection is typically against morbidity and mortality, and while viral replication in animal models is reduced, NA-based immunity is often infection permissive. Here, we use the well-established guinea pig influenza virus transmission model (22) to determine if vaccination with recombinant influenza B virus neuraminidase can break viral transmission chains and which factors may influence the efficiency of transmission in the background of mucosal NA immunity.
RESULTS
Intranasal vaccination with B/Malaysia/2506/2004 NA limits transmission between co-caged guinea pigs, although this is inoculation titer-dependent
Our previous work found that transmission from naïve B/Malaysia/2506/2004-infected donors to B/Malaysia/2506/2004 NA-vaccinated recipients in a contact transmission setting results in transmission to three of three vaccinated recipients—meaning in that setting, transmission was not prevented (23). However, we noted in this work that these vaccinated guinea pigs had very low nasal wash titers and a short duration of shedding. Here, we wanted to determine if these infected, but vaccinated guinea pigs, could allow subsequent infection ( Fig. 1).
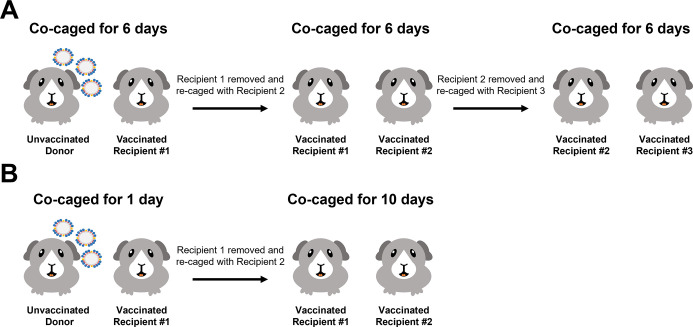
Fig 1.
Schematic depicting the transmission settings used in these studies. The experimental setup for the 6-day contact transmission setting (A) and 1-day contact transmission setting (B).
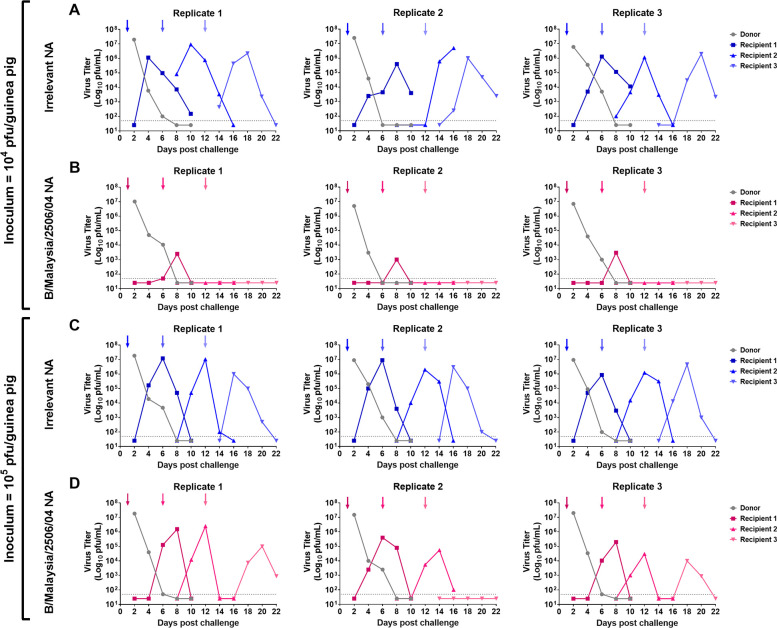
We initially infected naïve donor guinea pigs with 104 plaque-forming units (PFU) of B/Malaysia/2506/2004 virus. The following day, donor guinea pigs were co-caged with A/Michigan/45/2015 N1- (negative control group, Fig. 2A) or B/Malaysia/2506/2004 NA- (Fig. 2B) vaccinated guinea pigs (recipient 1). On day 6 following the initial donor infection, recipient 1 guinea pigs were co-caged with another set of vaccinated guinea pigs (recipient 2). On day 12 following the initial donor infection, recipient 2 guinea pigs were co-caged with another set of vaccinated guinea pigs (recipient 3). The experimental design is shown in Figure 1A. We assessed virus titers in the nasal washes at days 2, 4, 6, 8, and 10 post initial contact in all cases. Virus titers in the nasal wash indicate that virus was transmitted to each recipient in the irrelevant NA-vaccinated guinea pigs but virus transmission did not progress past recipient 1 in the B/Malaysia/2506/2004 NA-vaccinated guinea pigs.
Fig 2.
Assessment of B/Malaysia/2506/2004 transmission between vaccinated guinea pigs in a 6-day contact transmission setting. Naïve donor guinea pigs were anaesthetized and challenged with 104 (A and B) or 105 (C and D) PFU of B/Malaysia/2506/2004. The following day, donor and vaccinated recipient (recipient 1) transmission pairs were co-caged (contact transmission). On day 6 post initial donor challenge, the recipient guinea pig (recipient 1) was removed and rehoused with another vaccinated recipient guinea pig (recipient 2). Recipient 2 was re-homed again on day 12 post initial donor challenge with vaccinated recipient 3. On days 2, 4, 6, 8, and 10 post contact, nasal washes were collected from anaesthetized donor (gray) and recipient (non-gray) guinea pigs. Arrows depict the addition of a recipient and the removal of a donor/recipient. The experiment was repeated three times with each replicate containing an unvaccinated donor and recipient 1, recipient 2, and recipient 3.
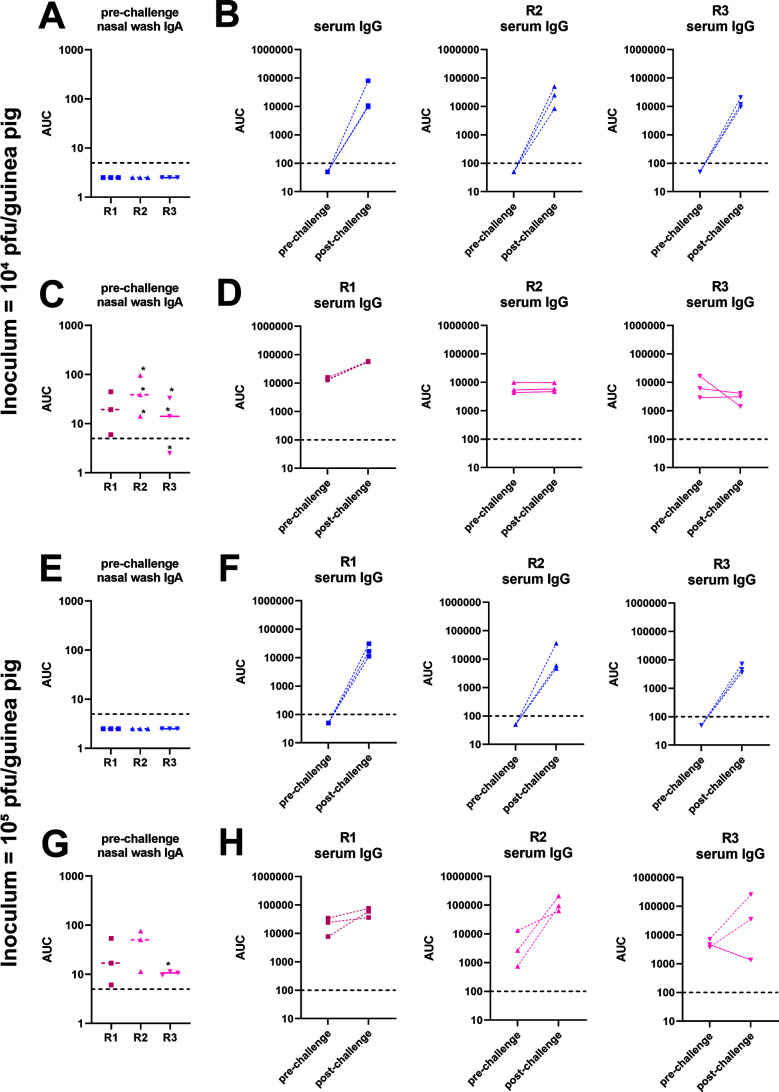
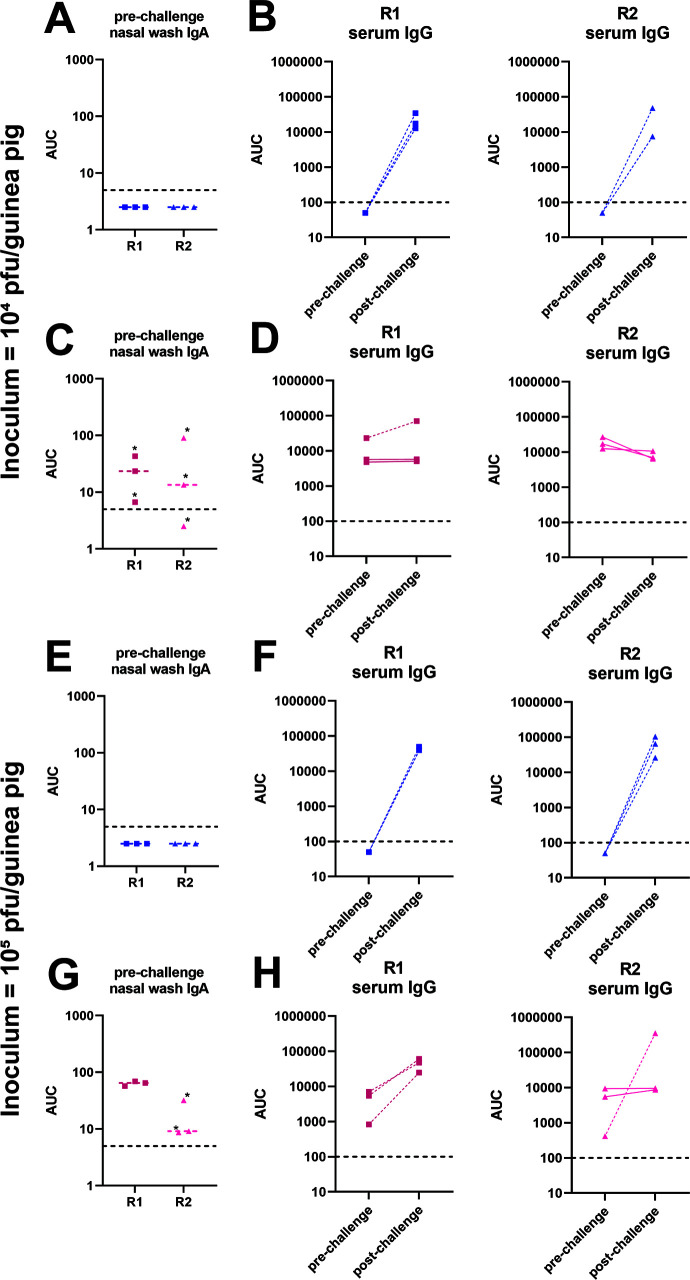
We next wanted to determine if increasing the inoculum titer would result in more efficient subsequent infection. Here, we infected naïve donor guinea pigs with 105 PFU of B/Malaysia/2506/2004 virus and performed recipient co-caging and nasal washes as described above. We found that, like above, virus transmitted to all of the irrelevant NA-vaccinated guinea pigs (Fig. 2C). Interestingly, we observed that virus transmitted from recipient 1 to recipient 2 in all of the B/Malaysia/2506/2004 NA replicates and virus transmitted from recipient 2 to recipient 3 in 2 out 3 B/Malaysia/2506/2004 NA replicates. We did not observe a strong correlation of nasal wash IgA titers to the virus with breakthrough infection in the vaccinated animals (Fig. 3A, C, E and G). In addition, infection as detected by virus shedding agreed well with serological data pre- and post-infection (Fig. 3B, D, F and H).
Fig 3.
Serology for 6day contact transmission setting shown in Fig. 2. Pre-challenge nasal wash IgA ELISA titers against purified B/Malaysia/2506/2004 virus preparations of animals from the 104 (A and C) and 105 (E and G) PFU challenge experiments. Stars indicate animals in which transmission did not occur. Limit of detection was 5, negatives are shown as 50% of the limit of detection, and the limit of detection is indicated by a horizontal dashed line. Pre- and post-challenge serum IgG titers against purified B/Malaysia/2506/2004 virus preparations of animals from the 104 (B and D) and 105 (F and H) PFU challenge experiments. Full lines indicate animals in which transmission did not occur; dashed lines indicate animals in which transmission did occur. Limit of detection was 100, negatives are shown as 50% of the limit of detection, and the limit of detection is indicated by a horizontal dashed line. R1, recipient 1; R2, recipient 2; R3, recipient 3; AUC, area under the curve.
These data suggest that vaccination with B/Malaysia/2506/2004 NA is infection permissive, but subsequent transmission from NA-vaccinated guinea pigs to other NA-vaccinated guinea pigs can be blocked in a virus inoculum-dependent manner.
Inhibition of transmission in recombinant NA-vaccinated guinea pigs is dependent on the length of exposure to infected donor animals
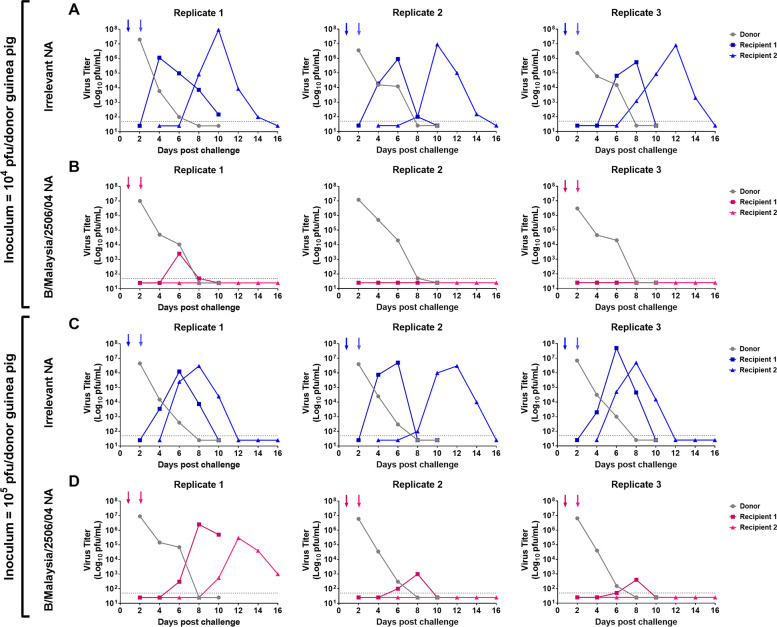
After determining that B/Malaysia/2506/2004 NA-vaccinated guinea pigs are susceptible to infection when exposed to infected guinea pigs for 6 days, we wanted to learn if B/Malaysia/2506/2004 NA-vaccinated guinea pigs would be susceptible if exposure time is limited in duration (24). In these next experiments (schematic shown in Figure 1B), we infected naïve donor guinea pigs with 104 (Fig. 4A and B) or 105 (Fig. 4C and D) PFU of B/Malaysia/2506/2004 virus. The following day, donor guinea pigs were co-caged with A/Michigan/45/2015 N1- (negative control group, Fig. 4A or Fig. 4 C) or B/Malaysia/2506/2004 NA- (Fig. 4B or Fig. 4 D) vaccinated guinea pigs (recipient 1). On day 2 following the initial donor infection, recipient 1 guinea pigs were co-caged with vaccinated guinea pigs (recipient 2) for the remainder of the experiment. We assessed virus titers in the nasal washes at days 2, 4, 6, 8, and 10 post donor infection in donors and recipient 1 and at days 4, 6, 8, 10, 12, 14, and 16 post donor infection for recipient 2 guinea pigs.
Fig 4.
Assessment of B/Malaysia/2506/2004 transmission between vaccinated guinea pigs in a 1-day contact transmission setting. Naïve donor guinea pigs were anaesthetized and challenged with 104 (A and B) or 105 (C and D) PFU of B/Malaysia/2506/2004. The following day, donor and vaccinated recipient transmission pairs were co-caged. On the subsequent day, vaccinated recipient guinea pigs were removed and re-housed with another vaccinated recipient guinea pig. On days 2, 4, 6, 8, and 10 post contact, nasal washes were collected from anaesthetized donor (gray) and recipient (non-gray) guinea pigs. Recipient 2 guinea pigs received additional nasal washes on days 12 and 14 post contact. Arrows depict the addition of a recipient and the removal of a donor/recipient. The experiment was repeated 3 times with each replicate containing an unvaccinated donor and recipient 1 and recipient 2.
In experiments where naïve donor guinea pigs were infected with 104 PFU of B/Malaysia/2506/2004, virus titer data indicate that virus was transmitted from the naïve donor to recipient 1 then on to recipient 2 in the irrelevant NA-vaccinated guinea pigs in all 3 replicates (Fig. 4A). In the B/Malaysia/2506/2004 NA-vaccinated guinea pigs, virus was transmitted from the naïve donor to recipient 1 in only one out of three replicates (Fig. 4B) and recipient 2 guinea pigs remained uninfected in all replicates.
In studies where naïve donor guinea pigs were infected with 105 PFU of B/Malaysia/2506/2004 virus, virus titer data indicate that virus was transmitted from the naïve donor to recipient 1 then on to recipient 2 in the irrelevant NA-vaccinated guinea pigs in all three replicates (Fig. 4C). In the B/Malaysia/2506/2004 NA-vaccinated guinea pigs, virus transmitted from the naïve donor to recipient 1 in all three replicates (Fig. 4B) and from recipient 1 to recipient 2 in one out of three replicates. As in the initial experiment, we did not find a strong correlation between nasal wash IgA titers to the virus and protection or breakthrough in vaccinated animals (Fig. 5A, C, E and G) and pre- and post-challenge serology agreed with virological detection of infection (Fig. 5B, D, F and H).
Fig 5.
Serology for 1-day contact transmission setting shown in Fig. 4. Pre-challenge nasal wash IgA ELISA titers against purified B/Malaysia/2506/2004 virus preparations of animals from the 104 (A and C) and 105 (E and G) PFU challenge experiments. Stars indicate animals in which transmission did not occur. Limit of detection was 5, negatives are shown as 50% of the limit of detection, and the limit of detection is indicated by a horizontal dashed line. Pre- and post-challenge serum IgG titers against purified B/Malaysia/2506/2004 virus preparations of animals from the 104 (B and D) and 105 (F and H) PFU challenge experiments. Full lines indicate animals in which transmission did not occur; dashed lines indicate animals in which transmission did occur. Limit of detection was 100, negatives are shown as 50% of the limit of detection, and the limit of detection is indicated by a horizontal dashed line. R1, recipient 1; R2, recipient 2; AUC, area under the curve.
These data suggest that vaccination with B/Malaysia/2506/2004 NA, alongside relatively limited exposure to infected donors, resulted in reduced transmission to B/Malaysia/2506/2004 NA-vaccinated guinea pigs.
DISCUSSION
Optimal vaccines serve two important purposes. They should protect the vaccinated individual from disease and they should protect others—including immunocompromised or naïve individuals—from onward transmission. While the second purpose was well recognized in the vaccinology and public health community, it has become part of public discourse during the SARS-CoV-2 pandemic (25). Several licensed vaccines fulfill both purposes. However, especially for respiratory viruses, blocking transmission through vaccination is often challenging as demonstrated with SARS-CoV-2 but also influenza virus (1, 25). Part of the problem is that many vaccines are administered intramuscularly which makes them very inefficient in inducing mucosal immune responses (2 – 4). However, mucosal immune responses can block infection completely (sterilizing immunity) depending on the vaccine target, and they can blunt transmission by reducing titers and/or potentially by producing pathogen that is already coated in antibody when it leaves the upper respiratory tract and therefore perhaps reduce the infectiousness of an infected subject.
In the past, we have shown that intranasal vaccination of guinea pigs, which are an excellent model for influenza virus transmission (while they do not show symptoms of disease), with recombinant NA can block viral transmission (18). However, this depended on the setting, and efficacy was higher in an “aerosol” transmission setting in which animals were separated by perforated barriers as compared with a cohoused setting which allowed for direct contact. Interestingly, vaccinated guinea pigs, while supporting virus replication when directly infected, did not pass virus on to naïve animals (18). Vice versa, vaccinated guinea pigs exposed to naïve infected guinea pigs did get infected but experienced lower virus replication. Here, we wanted to investigate if NA vaccination could block transmission chains in a setting that supports transmission very well: directly cohousing vaccinated recipient animals with naïve infected donor animals. In this setting, we wanted to explore two variables: does virus dose of inoculation matter when initially infecting the donor guinea pig? And does the time donors and recipients are co-housed have an impact on transmission? We found that mucosal vaccination with recombinant NA can efficiently break transmission chains but this depends on the “intensity” of exposure. When donor animals were inoculated with a lower dose of virus and cohoused for a long period of time (6 days) with vaccinated recipients, transmission to recipients occurred but only low viral titers were measured and virus was not further transmitted. If the initial viral inoculum was increased by one log, transmission chains were only broken in one out of three replicates. If the same experiment was performed with a short cohousing period (24 hours), transmission chains were blocked efficiently with low and high inocula; at the low inoculum dose, even transmission to the first recipient was blocked in two out of three replicates. These different scenarios may be similar to situations that humans experience during the influenza season as well. The short exposure experiment may resemble short contacts with infected individuals, e.g., in public transport, during a dinner, or at work. The long exposure is perhaps akin to exposure to infected family members within a household. The low and high inocula perhaps resemble close contact without a mask versus less close contact or masking. Irrespectively, in three out of four scenarios, mucosal immunity to NA was able to break transmission chains and similar immunity in the human population may restrict influenza virus circulation during the influenza season to a large degree. An interesting finding was that the inoculation dose did not change the titer in the initially infected naïve donor animals. However, it did change the frequency of breakthrough infections in transmission chains of vaccinated animals. We do not have a good explanation for this phenomenon but would speculate that there is a qualitative difference in the shed virus or the way it is shed. Additional sampling (e.g., air sampling and environmental sampling) may provide insight into this question in the future. In addition, we also measured mucosal immune responses to determine if there was a correlation with protection or breakthrough infection. We did not find a correlation, but a larger experiment is likely needed to determine correlates of protection. Of note, this study was an exploratory study since we were uncertain of what we would find in terms of transmission/block of transmission. The study was therefore not powered for statistical analysis. However, it provides information for power calculations for follow-up studies. Furthermore, we cannot exclude that recombinant HA would have the same effect. Indeed, it is likely that a recombinant HA vaccine administered the same way would perform well. However, antigenic drift may affect HA more than NA and we therefore think, based on the data presented here and a large number of studies by us and others that show benefits of NA-based immunity, further (clinical) development of NA-based mucosal vaccines is warranted (17, 20).
MATERIALS AND METHODS
Viruses and cells
Sf9 cells (CRL-1711, ATCC) for baculovirus rescue were grown in Trichoplusia ni medium-formulation Hink insect cell medium (TNM-FH, Gemini Bioproducts) supplemented with 10% fetal bovine serum (FBS; Sigma) and penicillin (100 U/mL)-streptomycin (100 µg/mL) solution (Gibco). BTI-TN-5B1-4 (High Five, ATCC) cells for protein expression were grown in serum-free Express Five SFM media (Gibco) supplemented with penicillin (100 U/mL)-streptomycin (100 µg/mL) solution. Madin Darby canine kidney (MDCK, ATCC) cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10% FBS and penicillin (100 U/mL)-streptomycin (100 µg/mL) solution. B/Malaysia/2506/04 virus was grown in 10-day-old embryonated chicken eggs (Charles River) for 72 hours at 33°C. Eggs were then cooled overnight at 4°C before harvesting the allantoic fluid. Harvested allantoic fluid was centrifuged at 4,000 × g for 10 min at 4°C to pellet debris. Viruses were then aliquoted and stored at −80°C prior to determining stock titers via plaque assay.
Protein production
Recombinant NAs from A/Michigan/45/15 (H1N1) or B/Malaysia/2506/04 virus were expressed in High Five insect cells as a fusion protein with an N-terminal vasodilator-stimulated phosphoprotein (VASP) tetramerization domain (26) and the globular head domain of the NA. Proteins were purified from the cell culture supernatant via Ni2+-nitrilotriacetic acid (Ni-NTA) chromatography (27, 28).
Guinea pig vaccination
All animal experiments were conducted in concordance with protocols approved by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee. Five- to 6-week-old female guinea pigs were purchased from Charles River Laboratory and randomly assigned to different vaccination groups. Guinea pigs were primed intranasally (I.N.) with 10 µg of A/Michigan/45/15 (N1) or B/Malaysia/2506/04 NA adjuvanted with 10 µg of poly(I⋅C) (InvivoGen). Four weeks after the prime, a boost via the I.N. route with 10 µg of poly(I⋅C)-adjuvanted recombinant protein was administered. At 4 weeks post boost, vaccinated guinea pigs were used in transmission studies.
Transmission experiments
Co-caged guinea pig transmission experiments were performed as previously described (23). For transmission studies where guinea pigs were co-caged with initial donors for 6 days (Fig. 1A), naïve donor guinea pigs were anaesthetized with ketamine (30 mg/kg) and xylazine (5 mg/kg) before being challenged I.N. with 104 or 105 plaque-forming units of B/Malaysia/2506/04 in 300 µL of phosphate-buffered saline (PBS). The following day, donor and vaccinated recipient (recipient 1) transmission pairs were co-caged (contact transmission). On day 6 post initial donor challenge, the recipient guinea pig (recipient 1) was removed and rehoused with another vaccinated recipient guinea pig (recipient 2). Recipient 2 was re-homed again on day 12 post initial donor challenge with vaccinated recipient 3. On days 2, 4, 6, 8, and 10 post contact, nasal washes were collected from anaesthetized donor and recipient guinea pigs. Recipient 2 guinea pigs received additional nasal washes on days 12 and 14 post contact.
For transmission studies where guinea pigs were co-caged with initial donors for 1 day (Fig. 1B), naïve donor guinea pigs were anaesthetized and challenged as described above. The following day, donor and vaccinated recipient transmission pairs were co-caged (contact transmission). On the subsequent day, vaccinated recipient guinea pigs were removed and re-housed with another vaccinated recipient guinea pig. On days 2, 4, 6, 8, and 10 post contact, nasal washes were collected from anaesthetized donor and recipient guinea pigs. Recipient guinea pig 2 received two additional nasal washes on days 12 and 14 post contact.
Plaque assays
Virus titers were determined by plaque assay on MDCK cell monolayers. Virus stocks and nasal washes were diluted 10-fold in 1× minimum essential medium (MEM) [10% 10× minimal essential medium (Gibco), 2 mM L-glutamine, 0.1% of sodium bicarbonate (wt/vol; Gibco), 10 mM 4-(2-hydroxyethyl)−1-piperazineethanesulfonic acid (HEPES) (Gibco), 100 U/mL penicillin–100 μ/mL streptomycin, and 0.2% bovine serum albumin (BSA)], and 0.1% (wt/vol) diethylaminoethyl (DEAE)-dextran was added to the cells and incubated on MDCK cells for 1 hour before the an agarose overlay containing a final concentration of 0.64% agarose (Oxoid), 1× MEM and 1 U/mL tolylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin was added to the cells. The cells were then incubated for 72 hours at 33°C, and visible plaques were counted after fixation with 3.7% formaldehyde and visualization with a crystal violet counterstain (Sigma-Aldrich). All virus titers are presented as the log10 PFU/mL. The limit of detection for these assays was 50 PFU/mL.
Seroconversion ELISA
Immulon plates (Immulon 4HBX; Thermo Scientific) were coated with 2 µg/mL of B/Malaysia/2506/2004 purified virus (50 µL/well) in PBS at 4°C overnight. The following day, the plates were washed three times with PBS containing 0.1% Tween 20 (PBS-T) and blocked in blocking solution (3% goat serum and 0.5% milk in PBS-T) for 1 hour at room temperature. After blocking, prediluted serum was added to the first well at a final concentration of 1:100 in blocking solution. Serum was then serially diluted and incubated at room temperature for 2 hours. Plates were then washed three times with PBS-T before the addition of donkey anti-guinea pig IgG-horseradish peroxidase (IgG-HRP; EMD Millipore) in blocking solution for 1 hour at room temperature. Plates were then washed four times with PBS-T with shaking, and then, the O-phenylenediamine dihydrochloride (OPD) substrate (SigmaFast OPD; Sigma-Aldrich) was added. After 10 min of incubation at room temperature, the reaction was stopped by adding 50 µL of 3 M HCl to the mixture. The optical density (OD) was measured at 490 nm on a Synergy 4 plate reader (BioTek). A cutoff value of the average of the OD values of blank wells plus three standard deviations was established for each plate and used for calculating the AUC values of the sera, which were the readout for this assay. The limit of detection of the assay was an AUC of 100. Values below 100 were assigned 50 AUC for graphing purposes.
IgA ELISA
Immulon plates (Immulon 4HBX; Thermo Scientific) were coated with 2 µg/mL of B/Malaysia/2506/2004 purified virus (50 µL/well) in PBS at 4°C overnight. The following day, the plates were washed three times with PBS containing 0.1% Tween 20 (PBS-T) and blocked in blocking solution (3% goat serum and 0.5% milk in PBS-T) for 1 hour at room temperature. After blocking, nasal washes were added to the first well at a final concentration of 1:5. Nasal washes were then serially diluted and incubated at room temperature for 2 hours. Plates were then washed three times with PBS-T before the addition of sheep anti-guinea pig IgA-horseradish peroxidase (IgA-HRP; Invitrogen) in blocking solution for 1 hour at room temperature. The remaining assay was then ran as described above. The limit of detection of the assay was an AUC of 5; any serum that did not reach this level was assigned a value of 2.5.
ACKNOWLEDGMENTS
This work was supported in part by the National Institute of Allergy and Infectious Disease (NIAID) grant AI117287, the Collaborative Influenza Vaccine Innovation Centers (CIVIC) contract 75N93019C00051 (F.K.), and the NIAID Centers of Excellence for Influenza Research and Response (CEIRR) contract 75N93021C00014 (F.K.).
Contributor Information
Florian Krammer, Email: florian.krammer@mssm.edu.
Mark T. Heise, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA
DATA AVAILABILITY
Study data is vailable in ImmPort under ID SDY2320.
REFERENCES
- 1. Javaid W, Ehni J, Gonzalez-Reiche AS, Carreño JM, Hirsch E, Tan J, Khan Z, Kriti D, Ly T, Kranitzky B, Barnett B, Cera F, Prespa L, Moss M, Albrecht RA, Mustafa A, Herbison I, Hernandez MM, Pak TR, Alshammary H, Sebra R, Smith M, Krammer F, Gitman MR, Sordillo EM, Simon V, van Bakel H. 2020. Real-time investigation of a large nosocomial influenza A outbreak informed by genomic epidemiology. Clin Infect Dis. doi: 10.1111/myc.13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Azzi L, Dalla Gasperina D, Veronesi G, Shallak M, Ietto G, Iovino D, Baj A, Gianfagna F, Maurino V, Focosi D, Maggi F, Ferrario MM, Dentali F, Carcano G, Tagliabue A, Maffioli LS, Accolla RS, Forlani G. 2022. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. EBioMedicine 75:103788. doi: 10.1016/j.ebiom.2021.103788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sano K, Bhavsar D, Singh G, Floda D, Srivastava K, Gleason C, Group PS, Carreño JM, Simon V, Krammer F. 2021. Efficient Mucosal antibody response to SARS-Cov-2 vaccination is induced in previously infected individuals. medRxiv. doi: 10.1101/2021.12.06.21267352 [DOI]
- 4. Brokstad KA, Eriksson JC, Cox RJ, Tynning T, Olofsson J, Jonsson R, Davidsson A. 2002. Parenteral vaccination against influenza does not induce a local antigen-specific immune response in the nasal mucosa. J Infect Dis 185:878–884. doi: 10.1086/339710 [DOI] [PubMed] [Google Scholar]
- 5. Krammer F, Fouchier RAM, Eichelberger MC, Webby RJ, Shaw-Saliba K, Wan H, Wilson PC, Compans RW, Skountzou I, Monto AS, Garsin DA. 2018. NAction! How can neuraminidase-based immunity contribute to better influenza virus vaccines? mBio 9. doi: 10.1128/mBio.02332-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McAuley JL, Gilbertson BP, Trifkovic S, Brown LE, McKimm-Breschkin JL. 2019. Influenza virus neuraminidase structure and functions. Front Microbiol 10:39. doi: 10.3389/fmicb.2019.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wohlbold TJ, Krammer F. 2014. In the shadow of hemagglutinin: a growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses 6:2465–2494. doi: 10.3390/v6062465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen M, Zhang XQ, Senaati HP, Chen HW, Varki NM, Schooley RT, Gagneux P. 2013. Influenza A penetrates host mucus by cleaving sialic acids with neuraminidase. Virol J 10:321. doi: 10.1186/1743-422X-10-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang X, Steukers L, Forier K, Xiong R, Braeckmans K, Van Reeth K, Nauwynck H, Bouvier NM. 2014. A beneficiary role for neuraminidase in influenza virus penetration through the respiratory mucus. PLoS ONE 9:e110026. doi: 10.1371/journal.pone.0110026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Palese P, Compans RW. 1976. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J Gen Virol 33:159–163. doi: 10.1099/0022-1317-33-1-159 [DOI] [PubMed] [Google Scholar]
- 11. Sandbulte MR, Westgeest KB, Gao J, Xu X, Klimov AI, Russell CA, Burke DF, Smith DJ, Fouchier RAM, Eichelberger MC. 2011. Discordant antigenic drift of neuraminidase and hemagglutinin in H1N1 and H3N2 influenza viruses. Proc Natl Acad Sci USA 108:20748–20753. doi: 10.1073/pnas.1113801108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kilbourne ED, Johansson BE, Grajower B. 1990. Independent and disparate evolution in nature of influenza A virus hemagglutinin and neuraminidase glycoproteins. Proc Natl Acad Sci USA 87:786–790. doi: 10.1073/pnas.87.2.786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawai A, Yamamoto Y, Nogimori T, Takeshita K, Yamamoto T, Yoshioka Y. 2021. The potential of neuraminidase as an antigen for nasal vaccines to increase cross-protection against influenza viruses. J Virol 95:e0118021. doi: 10.1128/JVI.01180-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wohlbold TJ, Nachbagauer R, Xu H, Tan GS, Hirsh A, Brokstad KA, Cox RJ, Palese P, Krammer F. 2015. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous. MBio 6. doi: 10.1128/mBio.02556-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deroo T, Jou WM, Fiers W. 1996. Recombinant neuraminidase vaccine protects against lethal influenza. Vaccine 14:561–569. doi: 10.1016/0264-410x(95)00157-v [DOI] [PubMed] [Google Scholar]
- 16. Liu WC, Lin CY, Tsou YT, Jan JT, Wu SC. 2015. Cross-reactive neuraminidase-inhibiting antibodies elicited by immunization with recombinant neuraminidase proteins of H5N1 and pandemic H1N1 influenza A viruses. J Virol 89:7224–7234. doi: 10.1128/JVI.00585-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Strohmeier S, Amanat F, Zhu X, McMahon M, Deming ME, Pasetti MF, Neuzil KM, Wilson IA, Krammer F, Schultz-Cherry S. 2021. A novel recombinant influenza virus neuraminidase vaccine candidate stabilized by a measles virus phosphoprotein tetramerization domain provides robust protection from virus challenge in the mouse model. mBio 12:e0224121. doi: 10.1128/mBio.02241-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McMahon M, Kirkpatrick E, Stadlbauer D, Strohmeier S, Bouvier NM, Krammer F. 2019. Mucosal immunity against neuraminidase prevents influenza B virus transmission in Guinea pigs. mBio 10:e00560-19. doi: 10.1128/mBio.00560-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strohmeier S, Carreño JM, Brito RN, Krammer F. 2021. Introduction of Cysteines in the stalk domain of recombinant influenza virus N1 Neuraminidase enhances protein stability and Immunogenicity in mice. Vaccines (Basel) 9. doi: 10.3390/vaccines9040404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strohmeier S, Amanat F, Campbell JD, Traquina P, Coffman RL, Krammer F. 2022. A Cpg 1018 adjuvanted neuraminidase vaccine provides robust protection from influenza virus challenge in mice. NPJ Vaccines 7:81. doi: 10.1038/s41541-022-00486-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McMahon M, Strohmeier S, Rajendran M, Capuano C, Ellebedy AH, Wilson PC, Krammer F. 2020. Correctly folded - but not necessarily functional - influenza virus neuraminidase is required to induce protective antibody responses in mice. Vaccine 38:7129–7137. doi: 10.1016/j.vaccine.2020.08.067 [DOI] [PubMed] [Google Scholar]
- 22. Lowen AC, Mubareka S, Tumpey TM, García-Sastre A, Palese P. 2006. The Guinea pig as a transmission model for human influenza viruses. Proc Natl Acad Sci USA 103:9988–9992. doi: 10.1073/pnas.0604157103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McMahon M., Kirkpatrick E, Stadlbauer D, Strohmeier S, Bouvier NM, Krammer F, Schultz-Cherry S. 2019. Mucosal immunity against neuraminidase prevents influenza B virus transmission in Guinea pigs. mBio 10:e00560–19. doi: 10.1128/mBio.00560-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frise R, Bradley K, van Doremalen N, Galiano M, Elderfield RA, Stilwell P, Ashcroft JW, Fernandez-Alonso M, Miah S, Lackenby A, Roberts KL, Donnelly CA, Barclay WS. 2016. Contact transmission of influenza virus between ferrets imposes a looser bottleneck than respiratory droplet transmission allowing propagation of antiviral resistance. Sci Rep 6:29793. doi: 10.1038/srep29793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krammer F. 2023. The role of vaccines in the COVID-19 pandemic: what have we learned? Semin Immunopathol. doi: 10.1007/s00281-023-00996-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu X, Zhu X, Dwek RA, Stevens J, Wilson IA. 2008. Structural characterization of the 1918 influenza virus H1N1 neuraminidase. J Virol 82:10493–10501. doi: 10.1128/JVI.00959-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Margine I, Palese P, Krammer F. 2013. Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the Baculovirus expression system. J Vis Exp 6:e51112. doi: 10.3791/51112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krammer F, Margine I, Tan GS, Pica N, Krause JC, Palese P. 2012. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS One 7:e43603. doi: 10.1371/journal.pone.0043603 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Study data is vailable in ImmPort under ID SDY2320.