Abstract
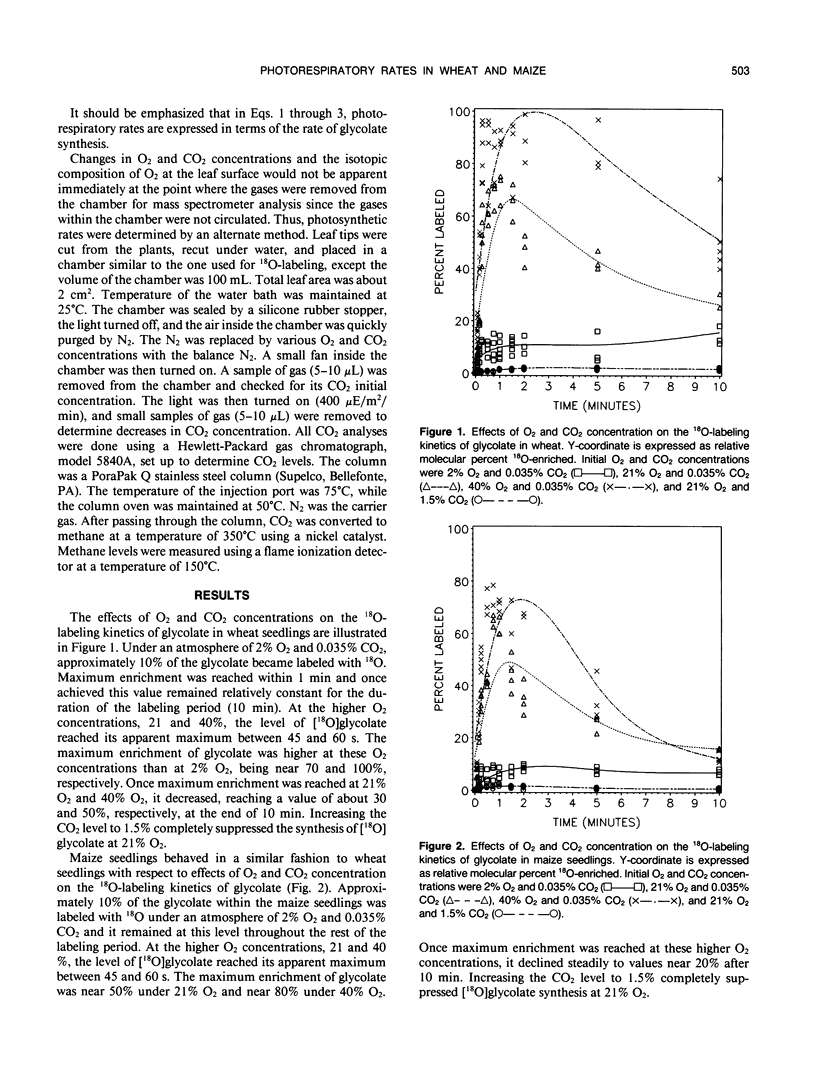
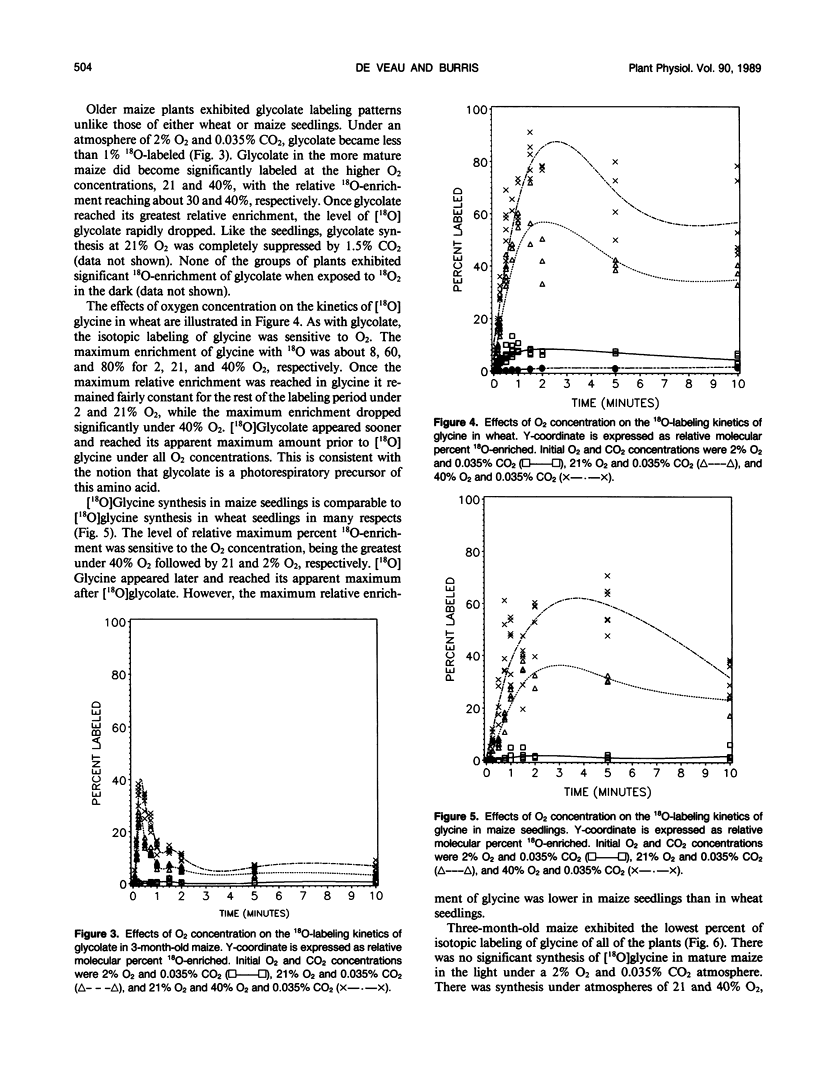
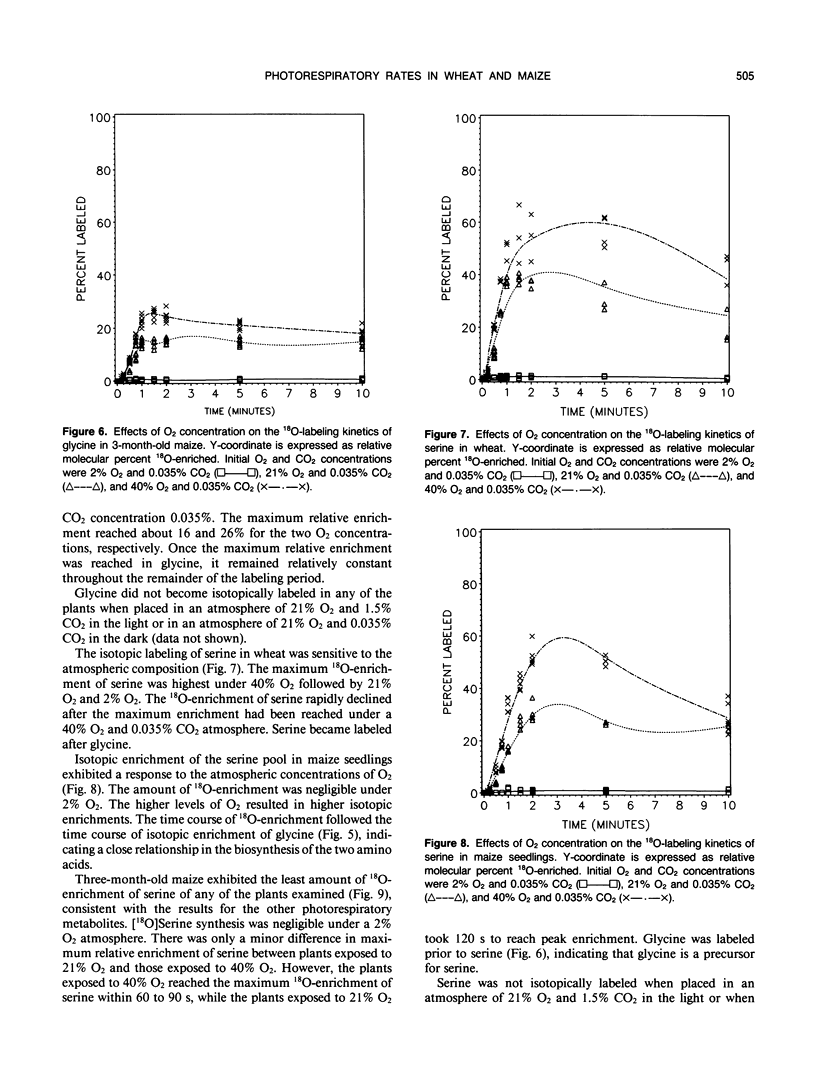
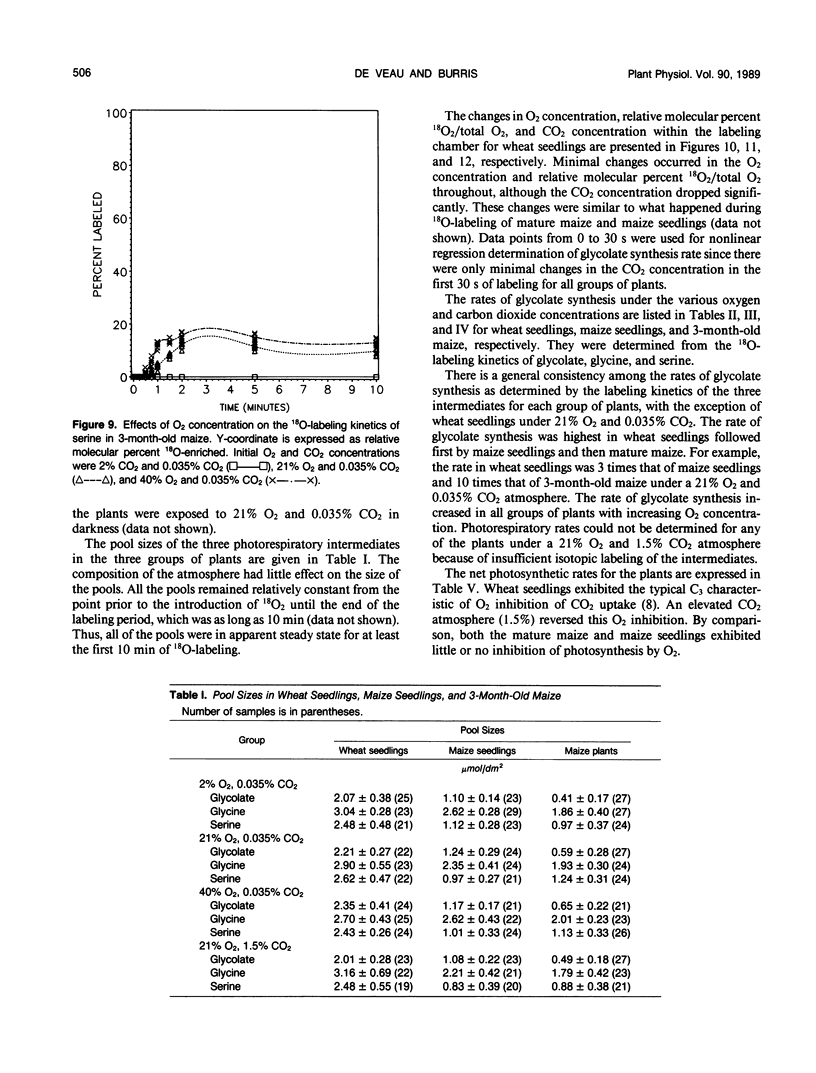
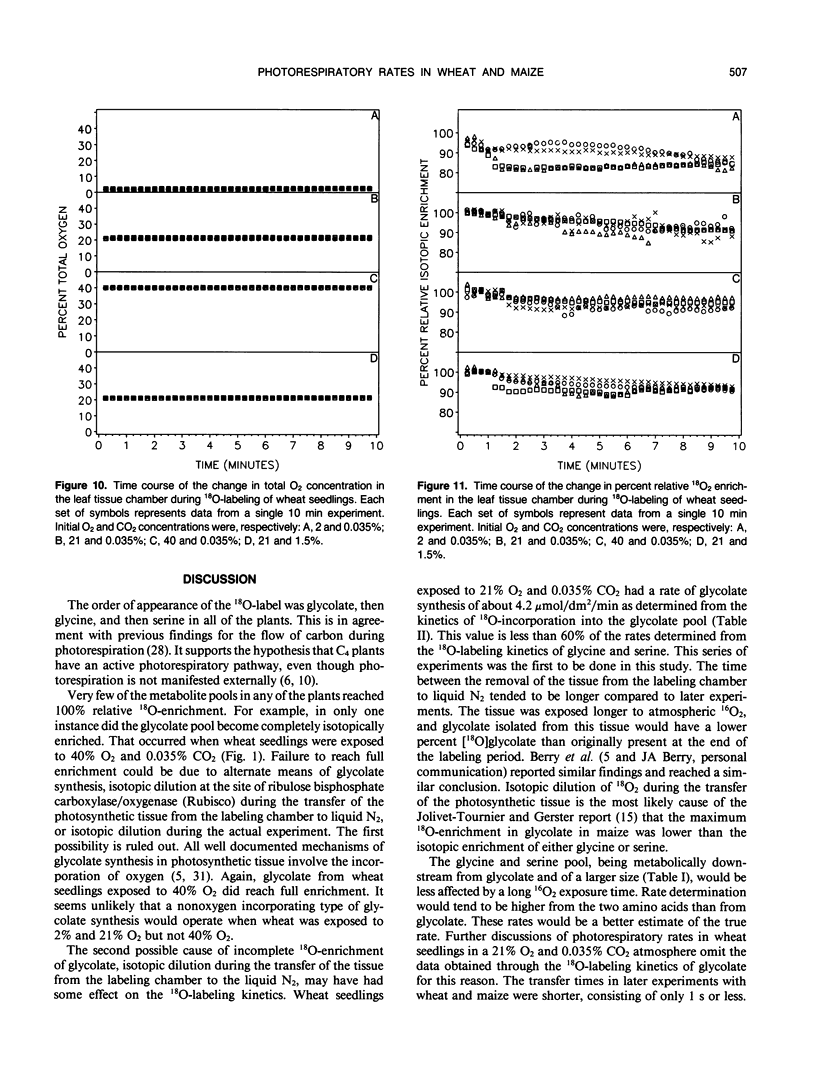
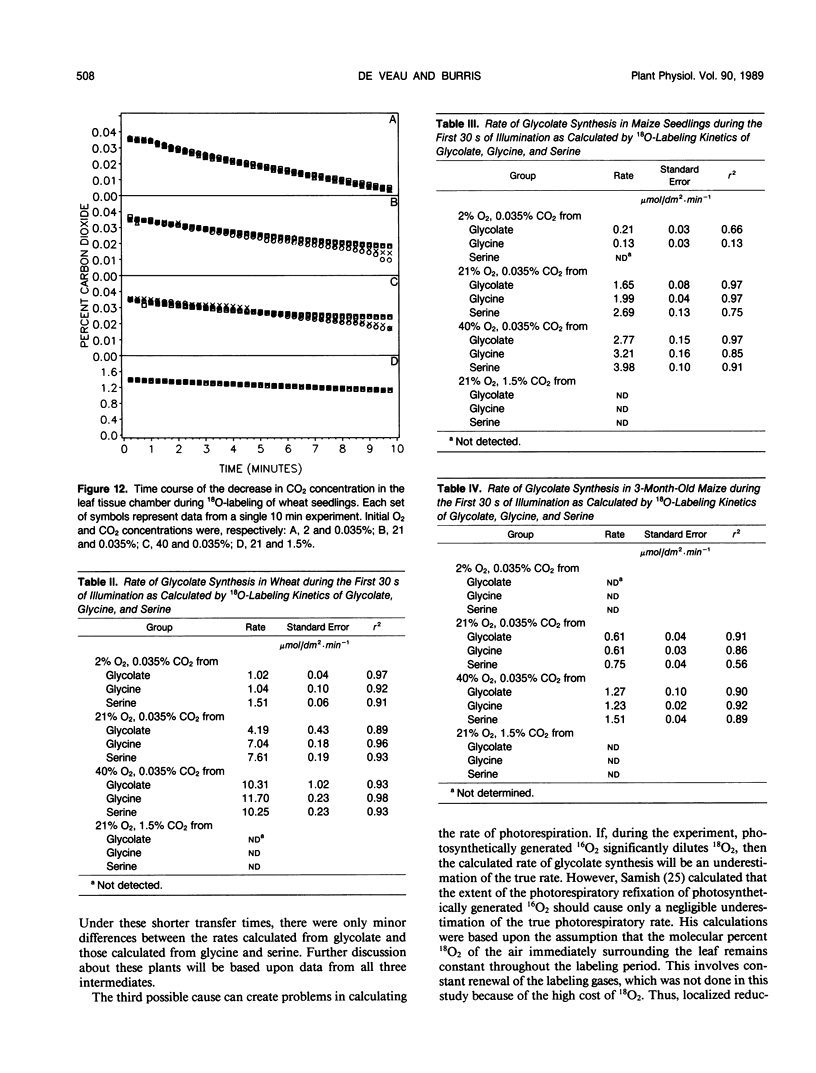
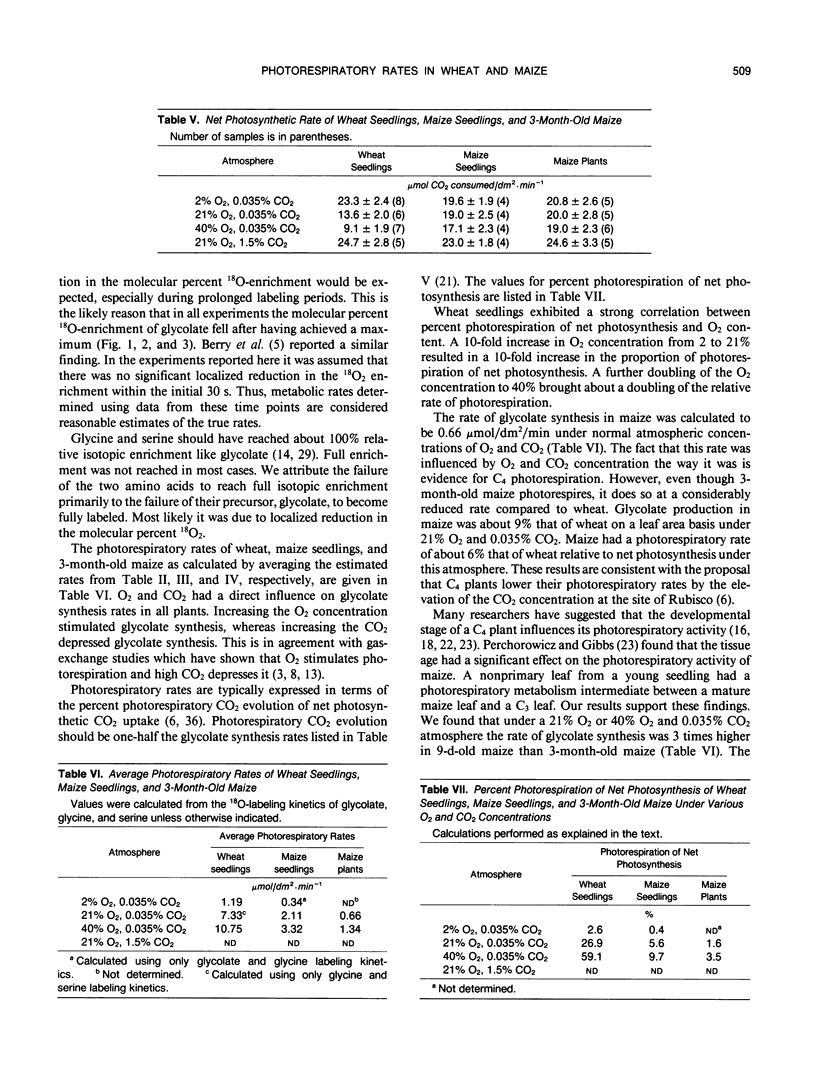
A method was devised to quantify short-term photorespiratory rates in terrestrial plants using 18O-intermediates of the glycolate pathway, specifically glycolate, glycine, and serine. The pathway intermediates were isolated and analyzed on a GC/MS to determine molecular percent 18O-enrichment. Rates of glycolate synthesis were determined from 18O-labeling kinetics of the intermediates, derived rate equations, and nonlinear regression techniques. Glycolate synthesis in wheat (Triticum aestivum L.), a C3 plant, and maize (Zea mays L.), a C4 plant, was stimulated by high O2 concentrations and inhibited by high CO2 concentrations. The synthesis rates were 7.3, 2.1, and 0.7 micromoles per square decimeter per minute under a 21% O2 and 0.035% CO2 atmosphere for leaf tissue of wheat, maize seedlings, and 3-month-old maize, respectively. Photorespiratory CO2 evolution rates were estimated to be 27, 6, and 2%, respectively, of net photosynthesis for the three groups of plants under the above atmosphere. The results from maize tissue support the hypothesis that C4 plants photorespire, albeit at a reduced rate in comparison to C3 plants, and that the CO2/O2 ratio in the bundle sheath of maize is higher in mature tissue than in seedling tissue. The pool size of the three photorespiratory intermediates remained constant and were unaffected by changes in either CO2 or O2 concentrations throughout the 10-minute labeling period. This suggests that photorespiratory metabolism is regulated by other mechanism besides phosphoglycolate synthesis by ribulose-1,5-bisphosphate carboxylase/oxygenase, at least under short-term conditions. Other mechanisms could be alternate modes of synthesis of the intermediates, regulation of some of the enzymes of the photorespiratory pathway, or regulation of carbon flow between organelles involved in photorespiration. The glycolate pool became nearly 100% 18O-labeled under an atmosphere of 40% O2. This pool failed to become 100% 18O-enriched under lower O2 concentrations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Lorimer G. H., Tolbert N. E. Incorporation of molecular oxygen into glycine and serine during photorespiration in spinach leaves. Biochemistry. 1971 Dec 7;10(25):4777–4782. doi: 10.1021/bi00801a027. [DOI] [PubMed] [Google Scholar]
- BASSHAM J. A., KIRK M. The effect of oxygen on the reduction of CO2 to glycolic acid and other products during photosynthesis by Chlorella. Biochem Biophys Res Commun. 1962 Nov 27;9:376–380. doi: 10.1016/0006-291x(62)90019-0. [DOI] [PubMed] [Google Scholar]
- Bergström K., Gürtler J., Blomstrand R. Trimethylsilylation of amino acids. I. Study of glycine and lysine TMS derivatives with gas-liquid chromatography-mass spectrometry. Anal Biochem. 1970 Mar;34:74–87. doi: 10.1016/0003-2697(70)90088-6. [DOI] [PubMed] [Google Scholar]
- Berry J. A., Osmond C. B., Lorimer G. H. Fixation of O(2) during Photorespiration: Kinetic and Steady-State Studies of the Photorespiratory Carbon Oxidation Cycle with Intact Leaves and Isolated Chloroplasts of C(3) Plants. Plant Physiol. 1978 Dec;62(6):954–967. doi: 10.1104/pp.62.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Huang A. H. Metabolism of Glycolate in Isolated Spinach Leaf Peroxisomes : KINETICS OF GLYOXYLATE, OXALATE, CARBON DIOXIDE, AND GLYCINE FORMATION. Plant Physiol. 1981 May;67(5):1003–1006. doi: 10.1104/pp.67.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of Oxygen on Photosynthesis, Photorespiration and Respiration in Detached Leaves. II. Corn and other Monocotyledons. Plant Physiol. 1966 Mar;41(3):428–431. doi: 10.1104/pp.41.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbaud A., Andre M. Photosynthesis and photorespiration in whole plants of wheat. Plant Physiol. 1979 Nov;64(5):735–738. doi: 10.1104/pp.64.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbaud A., André M. Effect of CO(2), O(2), and Light on Photosynthesis and Photorespiration in Wheat. Plant Physiol. 1980 Dec;66(6):1032–1036. doi: 10.1104/pp.66.6.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. L., Tolbert N. E. Glycolate, glycine, serine, and glycerate formation during photosynthesis by tobacco leaves. J Biol Chem. 1966 Dec 10;241(23):5705–5711. [PubMed] [Google Scholar]
- Jolivet-Tournier P., Gerster R. Incorporation of Oxygen into Glycolate, Glycine, and Serine during Photorespiration in Maize Leaves. Plant Physiol. 1984 Jan;74(1):108–111. doi: 10.1104/pp.74.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R., Sinha S. K. Change in the predominance from C 4 to C 3 pathway following anthesis in sorghum. Biochem Biophys Res Commun. 1973 May 1;52(1):121–124. doi: 10.1016/0006-291x(73)90962-5. [DOI] [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neal D., Hew C. S., Latzko E., Gibbs M. Photosynthetic carbon metabolism of isolated corn chloroplasts. Plant Physiol. 1972 Apr;49(4):607–614. doi: 10.1104/pp.49.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Gibbs M. Carbon dioxide fixation and related properties in sections of the developing green maize leaf. Plant Physiol. 1980 May;65(5):802–809. doi: 10.1104/pp.65.5.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REINER J. M. The study of metabolic turnover rates by means of isotopic tracers. I. Fundamental relations. Arch Biochem Biophys. 1953 Sep;46(1):53–79. doi: 10.1016/0003-9861(53)90170-2. [DOI] [PubMed] [Google Scholar]
- Samish Y. B. The rate of photorespiration as measured by means of oxygen uptake and its respiratory quotient. Plant Physiol. 1971 Sep;48(3):345–348. doi: 10.1104/pp.48.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler R. F., Brown A. S., Brown S. B. Bile pigment synthesis in plants. Mechanism of 18O incorporation into phycocyanobilin in the unicellular rhodophyte. Cyanidium caldarium. J Biol Chem. 1979 May 10;254(9):3411–3418. [PubMed] [Google Scholar]
- Volk R. J., Jackson W. A. Photorespiratory phenomena in maize: oxygen uptake, isotope discrimination, and carbon dioxide efflux. Plant Physiol. 1972 Feb;49(2):218–223. doi: 10.1104/pp.49.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]


