Abstract
Significantly fewer thraustochytrid protists (zoosporic fungi) were observed in association with healthy leaf tissue of the marine angiosperm Thalassia testudinum than in association with sterilized samples that were returned to the collection site for 48 h. In support of the hypothesis that sea grass secondary metabolites were responsible for these differences, extracts of healthy T. testudinum leaf tissues inhibited the growth of the co-occurring thraustochytrid Schizochytrium aggregatum and deterred the attachment of S. aggregatum motile zoospores to an extract-impregnated substrate. By using S. aggregatum for bioassay-guided chemical fractionation, a new flavone glycoside was isolated and structurally characterized as luteolin 7-O-β-d-glucopyranosyl-2"-sulfate. Whole-leaf tissue concentrations of this metabolite (4 mg/ml of wet leaf tissue) inhibited S. aggregatum attachment, and a significantly lower concentration (270 μg/ml) reduced thraustochytrid growth by 50%, suggesting that natural concentrations are at least 15 times greater than that needed for significant microbiological effects. These results offer the first complete chemical characterization of a sea grass sulfated flavone glycoside and provide evidence that a secondary metabolite chemically defends T. testudinum against fouling microorganisms.
The sea grass Thalassia testudinum (turtle grass) is an important component of nearshore marine ecosystems, providing nursery grounds for commercially relevant fish and invertebrate species, stabilizing sediments, and fixing significant amounts of inorganic carbon. The health of T. testudinum communities, however, is periodically jeopardized by mass mortality events that are believed to be caused by pathogenic Labyrinthula spp. (9, 34), the same zoosporic fungi responsible for wasting epidemics in the temperate sea grass Zostera marina (28, 37). In addition, it has been shown that epiphytes reduce sea grass photosynthetic rates and proposed that this stress reduces fitness (35). Given the potential threat imposed by Labyrinthula spp. and the detrimental effects of a heavy epiphyte load, there is ample ecological rationale for marine macrophytes to maintain some type of antimicrobial chemical defense to reduce the rates of infection and surface fouling. Although the secondary metabolites of marine plants have been studied in detail (reference 10 and references therein) and antimicrobial chemical defenses are well documented for some terrestrial plants (23), the effects of marine plant secondary metabolites on co-occurring microorganisms remain largely undocumented (16).
Sea grasses are a rich source of secondary metabolites, particularly phenolic compounds (25). Phenolic compounds are well known as allelopathic agents in terrestrial plants (40), and similar ecological functions have been proposed for sea grasses (45). In support of this suggestion, polyphenolic compounds have long been associated with reduced fouling in seaweeds (38; but see reference 20), and it has been proposed that eelgrass chemistry alters the composition of the epiphytic community (15). In addition, the total concentration of phenolic compounds in Z. marina was shown to increase in response to infection by L. zostera (42), and a phenolic compound purified from this sea grass had antifouling activity (41). Sea grass phenolic compounds include sulfated flavonoids, a group of conjugated metabolites for which the sulfate component is believed to represent a marine adaptation (14). Although ecological roles have been suggested for these conjugated metabolites (13), the ecological effects of purified flavones (or, for that matter, virtually any marine secondary metabolites) against ecologically relevant microorganisms have yet to be documented.
The thraustochytrids, a group of zoosporic marine microorganisms that can functionally (not phylogenetically) be described as fungi (2), represent one group of marine microorganisms for which questions about the ecological effects of marine secondary metabolites need to be addressed. Thraustochytrids (kingdom Stramenopila, phylum Labyrinthulomycota, family Thraustochytriaceae) are obligate marine protists characterized by the production of motile, heterokont zoospores and a globose somatic thallus and ectoplasmic network (31). Although the ecological roles of these microbes are not well defined, they have been isolated in greatest numbers from coastal and estuarine environments (27) and have been reported parasitizing marine invertebrates (30) and, in at least one case, marine algae (11). In addition, thraustochytrids are saprobes and have been found in greater numbers on cast seaweeds than on living tissue (26), suggesting a role in algal decay. Reduced numbers of thraustochytrids observed on healthy algae have led to proposals that an undefined inhibiting factor (26), or more specifically antibiosis (32, 36), is responsible. To date, however, although algae have been shown to produce antimicrobial metabolites (10), exude into seawater metabolites that induce negative chemotactic responses in bacteria (3), and reduce the settlement of invertebrate larvae (44), quantitative studies documenting ecologically relevant microbiological activities for naturally occurring concentrations of chemically defined marine plant metabolites have not been performed.
This study documents the occurrence of thraustochytrids on healthy (GREEN) and dead (BROWN) T. testudinum that had been sterilized by autoclaving and reintroduced to the collection site. The observation that these microbes occurred less frequently on healthy sea grass led us to investigate the secondary metabolites of T. testudinum and subsequently to isolate and structurally define the new, sulfated flavone glycoside luteolin 7-O-β-d-glucopyranosyl-2"-sulfate. This compound possesses significant ecologically relevant microbiological activity against the co-occurring thraustochytrid Schizochytrium aggregatum, supporting the hypothesis that T. testudinum maintains an effective antimicrobial chemical defense as a mechanism to reduce surface fouling.
MATERIALS AND METHODS
Sea grass collection.
The experiments described here were initiated during a research expedition to the island of Little San Salvador, Bahamas Islands, in June 1996 aboard the R/V Seward Johnson (Harbor Branch Oceanographic Institution). During this expedition, samples of the sea grass T. testudinum were collected from an extensive shallow-water sea grass bed located in a mangrove lagoon within the interior of the island. To assess thraustochytrid occurrence, 10 replicate sea grass samples, each consisting of 10 to 15 healthy sea grass leaves, were collected underwater in sterile 50-ml screw-cap centrifuge tubes. The sea grass leaves were mature, green, without obvious epiphytes, and with no visible sign of infection or injury. These samples were immediately brought back to the shipboard laboratory for processing. In addition, a large collection of approximately 1 kg of green sea grass leaves was made for subsequent chemical workup.
Enumeration of zoosporic fungi.
To compare the occurrence of zoosporic fungi (thraustochytrids) on healthy (GREEN) and dead (BROWN) T. testudinum leaves, the following experiments were undertaken. Two healthy blades from each T. testudinum replicate were rinsed with 25 ml of filtered seawater (pore size, 0.2 μm) and cut into pieces ca. 1 cm long. Five pieces (subsamples) from each replicate were placed onto a single plate of KMV medium for the enumeration of zoosporic fungi (31). All of the plates were sealed with Parafilm after inoculation and maintained in the laboratory (ca. 27°C), where they were monitored for 15 days through a Spencer AO binocular microscope at magnifications of ×10, ×30, and ×60. Thraustochytrids could be readily visualized at low-power magnification by their characteristic colony morphology as they grew away from the sea grass leaf tissue and out onto the agar surface. Representative colonies were isolated by repeated transfer on KMV medium and examined at higher-power magnification to confirm their identity. After 15 days of observation, the number of subsamples positive for thraustochytrids was recorded for each replicate and the replicates were assigned a cumulative numeric value of 0 (none of five subsamples positive) or 1 (five of five subsamples positive). The mean thraustochytrid occurrence on the 10 replicates (± standard deviation) was calculated from these cumulative numbers. This method of scoring (positive or negative) gave equal weight to large and small colonies and thereby eliminated the effects of differential growth rates as long as colony growth was sufficient to be visualized under the dissecting microscope.
To document thraustochytrid colonization of dead (BROWN) sea grass, healthy blades from each replicate collection were wrapped in aluminum foil and autoclaved (121°C) for 30 min. Two or three of the resulting BROWN blades from each replicate were placed in sterile 50-ml centrifuge tubes into which 10 holes (diameter, 0.6 cm) had been drilled with an ethanol-rinsed drill bit. The caps were secured on these tubes, and the tubes were placed in sterile whirl-packs for transport back to the collection site. Three controls were prepared in an analogous manner with nonautoclaved (GREEN) sea grass blades. At the collection site, the whirl-packs were opened underwater and the tubes were attached to the ends of 0.5-m lengths of three-ply polypropylene rope by insertion between a section of unwound strands. One tube was attached per rope, and the ropes were anchored to the sea grass bed at intervals of ca. 5 m. Because the ropes were buoyant, the tubes were held ca. 6 cm above the top of the sea grass bed. All the tubes were left in the field for 48 h, after which time they were placed in sterile whirl-packs underwater and brought back to the ship for immediate processing in the same manner as described above for GREEN samples, with five subsamples from each replicate (including controls) being inoculated onto KMV medium. Autoclaved controls were prepared by inoculating five subsamples from each BROWN replicate onto KMV medium immediately after autoclaving.
Sea grass extraction.
To determine if chemicals produced by T. testudinum affect thraustochytrid growth and attachment, organic compounds were extracted from the 1-kg green sea grass collection and the extracts were analyzed for mass, biological activity, and secondary-metabolite composition. All extractions were performed volumetrically by adding wet T. testudinum to a known volume of deionized water in a graduated cylinder and recording the volume of water displaced. The sea grass and water were blended for 5 min with a hand-held Hamilton Beach blender, and the resulting solution was passed through a Gelman Sciences type A/E 47-mm-diameter glass fiber filter. Filtered extracts were dried with a Savant SC200 SpeedVac and, for biological testing, dissolved in methanol (MeOH) in a volume equal to that of the sea grass extracted, resulting in solutions that contained what will be considered whole-leaf tissue concentrations of sea grass metabolites.
Disk diffusion assay.
Crude GREEN and BROWN T. testudinum extracts were tested at whole-leaf tissue concentrations for antibiotic activity against zoospores of the thraustochytrid S. aggregatum Goldstein and Belsky. This strain (T96-6) was isolated during the Bahamas expedition from a detached T. testudinum leaf collected as drift floating in Nassau Harbor, and the assay was performed by a modification of the standard antibiotic disk diffusion assay. In this modified assay, a zoospore suspension was prepared by adding an agar block containing a colony of S. aggregatum to a plate of B1 medium (0.25% peptone, 0.15% yeast extract, 0.15% glycerol, 1.6% agar, 100% seawater) and flooding this plate with 5 ml of autoclaved seawater. Within 24 to 36 h after seawater addition, S. aggregatum released highly motile zoospores (visible by low-power microscopy). The spore concentration was determined with the aid of a hemacytometer, and 100 μl of this suspension (105 to 106 spores/ml) was added to a plate containing B1 medium, spread with a sterile glass rod, and allowed to dry. GREEN T. testudinum extracts (25 μl, whole-leaf tissue concentration) or MeOH alone (control) were added to sterile paper disks, and the solvent was allowed to evaporate. Once dry, the paper disks were placed on the surface of the inoculated agar, and the plates were sealed with Parafilm and incubated at 30°C for 48 h. The spore inoculum was sufficient to produce confluent growth on the surface of the plate, and antibiotic activity was recorded as the diameter of clear zones of inhibited microbial growth around the paper disk.
Bioassay-guided fractionation and chemical characterization.
With the disk diffusion assay as a guide, the crude GREEN T. testudinum extract (2.2 g) was partitioned between water (1.4 g) and ethyl acetate (450 mg) and the active water fraction was dried, solubilized in MeOH, and subjected to Sephadex LH-20 (Pharmacia) size exclusion chromatography (100% MeOH; flow rate, 1.5 ml/min). The active fractions (188 mg), characterized by a yellow band on the column, were combined and further purified by semipreparative reversed-phase high-pressure liquid chromatography (HPLC) (8-μm Dynamax C18 column), using 40% MeOH–H2O with 0.1% trifluoroacetic acid. The major product of this separation was the biologically active flavone glycoside luteolin 7-O-β-d-glucopyranosyl-2"-sulfate (35 mg) with the following chemical characteristics: [α]D25 = −17.2° (H2O; concentration, 0.29). High-resolution-(−) fast atom bombardment mass spectrometry [M − H]− m/z calculated for C21H19O14S, 527.0496; observed, 527.0460 (Δ6.7 ppm); M− (relative intensity, 22%), 549 [(M + Na − H)−] (7.7), 447 (4.5), 419 (16.1), 285 (32.0), and 269 (11.9). UV λmax (log e) = 346 (4.13), 263 (4.06), and 251 (4.09) nm. IR νmax, 3352, 1637, 1257, 1091, and 1025 cm−1. 1H nuclear magnetic resonance spectroscopy (NMR) (multiplicity, J [hertz]; assignment): 300 MHz; dimethyl sulfoxide (DMSO)-d6: δ 3.27 (dd; 8.4; H4"), 3.49 (m; H5"), 3.50 (m; H6"), 3.60 (dd; 8.8; H3"), 3.71 (m; H6"), 4.02 (dd; 8.4; H2"), 5.29 (d; 7.4; H1"), 6.41 (d; 1.6; H6), 6.75 (s; H3), 6.76 (d; 1.6; H8), 6.90 (d; 8.3; H5′), 7.44 (s; H2′), and 7.46 (d; 8.3; H6′). 13C NMR (400 MHz, DMSO-d6): δ 95.0 (C-8), 99.6 (C-6), 103.1 (C-3), 105.4 (C-10), 113.5 (C-2′), 116.0 (C-5′), 119.1 (C-6′), 121.3 (C-1′), 145.7 (C-3′), 149.9 (C-4′), 156.8 (C-9), 161.1 (C-5), 162.7 (C-7), 164.4 (C-2), and 181.9 (C-4); sugar: 60.5 (C-6"), 69.4 (C-4"), 75.8 (C-3"), 76.8 (C-5"), 78.4 (C-2"), and 97.4 (C-1"). In addition, two related but less active compounds were obtained in low yield. These compounds were not characterized.
To obtain the acetate derivative, 2.5 mg of the flavone was treated with 0.5 ml of acetic acid-pyridine (1:1) and left overnight at room temperature. The reaction product was then dried under vacuum, dissolved in dichloromethane (CH2Cl2), and extracted with H2O. The CH2Cl2 layer was further purified by reversed-phase HPLC (70% MeOH–H2O) to yield the peracetate derivative. The following spectral data were obtained for the peracetate derivative: 1H NMR; 500 MHz, DMSO-d6 (multiplicity, J [hertz]; assignment): δ 2.07, 2.08, 2.13, 2.34, 2.36, 2.44 (s, 6 × Me), 3.90 (1H, m, H2"), 3.90 (1H, m, H5"), 4.19 (1H, dd, 12.0, 2.0, H6"), 4.29 (1H, dd, 12.0, 6.0, H6"), 5.12 (1H, dd, 9.6, H3"), 5.12 (1H, d, 7.0, H1"), 5.22 (1H, dd, 9.1, 9.6, H4"), 6.58 (1H, s), 6.76 (1H, d, 1.7), 7.06 (1H, d, 2.0), 7.37 (1H, d, 9.0), 7.70 (1H, br s), and 7.74 (1H, d, 8.5).
To determine the absolute stereochemistry of the sugar portion of the molecule, the natural product was subjected to acid hydrolysis (10% methanolic HCl) at 70°C for 3 h. The reaction products were neutralized with NaHCO3 and extracted with butanol. 1H NMR analysis of the aqueous layer indicated the presence of a glycoside, whose optical rotation was then determined.
Instrumental analyses.
NMR spectra were recorded in DMSO-d6 on Unity Plus 500-MHz, Gemini 400-MHz, and Unity Inova 300-MHz NMR spectrometers. The mass spectrum was measured in the negative FAB mode on a VG-ZAB mass spectrometer at the University of California, Riverside. The UV spectrum was measured with a Perkin-Elmer Lambda 4B UV/VIS spectrophotometer. Optical rotation was measured with a Rudolph Research Autopol III polarimeter. Quantitative HPLC was performed with a Hewlett-Packard model 1090 liquid chromatograph.
Quantification of the active compound in crude extract.
MeOH solutions of luteolin 7-O-β-d-glucopyranosyl-2"-sulfate were prepared at five concentrations (0.2 to 1.0 mg/ml), and 25 μl of each was injected into a Hewlett-Packard diode array HPLC instrument (5-μm ODS Hypersil C18 column) with 30% aqueous MeOH (plus 0.1% trifluoroacetic acid) as the eluent and UV detection at 350 nm. A standard curve relating concentration to peak area was generated, and the retention time and absorbance spectrum for the compound were recorded. Five replicate 1-cm3 T. testudinum water extracts were prepared as previously described. Following filtration (pore size, 0.2 μm), drying, and weighing, these extracts were brought up to 1 mg/ml in MeOH and 10 μl of each was injected as for the standard. Peak areas corresponding to luteolin 7-O-β-d-glucopyranosyl-2"-sulfate were recorded and converted to concentration by using the standard curve.
Settlement assay.
The effects of the crude GREEN T. testudinum extract and the purified flavone glycoside on the ability of S. aggregatum motile zoospores to attach to extract impregnated agar were measured by a modification of a previously described assay (43). For crude extracts, 8 ml of wet T. testudinum (GREEN) leaf tissue was extracted with deionized water, filtered, and dried and the residue was dissolved in 8 ml of molten B1 agar. The molten agar was poured into a sterile petri dish (60 by 16 mm) and, after solidification, cut into 1-cm2 blocks (treatment). Solvent-only B1 control blocks were also prepared. For luteolin 7-O-β-d-glucopyranosyl-2"-sulfate (which is yellow), Phytagel (Sigma Chemical Co., St. Louis, Mo.) was used as the compound rapidly diffused from agar, and Phytagel is reported to have superior retention properties in similar applications (18). The purified flavone glycoside was incorporated into Phytagel at whole-leaf tissue concentration (4 mg/ml) and prepared along with solvent controls as for the crude extracts.
To perform the settlement assay, a large volume of spore suspension was required relative to that used in the disk diffusion assay. This suspension was prepared by flooding a series of plates that had been inoculated with S. aggregatum (3 to 5 days earlier) with 10 ml of autoclaved seawater. The resulting spore suspensions were combined, and the spore concentration was determined with the aid of a hemacytometer (1.5 × 105 spores/ml). An 8-ml volume of this spore suspension was then added to each of 10 petri dishes. Five of the dishes were each given one treatment block and the remaining five were each given one control. Following a 2-h incubation, 1 ml of 37% formaldehyde (final concentration, 4%) was added to each dish to stop the experiment. After fixation for 5 min, each block was carefully removed with a spatula, dipped five times in a sterile seawater bath, and placed on a paper towel to remove excess water and then onto a microscope slide. A coverslip was placed on top of the agar block, and the block was viewed at ×400 magnification with an Olympus BHT phase-contrast microscope. Ten random microscope eyepiece grids (20 μm2) were counted for each replicate, and the mean values of the treatments and controls were compared (t test).
IC50 microtiter plate assay.
The concentration of luteolin 7-O-β-d-glucopyranosyl-2"-sulfate that inhibited the growth of S. aggregatum by 50% (IC50) was estimated (in triplicate) by serially diluting (1:4) the compound (starting concentration, 4.4 mg/ml in MeOH) in medium B1 (without agar) down the rows of a 96-well microtiter plate. MeOH controls were similarly prepared. Following compound dilution, 25 μl of S. aggregatum spore suspension (106 spores/ml), prepared as for the disk diffusion assay, was added to each well, and the plate was incubated at 30°C for 3 days. Following incubation, 10 μl from each well was spotted onto B1 medium and the plates were sealed with Parafilm and incubated at 30°C for 36 h. S. aggregatum formed well-developed colonies on B1 plates, and the compound concentration that reduced growth by 50% was determined visually by comparing the area of growth for each dilution to that from control wells.
RESULTS
Thraustochytrid occurrence on healthy and dead T. testudinum.
Four days after inoculation onto KMV medium, the occurrence of thraustochytrids on healthy (GREEN) specimens of T. testudinum was 60% (± 30%), indicating that, on average, three of five subsamples per replicate scored positive for thraustochytrid growth. Fifteen days after plating, the thraustochytrid occurrence on GREEN T. testudinum had increased to 78% (± 19%). It was observed that the increased occurrence of thrausochytrids over time on GREEN samples was correlated with a change in sea grass leaf tissue color from green to brown. The three GREEN T. testudinum controls (nonautoclaved blades returned to the field as for the BROWN samples) yielded similar results (on average, 73% of the subsamples were positive for thraustochytrids).
In contrast to the GREEN samples, sterilized (BROWN) T. testudinum incubated at the collection site for 48 h had a 100% thraustochytrid occurrence on KMV 3 days after being plated. All five subsamples of each of the 10 BROWN sea grass replicates produced luxuriant thraustochytrid growth around the entire margin of the sea grass. No growth was observed on the autoclaved controls, indicating that thraustochytrids colonized these sea grasses following reintroduction to the collection site. Growth on the GREEN samples, by contrast, was slow to develop and in general did not cover the entire margin of each subsample. These observations led us to investigate the possibility that secondary metabolites present in healthy (GREEN) sea grass leaf tissue account for reduced thrausochytrid growth and occurrence relative to autoclaved leaf tissue that had been reintroduced to the collection site.
Crude extract yield and antibiotic activity.
GREEN sea grass from the 1-kg collection was extracted volumetrically, with the average mass of 1 ml of leaf tissue aqueous extract being 156 ± 20 mg (n = 5, range = 125 to 181 mg). The whole-leaf tissue concentration at which extracts were tested was therefore 156 mg/ml. To determine if T. testudinum metabolites inhibited thraustochytrid growth, a GREEN extract was initially tested aboard ship at whole-leaf tissue concentration and proved to be active against S. aggregatum in the disk diffusion assay. Two additional GREEN extracts were subsequently prepared and tested in a similar manner at Scripps Institution of Oceanography and produced 10- and 12-mm-diameter zones of inhibited growth around extract-saturated disks.
Settlement activity in crude extract.
Because assays that measure antibiotic activity are not the most realistic method by which to study ecological effects (19), crude GREEN T. testudinum extracts were tested for their effects on the attachment of S. aggregatum zoospores to an extract-impregnated agar surface. In this assay, the mean number of thraustochytrids that attached to treated agar was 26.8 ± 9.1 (n = 5) while the number attaching to control blocks was 233 ± 21.8 (n = 5), demonstrating that GREEN extracts, tested at whole-leaf tissue concentrations, effectively deterred thraustochytrid attachment relative to controls. On average, T. testudinum extracts reduced the attachment of S. aggregatum by 88%, with the difference between treatment and control being highly significant (t test, P < 0.001).
Structure elucidation.
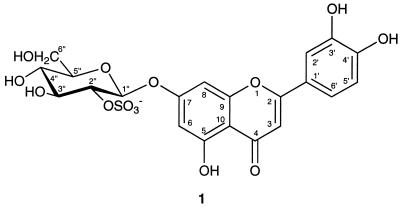
With the disk diffusion assay as a guide, it was possible to monitor the antibiotic activity detected in the GREEN T. testudinum crude extract through the chemical fractionation process and to obtain a pure, biologically active metabolite. The 1H NMR spectrum of this substance was indicative of a flavone glycoside, and the substance was identified as luteolin based on its UV spectrum. The 13C NMR data confirmed the identity of the flavone as luteolin with glycosylation at position 7 (24). The large coupling constant (7.4 Hz) exhibited by the anomeric proton provided evidence that the sugar was a β-glycoside, and it was tentatively identified as β-glucose due to the similarity of the 13C NMR shifts to published values (12). Because of coupling-pattern overlap in the NMR spectrum, the sugar was firmly established as β-glucose only upon NMR analysis of the acetylated product. Acid hydrolysis of the natural product yielded a sugar that exhibited a positive optical rotation ([α]d25 = +27.3°) indicative of a d-glycoside, thus confirming the presence of β-d-glucose in the molecule. The mass spectrum indicated a molecular formula of C21H19O14S for [M−] at m/z = 527.046. The presence of a sulfate group was confirmed by IR resonances at 1,257 and 1,091 cm−1. The position of the sulfate was assigned to C-2 of the sugar based on the downfield shift (+4 ppm) of the carbon at this position in the natural product; this was further supported by the relatively small shift (0.12 ppm) in the proton resonance of H-2 upon acetylation. Based on the above information, the final structure of the active substance was identified as luteolin 7-O-β-d-glucopyranosyl-2"-sulfate (Fig. 1). Although sea grasses have long been known to produce phenolic compounds including conjugated flavonoids of the type reported here (14), luteolin 7-O-β-d-glucopyranosyl-2"-sulfate represents a new structure and, to the best of our knowledge, the first complete chemical characterization of a sea grass sulfated flavone glycoside.
FIG. 1.
Chemical structure of the antibiotic flavone glycoside (luteolin 7-O-β-d-glucopyranosyl-2"-sulfate) isolated from T. testudinum.
Concentration of active compound in extract.
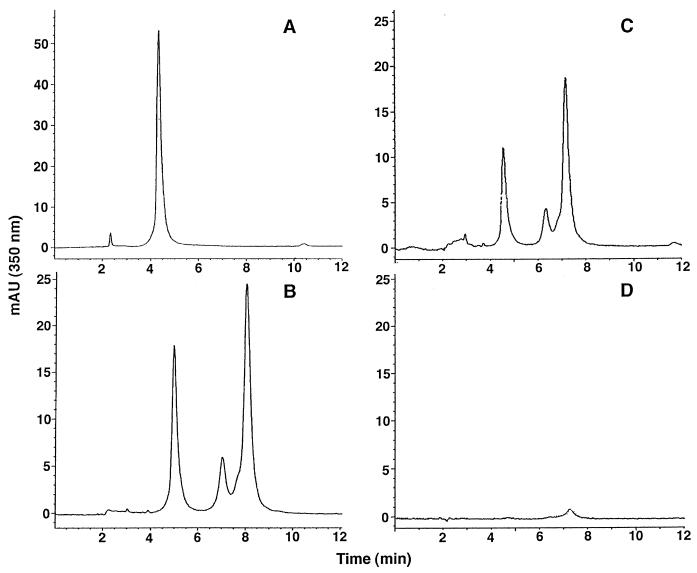
Given that the objective of this study was to determine if the biological activities associated with luteolin 7-O-β-d-glucopyranosyl-2"-sulfate have ecological relevance, the concentration of this substance in the GREEN extract was determined and used as a reference point for biological testing. Because this compound was water soluble and difficult to isolate quantitatively, it was necessary to calculate the concentration by using analytical-scale HPLC peak integration. By using this method, the mass of luteolin 7-O-β-d-glucopyranosyl-2"-sulfate in the water extract of 1 ml of wet T. testudinum tissue equaled 4.1 ± 1.3 mg (n = 5). This yield was equivalent to 2.6% of the extract mass (156 mg). For bioassay purposes, 4 mg/ml was considered the whole-leaf tissue or “natural” concentration of luteolin 7-O-β-d-glucopyranosyl-2"-sulfate in the GREEN sea grass tissue. The HPLC traces of this compound (standard) and of the crude GREEN extract are shown in Fig. 2. The absorbance spectra for the standard and the corresponding 5-min peak in the GREEN extract were identical, confirming that the compound being quantified was the flavone glycoside.
FIG. 2.
HPLC traces of luteolin 7-O-β-d-glucopyranosyl-2"-sulfate (A), extract from healthy (GREEN) T. testudinum (B), extract from autoclaved (BROWN) T. testudinum (C), and extract from autoclaved (BROWN) T. testudinum following a 48-h seawater incubation (D).
If luteolin 7-O-β-d-glucopyranosyl-2"-sulfate was responsible for the reduced numbers of thraustochytrids observed on healthy T. testudinum, it would be expected that this compound would not be present in the BROWN sea grass leaf tissue, since thraustochytrids occurred on 100% of these samples following a 48-h seawater incubation. However, extracts of autoclaved (BROWN) T. testudinum, prepared as for the GREEN extracts, showed no reduction in antibiotic activity, suggesting that the luteolin 7-O-β-d-glucopyranosyl-2"-sulfate is heat stable. HPLC analysis confirmed that this compound was intact and present at concentrations similar to those observed in the GREEN extract (Fig. 2). However, when BROWN sea grass was incubated in seawater for 48 h as for the in situ experiment, extract HPLC analysis revealed no trace of the flavone glycoside and the extract showed no antibiotic activity, indicating that the compound rapidly leached from dead sea grass leaf tissue during the incubation period.
Antibiotic and settlement activity.
The flavone glycoside isolated from the GREEN T. testudinum extract displayed antibiotic activity at whole-leaf tissue concentration (4 mg/ml) against S. aggregatum, producing a zone of inhibition 8 to 10 mm in diameter. This activity is slightly lower than that of the crude extract (zone of inhibition 10 to 12 mm in diameter), with the difference possibly being attributable to two related but low-yielding compounds that were detected in the crude extract but were not isolated. The IC50 of the purified flavone glycoside was equal to 270 μg/ml, indicating that the average sea grass leaf tissue concentration was ca. 15 times greater than the potency required to have a significant effect on thraustochytrid growth. The flavone, tested at the whole-leaf tissue concentration for settlement effects, reduced the attachment of S. aggregatum motile zoospores by 85% relative to that of controls, with the mean number of thraustochytrids attaching to the treated surface being 14.1 ± 3.3 (n = 5) and that of those attaching to the control being 2.2 ± 0.3 (n = 5). This difference was highly significant (t test, P = 0.008) and supports the ecological role of luteolin 7-O-β-d-glucopyranosyl-2"-sulfate in chemical defense against fouling microorganisms.
DISCUSSION
Marine plants and invertebrates are rich sources of biologically active secondary metabolites, many of which provide important ecological functions such as chemical defense against potential predators (1, 29). These metabolites may also provide antimicrobial chemical defense to ward off infection and fouling; however, to date we have gained only marginal insight into the ecological effects of marine secondary metabolites on co-occurring microorganisms (8, 16, 29). In cases where these issues have been addressed (see, e.g., references 5, 21, 22, 39, and 43), crude organic extracts have produced significant microbiological effects; however, the corresponding metabolites have not been isolated, characterized, or quantified in the host tissues, and therefore the chemical basis and ecological significance of the observed activities remain obscure.
In the present study, we observed reduced numbers of thraustochytrids on healthy (GREEN) T. testudinum relative to dead (BROWN) sea grass leaf tissue that had been sterilized by autoclaving and reintroduced to the collection site for 48 h. This observation led us to investigate the hypothesis that sea grass secondary metabolites regulate associated microbial populations and thereby provide a type of antimicrobial chemical defense that functions via reduced surface fouling. In support of this hypothesis, whole-leaf tissue concentrations of T. testudinum metabolites were antibiotic to the thraustochytrid S. aggregatum and subsequent bioassay-guided chemical fractionation of the crude extract led to the isolation and structural characterization of a new antibiotic flavone glycoside (luteolin 7-O-β-d-glucopyranosyl-2"-sulfate). This water-soluble sea grass metabolite occurred in volumetric sea grass leaf tissue extracts at a concentration of 4 mg/ml and significantly reduced the ability of highly motile and chemotactic S. aggregatum zoospores to attach to a compound-impregnated substrate. In addition, the IC50 of this compound for S. aggregatum was 270 μg/ml, suggesting that it can induce significant physiological effects at concentrations ca. 15 times lower than those occurring in sea grass leaf tissue. These results, coupled with the observation that the antibiotic flavone was undetectable in dead sea grass leaf tissue following a 48-h seawater incubation and that this loss of compound correlated with increased thraustochytrid occurrence, support our proposal that the antibiotic flavone glycoside functions within the sea grass as a chemical defense against fouling microorganisms.
There is a clear ecological rationale for sea grasses to maintain antimicrobial chemical defenses, since they are susceptible to periodic epidemics of Labyrinthula-induced diseases (9, 34, 37) and the shading effects of an unregulated fouling community can reduce plant fitness (35). That sea grasses are chemically defended is supported by the observation that thraustochytrids produce cellulases and have the independent ability to decompose submerged plant material yet do not begin this process in healthy plants (4) until some inhibiting factor ceases to be released (26). In the present study, the antibiotic luteolin 7-O-β-d-glucopyranosyl-2"-sulfate appears to be such a factor. Although thraustochytrids were isolated from healthy T. testudinum leaf tissue despite the presence of the antibiotic flavone glycoside, indicating that settlement deterrence is not complete, it is proposed that their reduced numbers are explained by the presence of this metabolite and that only after the leaves senesce and the compound has leached out from the tissue do increased growth and colonization occur and does the decomposition process begin.
It has long been suspected that phenolic compounds produced by marine plants function in chemical defense (see, e.g., reference 38) and that loss of phenolic compounds during the decomposition process is correlated with increased microbial biomass (7, 36). This concept was supported by past observations that thraustochytrid growth was inhibited on live algae relative to autoclaved seaweeds and inversely correlated with total phenolic compound content (32). In addition, it has been shown that phenolic compounds confer resistance to sea grass wasting diseases (6), increase in concentration as a result of infection by Labyrinthula (42), and have antifouling properties (41). The present study confirms that sea grass phenolic compounds have significant, ecologically relevant microbiological activity and indicates that this activity can be accounted for by the presence of a sulfated flavone glycoside, not simple phenolic acids similar to those implicated as the primary agents of chemical defense in land plants (33). By using bioassay-guided fractionation of the crude extract, it was evident that the majority of the biological activity could be accounted for by the presence of this conjugated metabolite, with the remainder being due to the presence of minor derivatives that could be recognized by NMR but were not characterized. These results indicate that simple phenolic compounds were not responsible for the biological activity of the crude extract and that the correlation of total phenolic compound concentration with biological activity may not accurately predict the potency of individual metabolites.
Elucidating the ecological effects of marine secondary metabolites at the microbiological level is made difficult by the lack of experimental methods by which these processes can be measured (19). Certainly, it is critical in any test of ecologically relevant biological activity that an attempt is made to reproduce the metabolite concentrations experienced by the assay organism(s) in nature. In this study and because of lack of information about compound release from sea grass tissues, we chose to test crude extracts and purified materials at average whole-leaf tissue concentrations. It has been estimated that as little as half of the total amount of compounds present in algae is recovered during the extraction and purification process (17), and, because sea grass leaves contain air bubbles that were not dislodged during volumetric measurements, it is likely that the concentrations tested were conservative relative to those occurring in sea grass tissues.
We propose that for luteolin 7-O-β-d-glucopyranosyl-2"-sulfate to have significant antifouling effects in nature, it must be continually released from the plant. Because this water-soluble metabolite was tested following incorporation into a matrix from which it could be observed to leach into the surrounding seawater (thereby creating a chemical gradient that potentially mimics that surrounding the healthy sea grass), we believe that the bioassay results are a good predictor of what occurs in nature. Even if in situ compound release rates are significantly lower than those generated in the laboratory bioassay, it remains possible that these rates are sufficient to account for the reduced occurrence of thraustochytrids observed on the surfaces of healthy T. testudinum tissues. Of course, alternative hypotheses, e.g., physical effects of the leaf surface, could explain the reduced numbers of thraustochytrids obtained from living sea grasses, and it remains to be determined if other factors are acting in concert with the observed chemical effects. That the antibiotic flavone did not lose biological activity following autoclaving and that only after seawater incubation and associated metabolite loss did thraustochytrid numbers increase support the proposal that the observed differences in thraustochytrid occurrence were due to chemical rather than physical effects. However, because we have no information about cellular location or in situ rates of compound release from sea grass leaf tissues and therefore no direct evidence that thraustochytrids contact the antibiotic in nature, it is not possible to draw absolute conclusions about the ecological effects of luteolin 7-O-β-d-glucopyranosyl-2"-sulfate on sympatric microorganisms. In addition, since dead (BROWN) leaves were created by autoclaving, it is not possible to extrapolate the results obtained here to naturally dead leaf tissues.
The present study is the first effort to fully characterize a sea grass sulfated flavonoid in an ecological context, and it resulted in the isolation of a new metabolite that exhibits significant ecologically relevant antibiotic and antifouling activity against a zoosporic marine fungus. Although sulfated flavone glycosides have been isolated from terrestrial plants, they are most prevalent in halophytes and their production is believed to represent a physiological adaptation to the marine environment (13). Although ecological functions for marine flavonoids have been proposed, the results presented here represent the first experimental evidence that these metabolites function in antimicrobial chemical defense.
REFERENCES
- 1.Bakus G J, Targett N M, Schulte B. Chemical ecology of marine organisms: an overview. J Chem Ecol. 1986;12:951–987. doi: 10.1007/BF01638991. [DOI] [PubMed] [Google Scholar]
- 2.Barr D J S. Evolution and kingdoms of organisms from the perspective of a mycologist. Mycologia. 1992;84:1–11. [Google Scholar]
- 3.Bell W, Mitchell R. Chemotactic and growth responses of marine bacteria to algal extracellular products. Biol Bull. 1972;142:265–277. [Google Scholar]
- 4.Bremer G B. Lower marine fungi (labyrinthulomycetes) and the decay of mangrove leaf litter. Hydrobiologia. 1995;295:89–95. [Google Scholar]
- 5.Bryan P J, Rittschof D, McClintock J B. Bioactivity of echinoderm ethanolic body-wall extracts: an assessment of marine bacterial attachment and macroinvertebrate larval settlement. J Exp Mar Biol Ecol. 1996;196:79–96. [Google Scholar]
- 6.Buchsbaum R N, Short F T, Cheney D P. Phenolic-nitrogen interactions in eelgrass, Zostera marina L.: possible implications for disease resistance. Aquat Bot. 1990;37:291–297. [Google Scholar]
- 7.Cundell A M, Brown M S, Stanford R, Mitchell R. Microbial degradation of Rhizophora mangle leaves immersed in the sea. Estuarine Coastal Mar Sci. 1979;9:281–286. [Google Scholar]
- 8.Davis A R, Targett N M, McConnell O J, Young C M. Epibiosis of marine algae and benthic invertebrates: natural products chemistry and other mechanisms inhibiting settlement and overgrowth. In: Scheuer P J, editor. Bioorganic marine chemistry. Berlin, Germany: Springer-Verlag KG; 1989. pp. 85–114. [Google Scholar]
- 9.Durako M J, Kuss K M. Effects of Labyrinthula infection on the photosynthetic capacity of Thalassia testudinum. Bull Mar Sci. 1994;54:727–732. [Google Scholar]
- 10.Faulkner D J. Marine natural products. Nat Prod Rep. 1996;13:75–125. doi: 10.1039/np9961300075. [DOI] [PubMed] [Google Scholar]
- 11.Gaertner A. Some fungal parasites found in the diatom populations of the Rosfjord area (South Norway) during March 1979. Veroeff Inst Meeresforsch Bremerhaven. 1979;16:139–157. [Google Scholar]
- 12.Gorin P A J, Mazurek M. Further studies on the assignment of signals in 13C magnetic resonance spectra of aldoses and derived methyl glycosides. Can J Chem. 1975;53:1212–1223. [Google Scholar]
- 13.Harborne J B. Flavonoid sulfates: a new class of natural products of ecological significance in plants. Prog Phytochem. 1977;4:189–208. [Google Scholar]
- 14.Harborne J B, Williams C A. Occurrence of sulfated flavones and caffeic acid esters in members of the Fluviales. Biochem Syst Ecol. 1976;4:37–44. [Google Scholar]
- 15.Harrison P G. Control of microbial growth and of amphipod grazing by water-soluble compounds from leaves of Zostera marina. Mar Biol. 1982;67:225–230. [Google Scholar]
- 16.Hay M E. Marine chemical ecology: what’s known and what’s next? J Exp Mar Biol Ecol. 1996;200:103–134. [Google Scholar]
- 17.Hay M E, Fenical W. Marine plant-herbivore interactions: the ecology of chemical defense. Annu Rev Ecol Syst. 1988;19:111–145. [Google Scholar]
- 18.Henrikson A A, Pawlik J R. A new antifouling assay method: results from field experiments using extracts of four marine organisms. J Exp Mar Biol Ecol. 1995;194:157–165. [Google Scholar]
- 19.Jenkins, K. M., P. R. Jensen, and W. Fenical. Bioassays with marine microorganisms. In K. Haynes and J. C. Millar (ed.), Methods in chemical ecology, in press. Chapman & Hall, New York, N.Y.
- 20.Jennings J G, Steinberg P D. Phlorotannins versus other factors affecting epiphyte abundance on the kelp Ecklonia radiata. Oecologia. 1997;109:461–473. doi: 10.1007/s004420050106. [DOI] [PubMed] [Google Scholar]
- 21.Jensen P R, Harvell C D, Wirtz K, Fenical W. Antimicrobial activity of extracts of Caribbean gorgonian corals. Mar Biol. 1996;125:411–419. [Google Scholar]
- 22.Kim K. Antimicrobial activity in gorgonian corals (Coelenterata: Octocorallia) Coral Reefs. 1994;13:75–80. [Google Scholar]
- 23.Levin D A. The chemical defenses of plants to pathogens and herbivores. Annu Rev Ecol Syst. 1976;7:121–159. [Google Scholar]
- 24.Markham K R, Chari V M, Mabry T J. Carbon-13 NMR spectroscopy of flavonoids. In: Harborne J B, Mabry T J, editors. The flavonoids: advances in research. New York, N.Y: Chapman & Hall; 1982. pp. 19–134. [Google Scholar]
- 25.McMillan C, Zapata O, Escobar L. Sulphated phenolic compounds in seagrasses. Aquat Bot. 1980;8:267–278. [Google Scholar]
- 26.Miller J D, Jones E B G. Observations on the association of thraustochytrid marine fungi with decaying seaweeds. Bot Mar. 1983;26:345–351. [Google Scholar]
- 27.Moss S T. Biology and phylogeny of the Labyrinthulales and Thraustochytriales. In: Moss S T, editor. The biology of marine fungi. New York, N.Y: Cambridge University Press; 1986. pp. 105–129. [Google Scholar]
- 28.Muehlstein L K, Porter D, Short F T. Labyrinthula sp., a marine slime mold producing the symptoms of wasting disease in eelgrass, Zostera marina. Mar Biol. 1988;99:465–472. [Google Scholar]
- 29.Paul V. Ecological roles of marine natural products. Ithaca, N.Y: Comstock Publishing Associates; 1992. [Google Scholar]
- 30.Porter D. Mycoses of marine organisms: an overview of pathogenic fungi. In: Moss S T, editor. The biology of marine fungi. New York, N.Y: Cambridge University Press; 1986. pp. 141–153. [Google Scholar]
- 31.Porter D. Labyrinthulomycota. In: Margulis L, Corliss J O, Melkonian M, Chapman D, editors. Handbook of Protoctista. Boston, Mass: Jones and Bartlett; 1990. pp. 388–398. [Google Scholar]
- 32.Raghukumar C, Nagarkar S, Raghukumar S. Association of thraustochytrids and fungi with living marine algae. Mycol Res. 1992;7:542–546. [Google Scholar]
- 33.Rice E L. Allelopathy. New York, N.Y: Academic Press, Inc.; 1974. [Google Scholar]
- 34.Roblee M B, Barber T R, Carlson P R, Jr, Durako M J, Fourqurean J W, Muehlstein L K, Porter D, Yarbro L A, Zieman R T, Zieman J C. Mass mortality of the tropical seagrass Thalassia testudinum in Florida Bay (USA) Mar Ecol Prog Ser. 1991;71:297–299. [Google Scholar]
- 35.Sand-Jensen K. Effect of epiphytes on eelgrass photosynthesis. Aquat Bot. 1977;3:55–63. [Google Scholar]
- 36.Sathe-Pathak V, Raghukumar S, Raghukumar C, Sharma S. Thraustochytrid and fungal component of marine detritus. I. Field studies on decomposition of the brown alga Sargassum cinereum. Indian J Mar Sci. 1993;22:159–167. [Google Scholar]
- 37.Short F T, Muehlstein L K, Porter D. Eelgrass wasting disease: cause and recurrence of a marine epidemic. Biol Bull. 1987;173:557–562. doi: 10.2307/1541701. [DOI] [PubMed] [Google Scholar]
- 38.Sieburth J M, Conover J T. Sargassum tannin, an antibiotic which retards fouling. Nature. 1965;208:52–53. [Google Scholar]
- 39.Slattery M, McClintock J B, Heine J N. Chemical defense in Antarctic soft corals: evidence for antifouling compounds. J Exp Mar Biol Ecol. 1995;190:61–77. [Google Scholar]
- 40.Swain T. Secondary compounds as protective agents. Annu Rev Plant Physiol. 1977;28:479–501. [Google Scholar]
- 41.Todd J S, Zimmerman R C, Crews P, Alberte R S. The antifouling activity of natural and synthetic phenolic acid sulfate esters. Phytochemistry. 1993;34:401–404. [Google Scholar]
- 42.Vergeer L H T, Aarts T L, de Groot J D. The ‘wasting disease’ and the effect of abiotic factors (light intensity, temperature, salinity) and infection with Labyrinthula zosterae on the phenolic content of Zostera marina shoots. Aquat Bot. 1995;52:35–44. [Google Scholar]
- 43.Wahl M, Jensen P R, Fenical W. Chemical control of bacterial epibiosis on ascidians. Mar Ecol Prog Ser. 1994;110:45–57. [Google Scholar]
- 44.Walters L J, Hadfield M G, Smith C M. Waterborne chemical compounds in tropical macroalgae: positive and negative cues for larval settlement. Mar Biol. 1996;126:383–393. [Google Scholar]
- 45.Zapata O, McMillan C. Phenolic acids in seagrasses. Aquat Bot. 1979;7:307–317. [Google Scholar]