Abstract
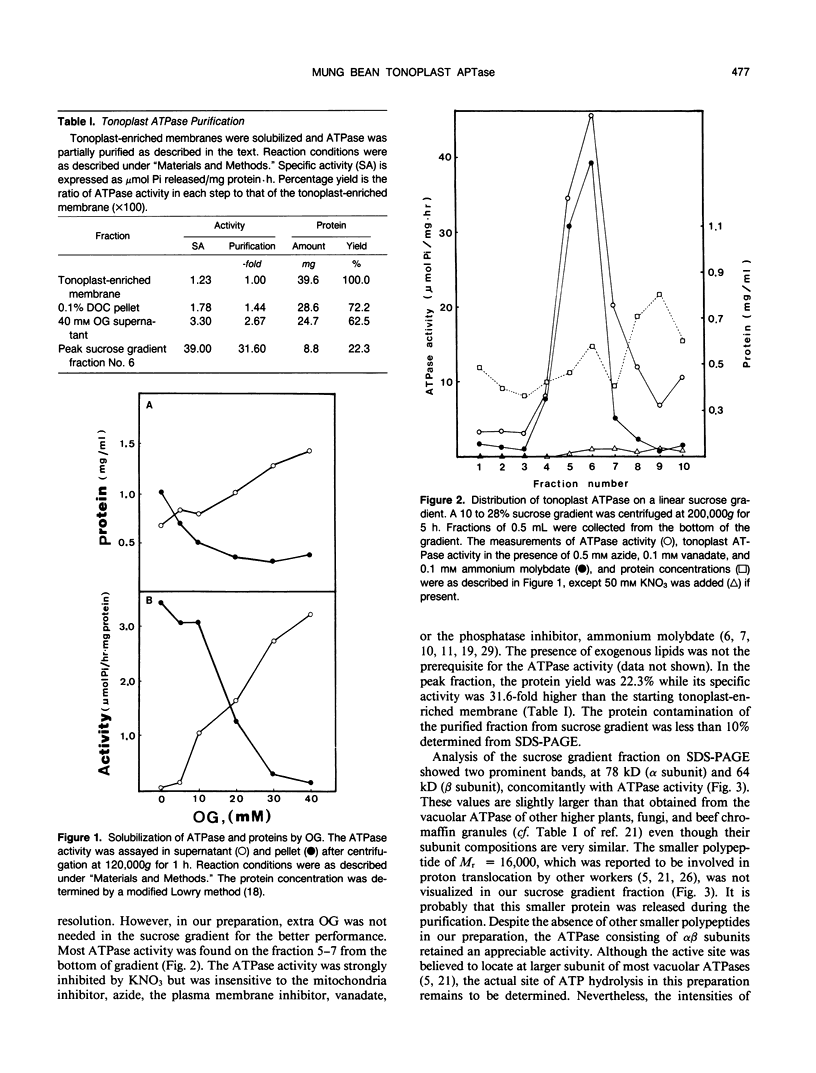
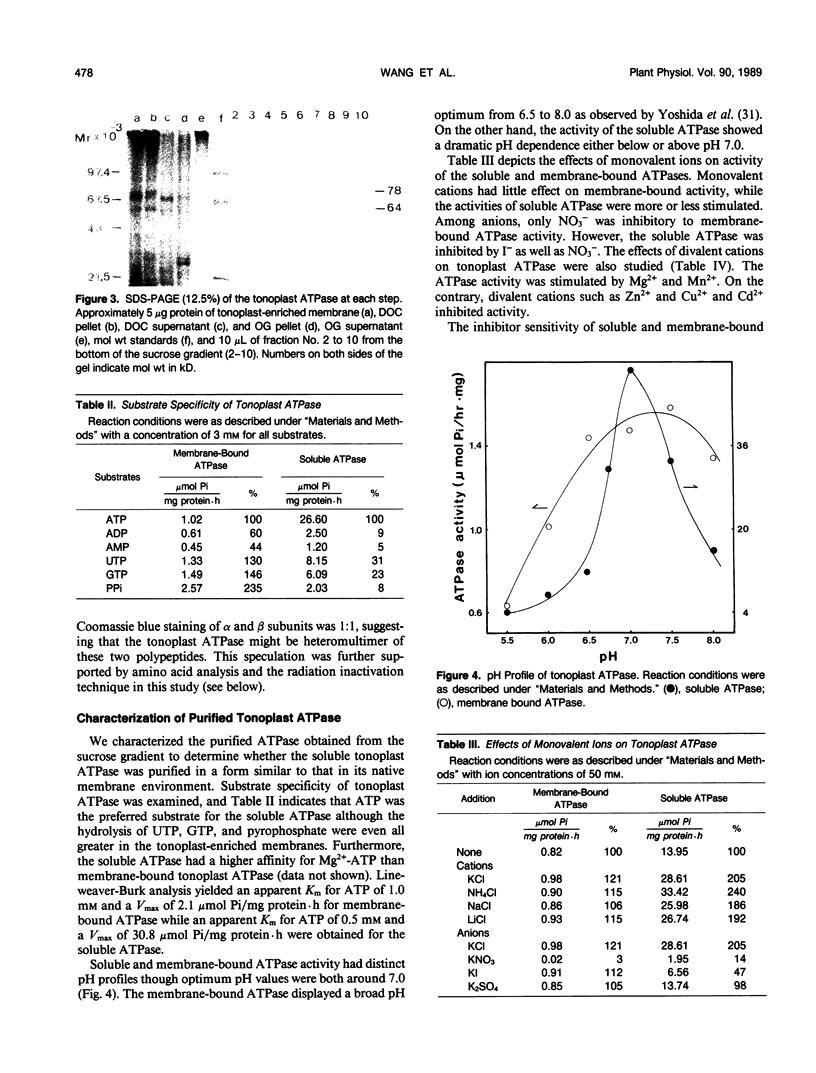
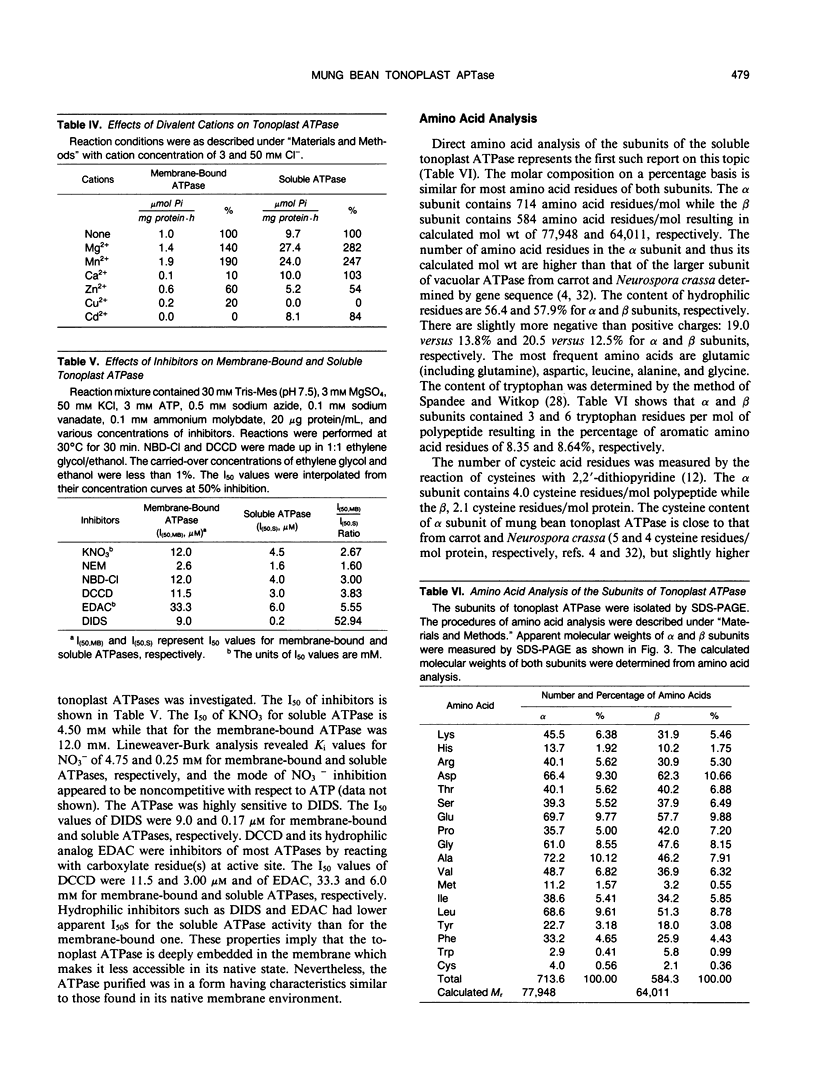
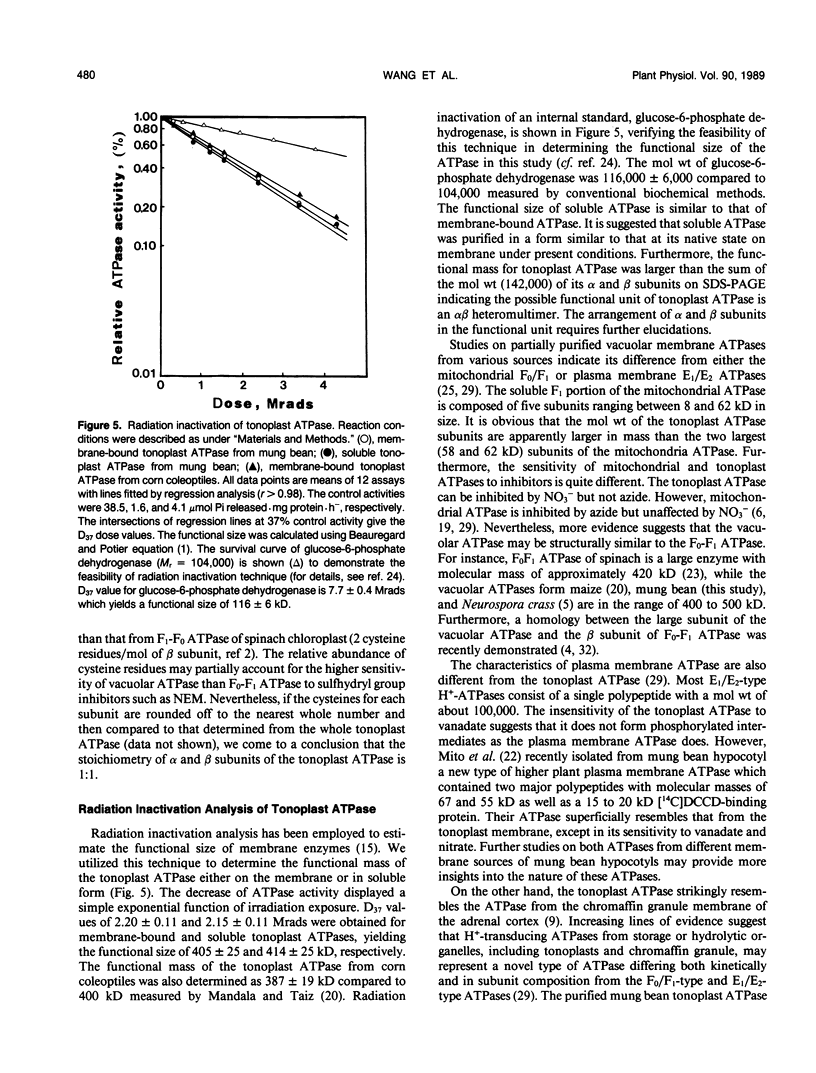
The tonoplast ATPase from etiolated seedlings of Vigna radiata L. (mung bean) was isolated using a two-step detergent solubilization modified from Mandala and Taiz (S Mandala, L Taiz [1985] Plant Physiol 78: 327-333). After ultracentrifugation on 10 to 28% sucrose gradient, the ATPase showed a 31.6-fold purification over the initial specific activity of the starting tonoplast-enriched membranes. The purified ATPase used Mg2+-ATP as the preferred substrate. The tonoplast ATPase was isolated in a form with characteristics similar to that on its native membrane environment. Analysis by SDS-PAGE revealed two prominent bands with molecular weights of 78,000 (α subunit) and 64,000 (β subunit). The intensity of Coomassie blue staining showed a 1:1 stoichiometry for α and β subunits. The amino acid composition of α and β subunits also confirmed the suggested stoichiometry of the subunit composition of the tonoplast ATPase. Moreover, radiation inactivation analysis yielded a functional size of 414 ± 24 and 405 ± 25 kilodaltons for soluble and membrane bound tonoplast ATPases, respectively. It is possible that the functioning tonoplast ATPase may be in a form of αβ-heteromultimer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauregard G., Potier M. Temperature dependence of the radiation inactivation of proteins. Anal Biochem. 1985 Oct;150(1):117–120. doi: 10.1016/0003-2697(85)90448-8. [DOI] [PubMed] [Google Scholar]
- Binder A., Jagendorf A., Ngo E. Isolation and composition of the subunits of spinach chloroplast coupling factor protein. J Biol Chem. 1978 May 10;253(9):3094–3100. [PubMed] [Google Scholar]
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Slayman C. W. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979 Apr 25;254(8):2928–2934. [PubMed] [Google Scholar]
- Bowman E. J., Tenney K., Bowman B. J. Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-1 encoding the 67-kDa subunit reveals homology to other ATPases. J Biol Chem. 1988 Oct 5;263(28):13994–14001. [PubMed] [Google Scholar]
- Churchill K. A., Sze H. Anion-sensitive, h-pumping ATPase in membrane vesicles from oat roots. Plant Physiol. 1983 Mar;71(3):610–617. doi: 10.1104/pp.71.3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cidon S., Nelson N. A novel ATPase in the chromaffin granule membrane. J Biol Chem. 1983 Mar 10;258(5):2892–2898. [PubMed] [Google Scholar]
- Dupont F. M., Burke L. L., Spanswick R. M. Characterization of a partially purified adenosine triphosphatase from a corn root plasma membrane fraction. Plant Physiol. 1981 Jan;67(1):59–63. doi: 10.1104/pp.67.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Higgins R. C., Dahmus M. E. Rapid visualization of protein bands in preparative SDS-polyacrylamide gels. Anal Biochem. 1979 Mar;93(2):257–260. doi: 10.1016/s0003-2697(79)80148-7. [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Sze H. Potential-dependent anion transport in tonoplast vesicles from oat roots. Plant Physiol. 1987 Mar;83(3):483–489. doi: 10.1104/pp.83.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson E., Howlett B., Jagendorf A. Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem. 1986 Jun;155(2):243–248. doi: 10.1016/0003-2697(86)90432-x. [DOI] [PubMed] [Google Scholar]
- Mandala S., Taiz L. Characterization of the subunit structure of the maize tonoplast ATPase. Immunological and inhibitor binding studies. J Biol Chem. 1986 Sep 25;261(27):12850–12855. [PubMed] [Google Scholar]
- Mandala S., Taiz L. Partial purification of a tonoplast ATPase from corn coleoptiles. Plant Physiol. 1985 Jun;78(2):327–333. doi: 10.1104/pp.78.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan R. S., Chien L. F., Wang M. Y., Tsai M. Y., Pan R. L., Hsu B. D. Functional size of photosynthetic electron transport chain determined by radiation inactivation. Plant Physiol. 1987 Sep;85(1):158–163. doi: 10.1104/pp.85.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall S. K., Sze H. Properties of the partially purified tonoplast H+-pumping ATPase from oat roots. J Biol Chem. 1986 Jan 25;261(3):1364–1371. [PubMed] [Google Scholar]
- Rea P. A., Griffith C. J., Sanders D. Purification of the N,N'-dicyclohexylcarbodiimide-binding proteolipid of a higher plant tonoplast H+-ATPase. J Biol Chem. 1987 Oct 25;262(30):14745–14752. [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Decrease of pH Gradients in Tonoplast Vesicles by NO(3) and Cl: Evidence for H-Coupled Anion Transport. Plant Physiol. 1987 Mar;83(3):490–496. doi: 10.1104/pp.83.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood J. B., Shouval D. Continuous production of erythropoietin by an established human renal carcinoma cell line: development of the cell line. Proc Natl Acad Sci U S A. 1986 Jan;83(1):165–169. doi: 10.1073/pnas.83.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Sze H. Similarities and differences between the tonoplast-type and the mitochondrial H+-ATPases of oat roots. J Biol Chem. 1985 Sep 5;260(19):10434–10443. [PubMed] [Google Scholar]
- Yoshida S., Kawata T., Uemura M., Niki T. Isolation and Characterization of Tonoplast from Chilling-Sensitive Etiolated Seedlings of Vigna radiata L. Plant Physiol. 1986 Jan;80(1):161–166. doi: 10.1104/pp.80.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimniak L., Dittrich P., Gogarten J. P., Kibak H., Taiz L. The cDNA sequence of the 69-kDa subunit of the carrot vacuolar H+-ATPase. Homology to the beta-chain of F0F1-ATPases. J Biol Chem. 1988 Jul 5;263(19):9102–9112. [PubMed] [Google Scholar]



