Abstract
Objective We aimed to evaluate both the long-term surgical outcomes and patient-reported outcomes of free scapular flap (FSF) phalloplasty. Method The same surgical team performed phalloplasty in 66 patients using a FSF between March 2000 and September 2018. All patients had at least 24 months of follow-up. The surgical techniques used, complications observed, and surgical and patient-reported outcomes were retrospectively described. Results A total of 66 patients with indications of penile trauma (n = 19), micropenis (n = 42), and self-amputation (n = 5) underwent FSF phalloplasty. Two patients (3%) had total flap necrosis and 1 (1.5%) had partial flap necrosis. The urethral complication rate was 18.2% (12/66 patients). All patients were able to void while standing after revision procedures or urethroplasty. We found that an FSF is a reliable donor site for penile reconstruction. Conclusion The FSF phalloplasty creates an esthetically pleasing penis and allows voiding while standing. Most patients can engage in sexual activity. The main drawbacks of using this method are that patients experience different degrees of sensory recovery, and patients undergoing surgery with the “tube-in-tube” technique may find they are be limited by the thickness of the flap. However, by making full use of residual tissue, such as the micropenis glans or scrotal skin, patients can obtain good tactile and erogenous sensation. We believe that using an FSF complements the existing phalloplasty techniques.
Keywords: penile reconstruction, free scapular flap, phalloplasty, penile sensation
Abstract
Résumé
Objectif Les chercheurs ont voulu évaluer les résultats chirurgicaux à long terme et les résultats cliniques déclarés par les patients d’une phalloplastie par lambeau scapulaire libre (LSL). Méthodologie La même équipe chirurgicale a effectué la phalloplastie de 66 patients au moyen d’un LSL entre mars 2000 et septembre 2018. Ceux-ci ont tous reçu un suivi d’au moins 24 mois. Les chercheurs ont décrit rétrospectivement les techniques chirurgicales utilisés, les complications observées et les résultats chirurgicaux et cliniques déclarés par les patients. Résultat Au total, 66 patients ayant des indications de traumatisme pénien (n=19), un micropénis (n=42) et une auto-amputation (n=5) ont subi une phalloplastie par LSL. Deux patients (3 %) ont subi une nécrose totale du lambeau et un (1,5 %) une nécrose partielle du lambeau. Le taux de complications urétrales s’est élevé à 18,2 % (12 patients sur 66). Tous les patients étaient en mesure d’uriner debout après les interventions de révision ou l’urétroplastie. Les chercheurs ont constaté que la région scapulaire est un siège de donneur fiable pour la reconstruction pénienne. Conclusion La phalloplastie par LSL crée un pénis à l’esthétique agréable, qui permet d’uriner debout. La plupart des patients peuvent se livrer à des activités sexuelles. Les principaux inconvénients de cette méthode proviennent du fait que les patients éprouvent divers degrés de récupération sensorielle et que ceux qui subissent la technique chirurgicale « à double tube » peuvent être limités par l’épaisseur du lambeau. Cependant, grâce au plein usage des tissus résiduels, tels que le gland du micropénis ou la peau du scrotum, les patients peuvent éprouver de bonnes sensations tactiles et érogènes. Les auteurs sont d’avis que l’utilisation du LSL complète les techniques de phalloplastie en place.
Mots-clés: lambeau scapulaire libre, phalloplastie, reconstruction pénienne, sensation pénienne
Introduction
The goal of phalloplasty is to construct an esthetically pleasing, functional penis in a single surgery, allowing the patient to urinate while standing and have a normal sexual life, without donor function or esthetic complications.1–3 However, due to the lack of appropriate substitutes for the corpora cavernosa and corpus spongiosum, penile reconstruction is a great challenge to plastic surgeons. Although more than a dozen techniques for penile reconstruction are applied, the optimal surgical approach has not been elucidated.4–11 Advances in microsurgical techniques enable free tissue transplantation to be an option for phalloplasty. Many free flaps such as the radial forearm free flap (RFFF), anterolateral thigh (ALT) flap, latissimus dorsi (LTD) flap, and scapular flap, have been used in penile reconstruction.4,5,9,12 To date, an RFFF is the most commonly used flap, and is recognized as the standard approach of penile reconstruction; 10 however, using this technique results in an unattractive donor site scar with the flap having a tendency to thin out over time. 13 For patients who do not desire forearm scars, we must choose a flap from a different donor site. The free scapular flap (FSF) has sufficient tissue, thick skin, constant anatomical location, and the proper pedicle length, and is thus a good option for phalloplasty.8,14–16 In this study, we retrospectively analyzed the data of patients with FSF penis reconstruction and evaluated the surgical and patient-reported outcomes, while also introducing our experience with this technique. Our study's results will provide valuable information to physicians and surgeons of other institutions for clinical decision-making.
Patients and Methods
From March 2000 to September 2018, a consecutive series of 66 patients with penile trauma (n = 19), micropenis (n = 42), and self-amputation (n = 5) underwent FSF phalloplasty.
FSF phalloplasty was conducted in 3 stages as follows: (1) penile and urethra reconstruction, (2) urethral anastomosis, and (3) penile prosthesis implantation.
At the beginning of this study (2000-2007; n = 23), the penile prosthesis was inserted routinely and immediately before flap anastomosis. Later (2008-2012; n = 16), we performed prosthesis implantation until protective sensation recovered, generally after the urethral anastomosis. In the latter part of the study (2012-2018; n = 27), we began using a dermal tissue to wrap the distal tip of the prosthesis to further reduce implant exposure. All patients signed an informed consent, and the study was approved by the medical ethics review board.
Flap Preparation
Typically, an FSF phalloplasty is carried out in 3 stages with 6 months between each stage. Complications arising at each stage are corrected at the subsequent stage.
Preoperatively, all patients are told to stop smoking for 3-4 weeks prior to surgery and their weight was controlled at a BMI <30 kg/m2. The skin of the scapular flap is verified to be healthy with no inflammation.
Flap Design
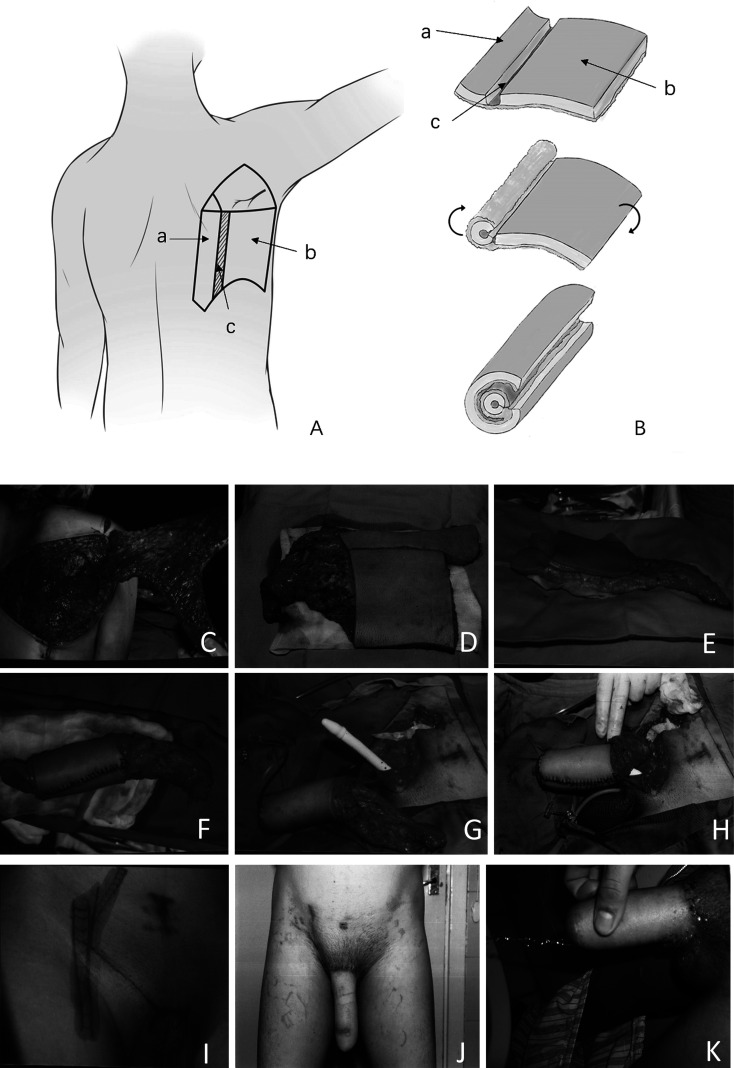
The anatomical distribution of the circumflex scapular artery is examined by Doppler ultrasound. Then, the flap was designed with the vascular and its branches. Flap a with area 16 × 4 cm2 is designed to form the neourethra, and a 16 × 14 cm2 wide flap b is designed to wrap the neourethra and form the penile shaft. Finally, the c flap with area 1.0 cm is designed for deepithelializing to facilitate easy closure (Figure 1).
Figure 1.
Preoperative, intraoperative of free scapular flap (FSF) phalloplasty and follow-up photographs. (A) Preoperative scapular flap design. (B) the “tube-in-tube” technique. (C) The scapular flap was raised. (D) The vascular pedicle was cut off and prepared for phallus creation. (E) The inner flap was exposed and sutured to form the urethra. (F) The outer flap was wrapped around the neourethra to form the shaft. (G) In the early phase of this study, the penile prosthesis was inserted routinely and immediately before flap anastomosis. (H) Vascular anastomosis is complete. (I) Preoperative design of the recipient site. (J) Six months after the first stage of phalloplasty. (K) Standing urination after urethral anastomosis.
The scapular flap and receptor vessels are dissected in the lateral position, and the penis reconstructed by anastomosing blood vessels in the spine position. Surgeons were divided into 2 groups based on dissection site in preparing the donor site and recipient site.
Donor Site Dissection
The flap is elevated with the transverse and descending cutaneous branches of the circumflex scapular artery and 2 vena comitantes. The dissection should start from the distal and medial margin of the flap towards the trilateral foramen because the distal and medial superficial fascia contains no important structures and is far from the vascular pedicle. After lifting the flap, the trilateral foramen is easily identified, and the vascular pedicle carefully cut down with the deep fascia around it. The donor site is closed directly. Any residual defect is covered with a full-thickness skin graft if complete closure cannot be performed.
Phallus Creation
(1) A 1 cm wide area is deepithelialized between the medial and lateral flaps. (2) The lateral flap is tubularized around the catheter to form the urethra. (3) The medial flap is wrapped around the neourethra in a tube-in-tube fashion to form the phallus. The arteries and veins of the pedicle are isolated and prepared for microsurgical anastomosis.
Then, the phallus is transferred to the recipient where the vascular anastomosis is performed. Circumflex scapular artery is connected to deep inferior epigastric artery, while vena comitans to deep inferior epigastric vein and superficial iliac circumflex vein or superficial inferior epigastric vein. We prefer to use the deep inferior epigastric vessels as the receptor vessels due to their large diameter and constant anatomical location.
Circulation of the grafted flap is observed for 30 min to ensure successful vascular anastomosis. A compressive vulvar bolster and a suction drain were placed routinely to prevent postoperative hematoma. Patients are transferred to a recovery room maintained at a temperature of 25°, the daily rehydration volume shall be at least 3000 mL, monitored for 2 days, and transferred again to the general ward to remain on bed rest for 1 week. The penis is raised up about 45° during this period. Subcutaneous heparin and aspirin are administered to prevent microvascular thrombosis. Antibiotics are also administered routinely to prevent infection.
Urethral Anastomosis
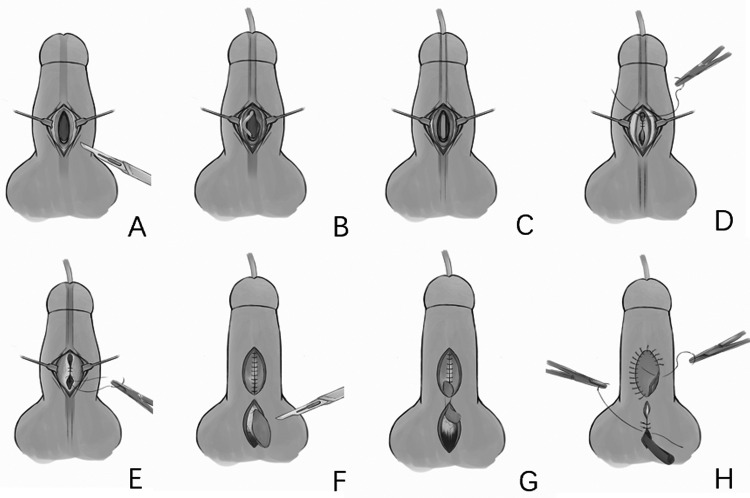
Urethral anastomosis is performed 6 months after the first surgical stage. A catheter is inserted into the bladder and passed through the 2 meatuses needing anastomosis. Urinary diversion is performed. Parallel incisions are made along the ventral side of the defective urethra to the superficial fascia. The skin is sutured around the catheter to form the urethra, and the fascia is sutured to form a second layer to cover the urethra. Then, a midline or lateral pedicled scrotal flap is transferred to cover the second layer, and the scrotum donor site is sutured directly. A latex drain is used to prevent scrotal hematoma. The drain is removed 24 h after surgery and the urethral catheter removed 48 h after surgery. Beginning at 3-days postoperatively, patients are asked to urinate once per day to flush the urethra. The urinary diversion is removed when patients can urinate satisfactorily (Figure 2).
Figure 2.
Procedures of urethral anastomosis.
Implantation of Prosthesis
Prosthesis implantation was performed as follows 6 months after urethral anastomosis. An incision is made along the previous scar from the right or left pubic area to the lower abdomen where a 7 × 3 cm2 area of tissue is harvested. We remove the epidermis and fat from the tissue for an area 5 × 2 cm2 of dermal tissue, which is used to wrap the tip of the prosthesis. The wound is sutured directly.
Then, a catheter is inserted into the bladder. An incision is made from the symphysis pubis to the periosteum. A 12 cm long prosthesis is sutured on the periosteum with a 4 to 0 steel wire. Then, we create a subcutaneous tunnel underneath the neophallus. The prosthesis wrapped with dermal tissue is placed into the tunnel. The incision is sutured layer by layer with absorbable thread. A drainage tube was placed in all cases.
Results
Complications were categorized according to the time period in which they were observed (2000-2007, 2008-2012, or 2013-2018) (Table 1). The total flap-related complications rate was 10.6%. A staged urethroplasty was performed in all patients. In total, 18.2% patients had urethral complications. All patients could eventually urinate while standing.
Table 1.
Complications.
| Overall (%) | 2000 to 2007 | 2008 to 2012 | 2013 to 2018 | |
|---|---|---|---|---|
| No. | 66 | 23 | 16 | 27 |
| Flap-related | ||||
| Anastomotic revision | 3 (4.5%) | 2 (8.7%) | 1 (6.2%) | 0 |
| Complete flap loss | 2 (3%) | 1 (4.3%) | 0 | 1 (3.7%) |
| Partial necrosis | 4 (6%) | 1 (4.3%) | 2 (12.5%) | 1 (3.7%) |
| Urologic | ||||
| Early fistula | 6 (9%) | 0 | 1 (6.2%) | 3 (11.1%) |
| Urethra necrosis | 2 (3%) | 2 (8.7%) | 0 | 0 |
| Strictures | 4 (6%) | 2 (8.7%) | 1 (6.2%) | 1 (3.7%) |
| Prosthesis | ||||
| No. | 45 | 21 | 10 | 14 |
| Revision surgery | 21 (46.7%) | 12 (57.1%) | 5 (50%) | 4 (28.6%) |
Of 66 patients, 45 underwent prosthesis implantation (Malleable 650, American Medical Systems, Inc.), and 21 required revision surgery due to prosthesis exposure or infection. We are constantly improving our implantation approach, from inserting the prosthesis immediately before flap anastomosis to the present approach of performing prosthesis implantation 6 months after urethral anastomosis, as well as using a dermal tissue to wrap the distal tip of the prosthesis. Complication rates decreased from 57.1% during the first third of the study duration to 28.6% during the last third. Although the differences between groups were not statistically significant due to the study's small sample size, we believe the results are still valuable and can provide some reference.
From 2000 to 2007, 2 patients experienced a venous thrombosis, where after immediate surgical exploration and secondary venous anastomosis, one case was salvaged successfully. Two patients experienced urethral flap necrosis because the flaps were not designed large enough, leading to tension closure with postoperative swelling and complete loss of the flaps. As a result, we modified our flap dimensions; thereafter, we did not observe any successive urethral flap necrosis or loss. Two patients experienced urethral stricture, in whom had successful revision by secondary urethroplasty. As mentioned above, the penile prosthesis was inserted routinely and immediately before flap anastomosis in this period, and high-incidence (57.1%) revision surgery related to prothesis implantation was observed. Twelve patients in this group answered the satisfaction questionnaire. One patient was unsatisfied with the hypertrophic appearance of the penis and is preparing for volume-reduction surgery. Four patients claimed they had minimal tactile sensation and 5 patients did not have any sexual sensation.
From 2008 to 2012, one patient experienced a venous thrombosis, which was salvaged successfully after secondary venous anastomosis. Two patients experienced partial necrosis. One fistula healed spontaneously, and one stricture underwent revision urethroplasty. Prothesis revision surgery was needed in 50% of patients when we implanted the prothesis in the second stage after protective sensation recovery. Thirteen patients in this group answered the satisfaction questionnaire; 2 were unsatisfied with the large size of the penis and cannot have sexual activity. One patient was unsatisfied with the tactile sensation and 3 patients do not have any sexual sensation.
From 2013 to 2018, one patient experienced complete flap loss in the 6 days postoperatively; a pedicled ALT flap was used to revise the phalloplasty 6 months later. Three patients had a fistula and healed spontaneously after 1 month. One patient had a stricture that was repaired successfully with secondary urethroplasty. In this period, the dermal tissue was applied to wrap the distal tip of the prosthesis. Revision surgery was needed in 28.6% of patients. Twenty-two patients in this group answered the satisfaction questionnaire. All patients were satisfied with the appearance of the penis and were able to have sexual activity. One patient was unsatisfied with the tactile sensation and did not have any sexual sensation.
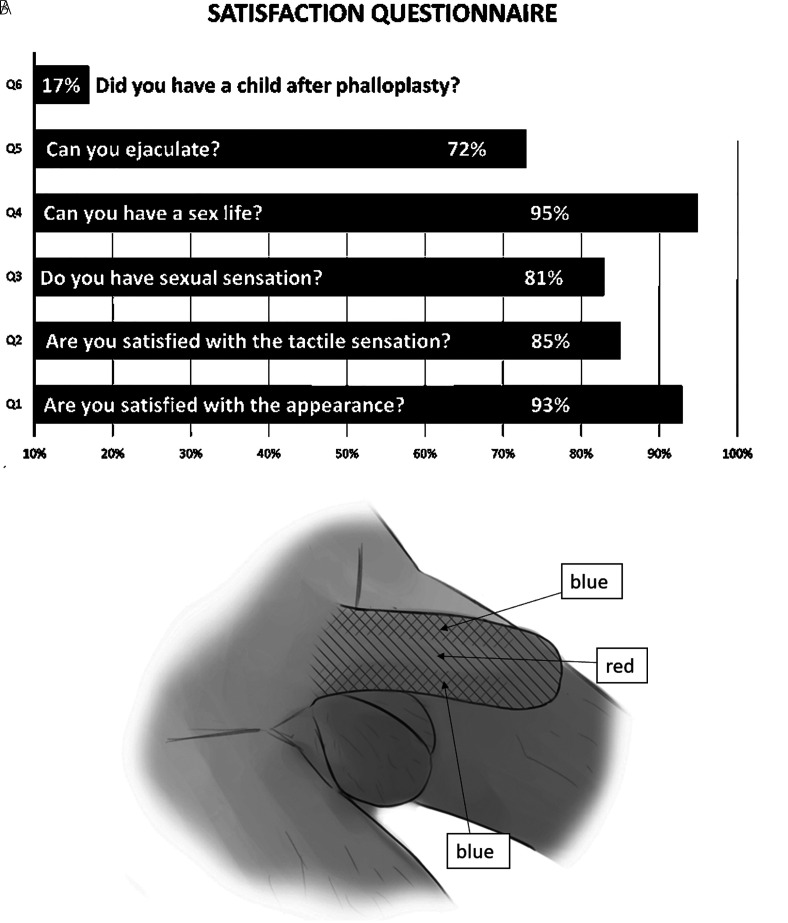
Patient-reported outcomes were obtained by following up with the patient by telephone or text message. Forty-seven patients answered the questionnaire (Figure 3) shows the questions and response rates.
Figure 3.
(A) The questions and outcomes of the questionnaire are shown. (B) Tactile sensation recovery of a patient (without glans or scrotal tissue application) postoperative month 6. Red: deep sensation. Blue: superficial sensation.
Discussion
The FSF flap can meet most standards for an ideal donor flap for penile reconstruction, including having sufficient blood supply and tissue mass, having the appropriate thickness, being hairless, and minimizing donor site morbidity. We believe it is one of the ideal flaps for penile reconstruction. In this study, we described the outcomes and patient-reported outcomes of 66 patients with FSF phalloplasty, which were performed by the same surgical team leading by an experienced surgeon. The total urethral complication rate was 18.2%. All complications such as flap necrosis and stricture were repaired successfully with revision surgery. All patients could void from the tip of the penis eventually. Forty-seven patients answered the questionnaire, which showed a 93% satisfaction with the penis appearance. Unexpectedly, the questionnaire showed that tactile and erogenous sensation, which is considered the main drawback of FSF flap, was 85% and 81%, respectively. We believe this is due to the full utilization of the residual glans and scrotum tissue, which is discussed later in the Sensation section of the discussion.
RFFF and LTD Flap
An RFFF is the most commonly used and considered to be the best flap for phalloplasty because of its appealing esthetic appearance and good sensation and because it allows voiding while standing and to have penetrative sexual intercourse after prosthesis implantation.10,17 The well-known drawbacks of RFFF for phalloplasty are donor site morbidity and a tendency to thin out over time. 13 Additionally, some patients have a limitation due to the amount of tissue in their forearm, and safe insertion of a prosthesis is not achievable. This is the main reason for the emergence of alternative techniques.
The LTD flap has been used as a “workhorse” for the reconstruction of a variety of anatomical and functional defects;18,19 it is currently another widely used option for total phalloplasty.4,20–23 The significant advantage of this flap is the concealed donor site, reliable vascular anatomy, sufficient amount of tissue for safe prosthesis implantation, and ability of the patient to void while standing after urethroplasty. The disadvantages are that the neophallus lacks tactile sensation, although the thoracodorsal nerve is connected to the ilioinguinal nerve or anterior branch of the obturator nerve. 24
FSF
The advantages of using the FSF are as follows. (1) The dermis of the back region is thick and tough, which translates well to maintaining the shape and rigidity of the neophallus with its volume staying relatively stable over time. This is evidenced in that, in our study, 4 patients could have penetrative sexual intercourse without a prosthesis. (2) The FSF is supplied by the superficial branch of the circumflex scapular artery, and both the transverse and descending branches can be harvested to ensure a stable blood supply. In addition, the anatomical position of the vascular pedicle is constant, and its diameter is about 2 to 3 mm, which is convenient for anastomosis. (3) The FSF can provide a large surface area, allowing urethroplasty and prosthesis implantation. (4) The donor site is concealed by clothing and the removal of the flap has little impact on esthetic appearance and function. Generally, there is little difference between the FSF and LTD flaps, but the former has an advantage over the latter. Dissection of the vascular pedicle of the FSF is much easier because the superficial branch of the circumflex scapular artery is superficially positioned and does not enter the muscle. Therefore, there is no need to separate and cut off the muscle, lending to less damage to the patient and retention of function of the LTD.
Sensation
Lacking tactile sensation is considered one of the main drawbacks of using the FSF in phalloplasty. 8 Because the nerves innervating the FSF spread extensively (as internal branches of the posterior branches of the second to fourth thoracic nerves), it is difficult to isolate them. Previously, we tried to dissect nerves to be used for transplantation but were unsuccessful. However, through follow-up, we found that the sensation gradually recovered at 6 months postoperatively (Figure 3B). Compared to other pedicled ALT flaps with nerves incorporated in our center or research on LTD flaps in the literature,4,20–23 we believe that nerve incorporation just accelerates nerve recovery in these 2 flaps. In our questionnaire, 85% of patients claimed that they were somewhat satisfied with tactile sensation after surgery. The optimal approach to harvesting an FSF while keeping sensory nerves intact so that they can be anastomosed still requires further study.
We believe making full use of residual penile tissue is the most effective way to restore erogenous sensation. In patients with micropenis, we preserve the glans with its neurovascular bundle, and incorporate it into the base of the neophallus on the ventral surface. In patients without a glans, we use the lateral or midline pedicle scrotal flap to cover the wound after urethral anastomosis as a waterproof layer. Importantly, the scrotal skin provides some erogenous sensation.
Urethroplasty
The ability to void while standing is a feature unique to men and is one of the most important factors for patients considering phalloplasty. 10 However, reconstruction of the penile urethra is the most complex part of penile reconstruction, with complication rates of 25% to 75%.25–27 In this study, the “tube-in-tube” technique was used for urethra reconstruction in all patients. Adequate blood supply and less closure tension, which are advantages of the FSF, can minimize urethral complications. Two-stage phalloplasty can significantly relieve pain during urination as well as reduce the likelihood of complications such as infection and flap loss. Delaying urethral anastomosis can eliminate the pressure from urine and contamination from it to surrounding transplanted tissues, which can significantly improve the success rate of urethroplasty and reduce the likelihood of complications. In our study, all patients could eventually void while standing.
This study has some limitations. This was a retrospective study with small sample size and no control group. More cases of FSF and LTD phalloplasty should be added in future studies to evaluate the advantages and drawbacks of using this flap in phalloplasty. However, we believe that the surgical methods used and resulting complication rate in this study can still provide a reference for penile reconstruction.
Conclusion
We found that FSF is a reliable donor site for penile reconstruction. The FSF phalloplasty creates an esthetically pleasing penis and allows voiding while standing. Most patients can engage in sexual activity. The main drawbacks of using this method are that patients experience difference degrees of sensory recovery, and patients undergoing surgery with the “tube-in-tube” technique may find that they are limited by the thickness of the flap. However, by making full use of residual tissue, such as the micropenis glans or scrotal skin, patients can obtain good tactile and erogenous sensation. We believe that using an FSF complements the existing phalloplasty techniques.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yangqun Li https://orcid.org/0000-0002-8961-4820
References
- 1.Gilbert DA, Horton CE, Terzis JK, et al. New concepts in phallic reconstruction. Ann Plast Surg. 1987;18(2):128-136. [DOI] [PubMed] [Google Scholar]
- 2.Hage JJ, De Graaf FH. Addressing the ideal requirements by free flap phalloplasty: some reflections on refinements of technique. Microsurg. 1993;14(9):592-598. [DOI] [PubMed] [Google Scholar]
- 3.Garaffa G, Christopher NA, Ralph DJ. Total phallic reconstruction in female-to-male transsexuals. Eur Urol. 2010;57(4):715-722. [DOI] [PubMed] [Google Scholar]
- 4.Perovic SV, Djinovic R, Bumbasirevic M, et al. Total phalloplasty using a musculocutaneous latissimus dorsi flap. Bju Int. 2007;100(4):899-905. [DOI] [PubMed] [Google Scholar]
- 5.Felici N, Felici A. A new phalloplasty technique: the free anterolateral thigh flap phalloplasty. J Plast Reconstr Aesthet Surg. 2006;59(2):153-157. [DOI] [PubMed] [Google Scholar]
- 6.Koshima I, Nanba Y, Nagai A, et al. Penile reconstruction with bilateral superficial circumflex iliac artery perforator (SCIP) flaps. J Reconstr Microsurg. 2006;22(3):137-142. [DOI] [PubMed] [Google Scholar]
- 7.Zielinski T. Phalloplasty using a lateral groin flap in female-to-male transsexuals. Acta Chir Plast. 1999;41(1):15-19. [PubMed] [Google Scholar]
- 8.Yang M, Zhao M, Li S, et al. Penile reconstruction by the free scapular flap and malleable penis prosthesis. Ann Plas Surg. 2007;59(1):95-101. [DOI] [PubMed] [Google Scholar]
- 9.Chang TS, Hwang WY. Forearm flap in one-stage reconstruction of the penis. Plast Reconstr Surg. 1984;74(2):251-258. [DOI] [PubMed] [Google Scholar]
- 10.Monstrey S, Hoebeke P, Selvaggi G, et al. Penile reconstruction: is the radial forearm flap really the standard technique? Plast Reconstr Surg. 2009;124(2):510-518. [DOI] [PubMed] [Google Scholar]
- 11.Lee GK, Lim AF, Bird ET. A novel single-flap technique for total penile reconstruction: the pedicled anterolateral thigh flap. Plast Reconstr Surg. 2009;124(1):163-166. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Li SK, Yang MY, et al. A free scapular skin flap for penile reconstruction. J Plast Reconstr Aesthet Surg. 2007;60(11):1200-1203. [DOI] [PubMed] [Google Scholar]
- 13.Arpa S D, Claes K, Lumen N, et al. Urethral reconstruction in anterolateral thigh flap phalloplasty. Plast Reconstr Surg. 2019;143(2):382e-392e. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert A, Teot L. The free scapular flap. Plast Reconstr Surg. 1982;69(4):601-604. [DOI] [PubMed] [Google Scholar]
- 15.Mayou BJ, Whitby D, Jones BM. The scapular flap--an anatomical and clinical study. Br J Plast Surg. 1982;35(1):8-13. [DOI] [PubMed] [Google Scholar]
- 16.Dos SL. The vascular anatomy and dissection of the free scapular flap. Plast Reconstr Surg. 1984;73(4):599-604. [DOI] [PubMed] [Google Scholar]
- 17.Doornaert M, Hoebeke P, Ceulemans P, et al. Penile reconstruction with the radial forearm flap: an update. Handchir Mikrochir Plast Chir. 2011;43(4):208-214. [DOI] [PubMed] [Google Scholar]
- 18.Baudet J, Guimberteau JC, Nascimento E. Successful clinical transfer of two free thoraco-dorsal axillary flaps. Plast Reconstr Surg. 1976;58(6):680-688. [DOI] [PubMed] [Google Scholar]
- 19.Lassen M, Krag C, Nielsen IM. The latissimus dorsi flap. An overview. Scand J Plast Reconstr Surg. 1985;19(1):41-51. [DOI] [PubMed] [Google Scholar]
- 20.Djordjevic ML, Bumbasirevic MZ, Vukovic PM, et al. Musculocutaneous latissimus dorsi free transfer flap for total phalloplasty in children. J Pediatr Urol. 2006;2(4):333-339. [DOI] [PubMed] [Google Scholar]
- 21.Vesely J, Hyza P, Ranno R, et al. New technique of total phalloplasty with reinnervated latissimus dorsi myocutaneous free flap in female-to-male transsexuals. Ann Plast Surg. 2007;58:544-550. [DOI] [PubMed] [Google Scholar]
- 22.Jun MS, Pusica S, Kojovic V, et al. Total phalloplasty With Latissimus dorsi musculocutaneous flap in female-to-male transgender surgery. Urology. 2018;120:269-270. [DOI] [PubMed] [Google Scholar]
- 23.Djordjevic ML, Bencic M, Kojovic V, et al. Musculocutaneous latissimus dorsi flap for phalloplasty in female to male gender affirmation surgery. World J Urol. 2019;37:631-637. [DOI] [PubMed] [Google Scholar]
- 24.Kojovic V, Marjanovic M, Radenkovic A, et al. Latissimus dorsi free flap phalloplasty: a systematic review. Int J Impot Res. 2020;12:1-8. [DOI] [PubMed] [Google Scholar]
- 25.Santucci RA. Urethral complications after transgender phalloplasty: strategies to treat them and minimize their occurrence. Clin Anat. 2018;31(2):187-190. [DOI] [PubMed] [Google Scholar]
- 26.Ascha M, Massie JP, Morrison SD, et al. Outcomes of single stage phalloplasty by pedicled anterolateral thigh flap versus radial forearm free flap in gender confirming surgery. J Urology. 2018;199:206-214. [DOI] [PubMed] [Google Scholar]
- 27.Frey JD, Poudrier G, Chiodo MV, et al. An update on genital reconstruction options for the female-to-male transgender patient: a review of the literature. Plast Reconstr Surg. 2017;139:728-737. [DOI] [PubMed] [Google Scholar]