Abstract
Background:
The diagnosis of infectious flexor tenosynovitis (FTS) has historically been made based on physical exam using Kanavel’s signs. The specificity of these findings has come into question. We looked to evaluate the use of contrast-enhanced computed tomography (CT) in increasing the successful diagnosis of FTS.
Methods:
Two adult cohorts were formed, one of patients with FTS confirmed in the operating room and the second of patients with ICD.10 identified finger cellulitis (FC), without concomitant FTS. Demographics, laboratory values, CT scans, and examination findings were evaluated. Axial CTs were evaluated in the coronal and sagittal planes and tendon sheath/tendon width were measured. The tendon sheath/tendon was recorded as a ratio in the coronal (CR) and sagittal (SR) planes. Continuous and dichotomous variables were analyzed and measures of sensitivity, specificity, and predictivity were calculated. Seventy patients were included, 35 in the FTS cohort and 35 with FC.
Result:
A higher number of Kanavel signs were present in the FTS group (2.9 vs. 0.5, P < .05), with CR and SR both being significantly larger in the FTS group (P < .05). CR and SR cutoffs ≥ 1.3 provided high sensitivity, specificity, and positive predictive value (PPV) for FTS. Likelihood of FTS increased 5.9% and 5.5% for every 0.1 increase in CR and SR, respectively, with a 14% increase for every additional Kanavel sign.
Conclusion:
In conclusion, CT ratios are useful in identifying FTS; and when used on their own or in combination with Kanavel’s signs, CR and SR objectively improve the diagnosis of FTS.
Keywords: hand, anatomy, tendon, basic science, infection, diagnosis, evaluation, research & health outcomes
Introduction
Flexor tenosynovitis (FTS) is a common orthopedic diagnosis leading to consultation, comprising 2% to 9% of hand infections, and can be difficult to diagnose.1-3 The source of infection may be penetrating injury or hematogenous spread. 1 The flexor tendon sheath provides nutrition while allowing optimal gliding and restraint to tendons. When bacteria, most commonly Staphylococcus or Streptococcus species, invade the sheath, they are difficult to treat using antibiotics alone, with surgery often required.4-6 Associated morbidity from FTS can be significant, with finger stiffness common, even with otherwise satisfactory outcomes. Tendon rupture, amputation, and systemic infection are a few complications stemming from FTS if diagnosis is missed or delayed.7,8 This being said, treatment within 48 hours of onset has shown 80% excellent outcomes, understating the importance of early, accurate diagnosis.9,10
Diagnosis of FTS has historically been by physical examination, specifically Kanavel’s signs that include uniform swelling, tenderness to palpation along the flexor tendon, pain with passive extension of the digit, and flexed posturing of the digit. 11 Kanavel’s signs have been thought to have high sensitivity, especially with multiple positive signs. 12 However, Hyatt and Bragg 4 reported up to 46% of patients with surgically proven FTS did not exhibit all 4 Kanavel signs preoperatively. This makes clinical diagnosis of FTS challenging, possibly delaying treatment. 13 Additionally, the specificity of Kanavel’s signs has been reported at 51% to 69%, indicating sometimes surgeons are operating on patients without FTS, causing unnecessary morbidity. 12 Other diagnostic tools include white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). However, these are nonspecific and may be normal, giving them minimal use diagnostically. 14 Radiographic soft tissue swelling also does not distinguish FTS from other causes. 15 Magnetic resonance imaging (MRI) has been described in aiding diagnosis but sensitivity and specificity have not been well studied. 16 Additionally, MRI access and time spent obtaining MRI may delay diagnosis, with a negative impact on patient outcomes. Ultrasound has been briefly studied as a diagnostic tool with high sensitivity, but mediocre specificity. 17 Most case reports have described severe FTS and may not represent the true predictive value of all FTS cases.18-20 Ultrasound also comes with inherent user variability and limitations with access and equipment quality.
The use of computed tomography (CT) in diagnosing FTS has not been studied. Reinus et al 21 reported on CT identification of infectious extensor tenosynovitis, where contrast enhancing fluid was seen within the tendon sheaths. Given widespread access to CT, the speed at which CT can be performed, and its objectivity compared to adjuncts like ultrasound, it may be a valuable adjunct to Kanavel’s signs in accurately diagnosing FTS. Furthermore, CT may distinguish FTS from other causes of hand infection. 22 Currently, a CT is not always ordered when diagnosing FTS, but some physicians obtain them when the diagnosis is not straightforward. At our institution, we found that many patients with finger infections had contrast-enhanced CT scans performed prior to the Emergency Department consulting orthopedics. In subsequent evaluation of these CTs, fluid within the tendon sheath, representing a “target sign,” was noted in FTS. The aim of this study is to evaluate the use of CT in providing objective data to aid in the prompt diagnosis of FTS. We hypothesize that contrast-enhanced hand CT scans will demonstrate sensitive and specific diagnostic measurements to help differentiate FTS from finger cellulitis (FC).
Materials and Methods
Following institutional review board approval, we retrospectively reviewed adult patients ≥ 18 years old who were admitted to one of 3 local hospitals with diagnoses of FTS or FC and who had all undergone a CT of their hand during admission. Clinician judgment by the attending emergency department physician was used regarding when a hand CT was believed necessary. In order to be included in the FTS cohort, a diagnosis of FTS must have been established in the operating room at the time of tendon sheath incision and drainage (I&D), based on purulence and subsequent positive culture growth. CPT and ICD.10 procedure codes were used to identify patients who had undergone tendon sheath I&D and charts were manually reviewed to ensure infection was noted at the time of I&D. The FC cohort was identified using ICD.10 diagnosis codes and any patients with concomitant FTS were not included in this group. Other exclusion criteria for both cohorts included any patient without a contrast-enhanced hand CT available for review, a CT with too much artifact that the reviewer felt an accurate measurement could not be reliably obtained, and those without physical examination documentation.
After inclusion criteria were met, a total of 35 patients made up the FTS group. These 35 comprised 23% of the 150 patients with operatively diagnosed FTS, with 111 patients excluded for having no CT, 2 did not have documented physical examination findings and 2 did not have positive culture growth to confirm infectious FTS. Next, 1265 patients were identified that met the ICD.10 diagnosis code for FC. Of these, 84 had received a CT scan and 31 were subsequently excluded due to insufficient physical examination documentation. Out of the remaining 53 patients, 35 were chosen at random to create a similarly sized cohort compared to the FTS group, with no specific matched variables. Additional demographic data are available in Table 1.
Table 1.
Demographic and Injury Data.
| Variable | Flexor tenosynovitis group* (n = 35) | Finger cellulitis group* (n = 35) | P value |
|---|---|---|---|
| Age (years) | 47 ± 13 | 45 ± 9.9 | .48 |
| Gender (male) | 22 (63%) | 22 (63%) | 1.0 |
| BMI (kg/m2) | 28.4 ± 7.5 | 27.5 ± 5.8 | .57 |
| Diabetic (Y) | 7 (20%) | 5 (14.3%) | .75 |
| History IV drug use (Y) | 14 (40%) | 14 (40%) | 1.0 |
| Number of Kanavel signs present | 2.9 ± 1.1 | 0.5 ± 0.8 | <.0001 |
| Kanavel signs | |||
| Fusiform swelling | 27 (77%) | 10 (29%) | <.0001 |
| Pain to palpation | 29 (83%) | 1 (3%) | <.0001 |
| Pain passive extension | 27 (77%) | 2 (6%) | <.0001 |
| Flexed digit posture | 19 (54%) | 3 (9%) | <.0001 |
| Febrile (Y) | 4 (11%) | 2 (6%) | .67 |
| WBC (K/mcL) | 12.0 ± 4.9 | 10.9 ± 3.4 | .28 |
| CRP (mg/L) | 81.4 ± 77.5 | 49.9 ± 79.7 | .17 |
| ESR (mm/h) | 36.5 ± 25.6 | 24.5 ± 23.7 | .09 |
Note. BMI = body mass index; IV= intravenous; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate.
Values are expressed as means ± standard deviations or as absolute values with percentages in parentheses.
Next, details of each patient’s presentation were recorded including number of Kanavel signs on physical exam, presence of fever, WBC count, CRP, and ESR (Table 1). A hand surgeon individually evaluated 86% of the patients in the study.
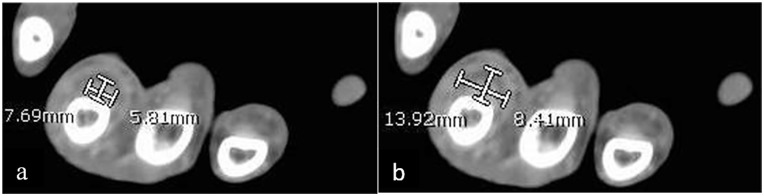
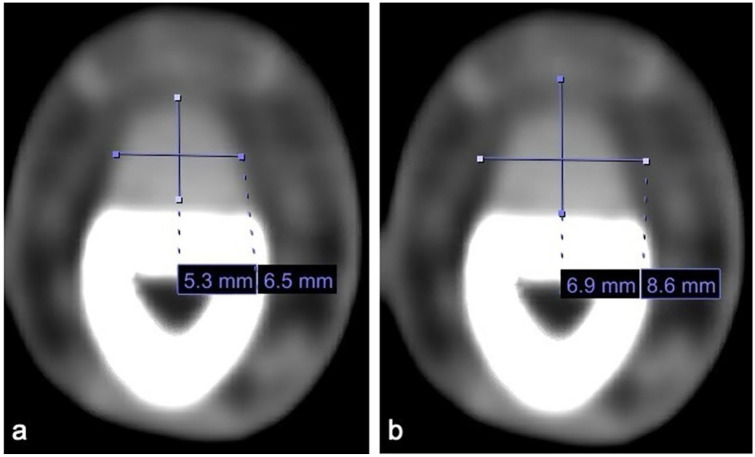
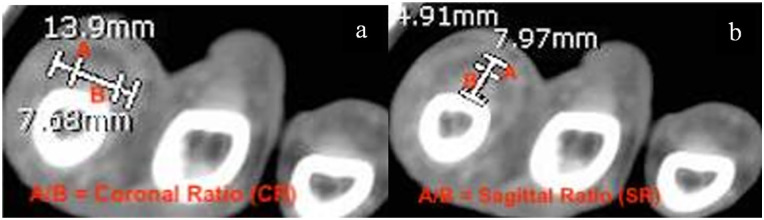
Additionally, each patient’s contrast-enhanced CT scan was closely evaluated and measured by one of the authors to maintain consistency with measurement techniques. The author was blinded as to which cohort the patient was in when reviewing their CT scans. Each CT was performed using a GE (General Electric, Boston, Massachusetts) unit with 2.5 mm slices in the axial plane. The finger in question was first identified based on charted notes and viewed in the axial plane on CT from proximal to distal, starting just proximal to the metacarpophalangeal (MCP) joint and moving distally toward the flexor tendon attachment at the base of the distal phalanx. Identification of any contrast enhancing fluid within the flexor tendon sheath was noted (Figure 1) and then measured at its site of largest enhancement, which was recorded. Measurements of the tendon width (Figure 2a) and the width of the entire tendon sheath including contrast enhancing fluid (Figure 2b) were recorded in the coronal and sagittal planes. These measurements are seen in Table 2. If no obvious fluid was identified, measurements were obtained at the level of the proximal phalanx in the axial plane (Figure 3a and 3b). In approximately 20% of patients where the dorsal aspect of the flexor tendon sheath was immediately adjacent to bone, the flexor tendon sheath was measured from the volar surface of the phalanx to the volar extent of the sheath. A ratio was then created by dividing the width of the tendon sheath by the width of the tendon in both coronal (Figure 4a) and sagittal (Figure 4b) planes in order to establish a fixed relationship that eliminated individual anatomic variances. The primary outcome measure compared CR and SR between the FTS group and the FC group. Secondarily, we evaluated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of these ratios, both alone and coupled with Kanavel’s signs, to best judge the likelihood of infectious FTS with the help of CT measurements.
Figure 1.
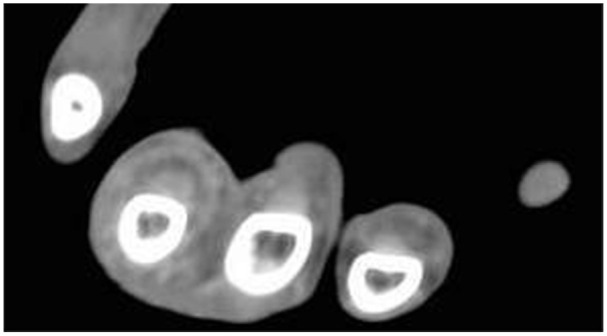
Axial computed tomography scan demonstrating “target sign,” with contrast enhanced fluid surrounding the flexor tendon and expanding the flexor tendon sheath at the level of the proximal phalanx.
Figure 2.
Axial computed tomography scan of flexor tenosynovitis patient demonstrating (a) flexor tendon width measurements in both the coronal and sagittal planes and (b) flexor tendon sheath width measurements in both the coronal and sagittal planes.
Table 2.
Radiographic Computed Tomography Findings.
| Variable | Flexor tenosynovitis group* (n = 35) | Finger cellulitis group* (n = 35) | P value |
|---|---|---|---|
| Radiologist reads fluid in tendon sheath (Y) | 18 (51%) | 0 (0%) | <.0001 |
| Study observer notes fluid in tendon sheath (Y) | 34 (97%) | 0 (0%) | <.0001 |
| Diameter of tendon sheath in coronal plane (mm) | 11.4 ± 2.5 | 9.62 ± 1.5 | <.001 |
| Diameter of tendon sheath in sagittal plane (mm) | 9.2 ± 2.8 | 6.2 ± 1.0 | <.0001 |
| Diameter of tendon in coronal plane (mm) | 7.6 ± 1.8 | 8.3 ± 1.4 | .09 |
| Diameter of tendon in sagittal plane (mm) | 5.5 ± 1.8 | 5.2 ± 0.9 | .33 |
| Ratio of tendon sheath diameter: tendon diameter in coronal plane (CR) | 1.5 ± 0.2 | 1.2 ± 0.08 | <.0001 |
| Ratio of tendon sheath diameter: tendon diameter in sagittal plane (SR) | 1.7 ± 0.3 | 1.2 ± 0.09 | <.0001 |
Note. CR = coronal plane; SR = sagittal plane.
Values are expressed as means ± standard deviations or as absolute values with percentages in parentheses.
Figure 3.
Axial computed tomography scan of finger cellulitis patient demonstrating no excess fluid in the flexor tendon sheath with (a) coronal and sagittal measurements of the flexor tendon, and (b) coronal and sagittal measurements of the flexor tendon sheath.
Figure 4.
Axial computed tomography scan of flexor tenosynovitis patient demonstrating (a) coronal plane ratio calculation (CR = A/B) and (b) sagittal plane ratio calculation (SR = A/B).
Statistical Analysis
After data collection, descriptive statistics were performed with means, ranges, and confidence intervals calculated for continuous variables and compared using 2-tailed student’s t-tests. Frequencies were calculated for dichotomous variables and compared using Fisher’s exact test for increased accuracy in small proportion analysis. Sensitivity, specificity, PPV, NPV, and + LR were calculated in regard to multiple clinical scenarios. To better identify the predictivity of FTS based on CT, bivariate logistic regression analysis was used to estimate the odds of FTS associated with multiple variables while adjusting for confounding factors. Holm-Bonferroni step-down method was used to account for increases in type 1 error due to multiple comparisons. A significance level of P < .05 was used.
Results
Overall, 70 patients were included in the study, with 35 in the FTS group and 35 in the FC group. There were no statistically significant differences in demographics between the 2 groups. However, when comparing vital signs, laboratory values, and physical examination findings, there were several notable differences (Table 1). The number of Kanavel signs identified was, on average, significantly higher in the FTS group at 2.9 compared to 0.5 in the FC group (P <.05). Additionally, all individual Kanavel signs had a higher incidence of detection in the FTS group, with pain to palpation being most common and flexed digit posture being least common in the FTS population. Uniform swelling was noted in 29% of FC patients, decreasing the specificity of this finding for FTS.
Computed tomography findings are described fully in Table 2. Radiologists only detected fluid in the flexor tendon sheath in 51% of the FTS group, compared to 97% that were detected retrospectively by our observer. Patients were noted to have maximal swelling in the tendon sheath at the level of proximal phalanx 77% of the time, with 20% at the level of the MCP joint or just proximal. Flexor tendon sheath width was larger in the FTS group in both coronal and sagittal planes, while the tendon width itself was not statistically different in either plane. The CR was 1.5 in the FTS group, compared to 1.2 in the FC group (P <.05), while the SR was 1.7 and 1.2 in the FTS and FC groups, respectively (P < .05). Sensitivity, specificity, PPV, NPV, and positive likelihood ratio (+LR) for CT abnormalities indicative of FTS are noted in Table 3. CR and SR cutoffs of ≥ 1.3 were chosen as they provided the highest combined sensitivity and specificity for both CR and SR and provided a simple reference number to be used in practice. Similar calculations were performed for Kanavel signs. Sensitivity and specificity were both improved with at least 2 Kanavel signs, with specificity increasing with the number of Kanavel signs. In an attempt to create a stronger, combined algorithm for predicting FTS, CT ratio findings ≥ 1.3 were combined with at least 2 positive Kanavel signs. This combination demonstrated a sensitivity/specificity of 66%/97% and 86%/97% for the coronal and sagittal plane measurements, respectively. The combination of a patient with ≥ 2 Kanavel signs and a ≥ 1.3 CR represents a 96% PPV, with a similar situation for SR ≥ 1.3 representing a 97% PPV.
Table 3.
Likelihood of Flexor Tenosynovitis Diagnosis from CT and/or Physical Exam.
| Variable | Sensitivity* | Specificity* | PPV* | NPV* | +LR |
|---|---|---|---|---|---|
| CR ≥ 1.3 | 83% | 97% | 97% | 85% | 28 |
| SR ≥ 1.3 | 100% | 94% | 95% | 100% | 17 |
| 4 Positive Kanavel signs | 34% | 100% | 100% | 60% | 34 |
| 3 Positive Kanavel signs | 74% | 100% | 100% | 80% | 24 |
| 2 Positive Kanavel signs | 86% | 83% | 83% | 85% | 5 |
| 1 Positive Kanavel sign | 97% | 66% | 74% | 96% | 3 |
| ≥ 3 Positive Kanavel signs and ≥ 1.3 CR | 60% | 97% | 95% | 71% | 20 |
| ≥ 3 Positive Kanavel signs and ≥ 1.3 SR | 69% | 97% | 96% | 76% | 23 |
| ≥ 2 Positive Kanavel signs and ≥ 1.3 CR | 66% | 97% | 96% | 74% | 22 |
| ≥ 2 Positive Kanavel signs and ≥ 1.3 SR | 86% | 97% | 97% | 87% | 29 |
Note. CT = computed tomography; PPV = positive predictive value; NPV = negative predictive value; LR = likelihood ratio; CR = coronal plane; SR = sagittal plane.
Values are expressed as means ± standard deviations or as absolute values with percentages in parentheses.
Finally, binary logistic regression analysis of all cases combined was performed. This analysis showed that no demographic variables were predictors of FTS. Every increase in 1 Kanavel sign demonstrated a 14% increase in likelihood of FTS. For every increase in CR and SR by 0.1 on CT, the likelihood of FTS increased by 5.9% and 5.5%, respectively.
Discussion
The primary goal of this study was to define whether contrast-enhanced CT scans can be used as another tool when differentiating between FTS and other hand infections. The aforementioned results support that CT does have a role as an adjunct to the commonly referenced Kanavel signs, especially when not all 4 signs are present. 11 With the alleged sensitivity of Kanavel’s signs, FTS has long been referred to as a clinical diagnosis, and supplementary imaging has been discouraged. 12 However, we demonstrated that up to 66% of patients did not present with all 4 Kanavel signs. Even more, 26% of patients with confirmed FTS only showed 2 Kanavel signs. Surprisingly, specificity in our study was higher than previously reported by others. 12 Based on the high PPV and +LR, they continue to have clinical utility. However, we demonstrate that some patients with FTS will still be misdiagnosed based on current practices, leading to delay in eventual treatment and increased patient morbidity.7,8 Unfortunately, other physical exam findings and laboratory markers do not provide much clarity in diagnosing FTS. In this study, no statistically significant increases in WBC, CRP, or ESR were noted to indicate usefulness in diagnosing FTS.
In order to decrease variability between patients based on stature, sex, and size of the injured finger, we created a ratio that compares both coronal and sagittal plane tendon sheath to tendon width. We found significantly higher ratios in both the coronal and sagittal planes in the FTS group compared to the FC group. These ratios are indicative of fluid, presumably infectious, surrounding the flexor tendon sheath, starving it of nutrients and likely altering its normal structure and function. We call this CT finding the “target sign,” with fluid surrounding the tendon. Both CR and SR were significantly higher in the FTS group at 1.5 and 1.7, respectively. SR ≥ 1.3 demonstrated sensitivity, specificity, PPV, and NPV of 100%, 94%, 95%, and 100%. CR ratio of ≥ 1.3 was also evaluated and demonstrated 83% sensitivity and 97% specificity. Additionally, a combined algorithm using CT ratios and Kanavel signs was designed based on the combination of variables with the highest +LR, which were those with ≥ 2 positive Kanavel signs with CR or SR ≥ 1.3. Specificity and PPV were universally high with any combination of ratio and number of Kanavel signs.
Binary logistic regression analysis was performed to help predict the presence of FTS demonstrated important findings. Considering the average number of Kanavel signs (2.9), CR (1.5), and SR (1.7) in the current FTS group, our model would correctly diagnose 91% of FTS cases. This compares to the averages in the FC group, which have a 5% likelihood of clinical FTS. For every 0.1 increase in CR and SR, the likelihood of FTS increases 5.9% and 5.5%, respectively. For every additional Kanavel sign, the likelihood of FTS increased 14%, offering benefit to our clinical algorithm.
Based on this data, a contrast-enhanced CT scan as an adjunct to evaluate for FTS is a reliable objective test that can help in patients that do not have a straightforward or “classic” presentation. CT evaluating extensor tendons in this manner has been mentioned by Reinus et al, 21 but no formal studies have objectified its usefulness with FTS. A primary limitation of CT is the risk of patient radiation exposure, which has previously made ultrasound and MRI other options for diagnosis. Unfortunately, ultrasound is user dependent with low specificity. For this reason, it is difficult to determine the need for operative intervention in the setting of a hand infection using only ultrasound. 17 MRI is considered the gold standard by many but is expensive and access is often limited in rural areas or during off-hours, significantly delaying diagnosis and affecting patient outcomes. 16
This study has several limitations. First, a larger sample size may help reinforce our findings and strengthen our conclusions. This being said, with FTS being largely a clinical diagnosis up to this point, it is difficult to gather larger cohorts with confirmed FTS who have CT scans to evaluate. The retrospective nature of the study may also contribute some selection bias with regard to which patients were chosen to receive CT scans. However, given the fact that more severe cases are often taken to the operating room without further imaging, most patients who received imaging likely had a borderline clinical presentation. This may increase value and external validity of our study, given the significant differences in CT measurements between cohorts. The study is also limited by possible imaging variability and the consistency of CT measurements performed by the study’s authors. While efforts were made to standardize this process, there may be some reliability issues if these measurements were being performed by an outside radiologist or surgeon. Despite this possible issue, we do feel as if the target sign is recognizable and the measurements described in this study are reproducible and not technically difficult.
In conclusion, patients with FTS must be recognized and treated promptly as previous studies have shown tendon necrosis, adhesion formation, spread to the deep fascial spaces, and permanent loss of hand function as possible sequela.7,9 CT measurements, particularly the CR and SR, when ≥ 1.3, increase the ability to radiographically differentiate FTS, possibly decreasing the amount of cases that are missed. While SR and CR ≥ 1.3 serve as reasonable cutoffs, the predictivity of these ratios continues to improve by almost 6% for every 0.1 increase in CR or SR. The use of CT does not eliminate the need for a proper physical examination, however. The combination of elevated SR/CR with ≥ 2 Kanavel signs maintains high sensitivity and PPV, with predictivity increasing 14% for every additional Kanavel sign recorded. Therefore, we recommend the use of CT as an adjunct to physical examination to provide objective data, tendon sheath coronal and sagittal ratios ≥ 1.3, to assist with the diagnosis of FTS.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects
Statement of Informed Consent: Informed consent was not required for this study as it is retrospective and all patient information was de-identified.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BT receives payments from ZimmerBiomet, Atricure, and Temple University. In addition to these he also has a relationship with Orthobullets.com. None of these are relevant to this manuscript. The remaining authors do not have any disclosures of a conflict of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Devon M. Myers  https://orcid.org/0000-0002-2907-1166
https://orcid.org/0000-0002-2907-1166
Craig Goubeaux  https://orcid.org/0000-0003-1059-4538
https://orcid.org/0000-0003-1059-4538
References
- 1. Pang HN, Teoh LC, Yam AK, et al. Factors affecting the prognosis of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2007;89(8):1742-1748. doi: 10.2106/JBJS.F.01356. [DOI] [PubMed] [Google Scholar]
- 2. Weinzweig N, Gonzalez M. Surgical infections of the hand and upper extremity: a county hospital experience. Ann Plast Surg. 2002;49(6):621-627. doi: 10.1097/00000637-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 3. Glass KD. Factors related to the resolution of treated hand infections. J Hand Surg Am. 1982;7(4):388-394. doi: 10.1016/s0363-5023(82)80150-0. [DOI] [PubMed] [Google Scholar]
- 4. Hyatt BT, Bagg MR. Flexor tenosynovitis. Orthoped Clin North Am. 2017;48(2):217-227. doi: 10.1016/j.ocl.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 5. Fowler JR, Ilyas AM. Epidemiology of adult acute hand infections at an urban medical center. J Hand Surg Am. 2013;38(6):1189-1193. doi: 10.1016/j.jhsa.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 6. Tosti R, Trionfo A, Gaughan J, et al. Risk factors associated with clindamycin-resistant, methicillin-resistant Staphylococcus aureus in hand abscesses. J Hand Surg Am. 2015;40(4):673-676. doi: 10.1016/j.jhsa.2014.12.044. [DOI] [PubMed] [Google Scholar]
- 7. Evgeniou E, Iyer S. Pyogenic flexor tenosynovitis leading to an amputation. BMJ Case Rep. 2012;2012:bcr2012006778. doi: 10.1136/bcr-2012-006778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boles SD, Schmidt CC. Pyogenic flexor tenosynovitis. Hand Clin. 1998;14(4):567-578. [PubMed] [Google Scholar]
- 9. Chapman T, Ilyas AM. Pyogenic flexor tenosynovitis: evaluation and treatment strategies. J Hand Surg Am. 2019;44(11):981-985. doi: 10.1016/j.jhsa.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 10. Giladi AM, Malay S, Chung KC. A systematic review of the management of acute pyogenic flexor tenosynovitis. J Hand Surg Eur Vol. 2015;40(7):720-728. doi: 10.1177/1753193415570248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanavel AB. Infections of the Hand. Philadelphia: Lea & Febiger; 1912. [Google Scholar]
- 12. Kennedy CD, Lauder AS, Pribaz JR, et al. Differentiation between pyogenic flexor tenosynovitis and other finger infections. Hand (N Y). 2017;12(6):585-590. doi: 10.1177/1558944717692089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dailiana ZH, Rigopoulos N, Varitimidis S, et al. Purulent flexor tenosynovitis: factors influencing the functional outcome. J Hand Surg Eur Vol. 2008;33(3):280-285. doi: 10.1177/1753193408087071. [DOI] [PubMed] [Google Scholar]
- 14. Bishop GB, Born T, Kakar S, et al. The diagnostic accuracy of inflammatory blood markers for purulent flexor tenosynovitis. J Hand Surg Am. 2013;38(11):2208-2211. doi: 10.1016/j.jhsa.2013.08.094. [DOI] [PubMed] [Google Scholar]
- 15. Yi A, Kennedy C, Chia B, et al. Radiographic soft tissue thickness differentiating pyogenic flexor tenosynovitis from other finger infections. J Hand Surg Am. 2019;44(5):394-399. doi: 10.1016/j.jhsa.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 16. Patel DB, Emmanuel NB, Stevanovic MV, et al. Hand infections: anatomy, types and spread of infection, imaging findings, and treatment options. Radiographics. 2014;34(7):1968-1986. doi: 10.1148/rg.347130101. [DOI] [PubMed] [Google Scholar]
- 17. Jardin E, Delord M, Aubry S, et al. Usefulness of ultrasound for the diagnosis of pyogenic flexor tenosynovitis: a prospective single-center study of 57 cases. Hand Surg Rehabil. 2018;37(2):95-98. doi: 10.1016/j.hansur.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 18. Sexton J, Pittman M, Morrow D. Flexor tenosynovitis using ultrasound. J Emerg Med. 2019;56(5):560-561. doi: 10.1016/j.jemermed.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 19. Hubbard D, Joing S, Smith SW. Pyogenic flexor tenosynovitis by point-of-care ultrasound in the emergency department. Clin Pract Cases Emerg Med. 2018;2(3):235-240. doi: 10.5811/cpcem.2018.3.37415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Padrez K, Bress J, Johnson B, et al. Bedside ultrasound identification of infectious flexor tenosynovitis in the emergency department. West J Emerg Med. 2015;16(2):260-262. doi: 10.5811/westjem.2015.1.24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reinus WR, De Cotiis D, Schaffer A. Changing patterns of septic tenosynovitis of the distal extremities. Emerg Radiol. 2015;22(2):133-139. doi: 10.1007/s10140-014-1258-5. [DOI] [PubMed] [Google Scholar]
- 22. Koshy JC, Bell B. Hand infections. J Hand Surg Am. 2019;44(1):46-54. doi: 10.1016/j.jhsa.2018.05.027. [DOI] [PubMed] [Google Scholar]