Abstract
Background and purpose
The Banff Patellofemoral Instability Instrument (BPII) 2.0 is a patient-reported outcome measure (PROM) designed specifically for patellofemoral instability. We translated and adapted the BPII 2.0 into Swedish and assessed its psychometric properties.
Patients and methods
The BPII 2.0 was forward- and back-translated. Children aged 10–16 years with patellar dislocation and instability or recurrent dislocation were recruited. Children completed the Swedish BPII 2.0 and KOOS-Child during their initial visit (t0) and 1 week later (t1). Internal consistency and test–retest reliability were evaluated using intraclass correlation coefficients (ICCs) for the BPII 2.0 and KOOS-Child scores comparison. Pearson correlation coefficients examined concurrent validity of the Swedish BPII 2.0 subscales with KOOS-Child subscales.
Results
64 children (46 females), mean age 13.8 (10.0–16.3) years, participated. Time after patellar dislocation or surgery was 3–24 months. 55 patients (86%) returned the second BPII 2.0 and KOOS-Child after an average of 9 (5–22) days. There were no ceiling or floor effects for the total score of the new Swedish BPII 2.0 or for its subscales. BPII 2.0 demonstrated excellent internal consistency at t0 (ICC 0.96, 95% confidence interval [CI] 0.95–0.97) and at t1 (ICC 0.97, CI 0.95–0.98), as well as excellent test–retest reliability (ICC 0.97, CI 0.96–0.98). Concurrent validity of the BPII 2.0 subscales with KOOS-Child subscales was moderate to strong (rho 0.40–0.88).
Conclusion
The Swedish BPII 2.0 showed excellent internal consistency as well as excellent test–retest reliability and is a reliable and valid questionnaire.
Lateral patellar dislocation (LPD) and recurrent instability are common in children and adolescents aged 12 to 18 years with an incidence rate of about 120–150/100,000 person years [1,2]. Patellar instability leads to a lack of trust in knee function, an increased fall risk and knee pain, and greatly affects quality of life [3-5]. Potential associated cartilage damage can result in early onset of osteoarthritis [6-8]. The recurrence rate after LPD is high and increases in the presence of predisposing anatomical risk factors; 30–70% will experience a redislocation [9-12]. To ascertain outcomes of surgical and non-surgical interventions, valid and reliable patient-reported outcome measures (PROMs) are needed in addition to objective clinical assessments [13].
The Banff Patellofemoral Instability Instrument (BPII) [14], the updated shorter version BPII 2.0 [15], and the Norwich Patellar Instability Score [16] are the only PROMs specifically designed for patients with patellofemoral instability [17]. Of these disease-specific outcome measures, only the BPII 2.0 has been validated in an adolescent population with LPD and after patellofemoral stabilization [18].
The BPII 2.0 has been adopted and recommended by the International Patellofemoral Study Group (IPSG). The English version of BPII 2.0 has been translated into German [19], Dutch [20], Portuguese [21], and Indonesian [22].
There are no validated Swedish PROMs for evaluating patellofemoral instability treatment. The Knee injury and Osteoarthritis Outcome Score for children (KOOS-Child) is a knee-specific questionnaire created for children 7–16 years of age [23] and has been validated in several languages including Swedish [23,24]. However, the KOOS-Child may not be effective for evaluating disabilities after LPD, thus possibly obscuring significant findings.
We aimed to translate and adapt the BPII 2.0 [15] for children and adolescents into Swedish and assess this new Swedish version of the BPII 2.0 for concurrent validity by comparing the BPII 2.0 scores with KOOS-Child [23,24] scores.
Patients and methods
The study complied with aspects of the COSMIN [25,26] framework in the assessment of questionnaire properties following survey translation and was reported according to COSMIN guidelines.
Translation
Forward- and back-translations of the BPII 2.0 [15] were performed according to international recommendations [25,26]. An individual (EF) proficient in English and Swedish, with extensive experience in the health condition under investigation, independently translated the BPII 2.0 from English to Swedish. A second individual proficient in both languages independently translated the Swedish version of the BPII 2.0 back into English (MK). Discrepancies between the back-translation and the original versions were reviewed by a native English researcher (MDI), but no inconsistencies were found. A team of researchers (MA and JvH) examined the clarity and comprehensibility of the Swedish version, and used a nominal group process to reconcile differences. The final version was piloted in 4 children aged 10–15 years. Based on this feedback, no changes were deemed necessary (see Supplementary data).
Recruitment and human subjects
The study sample size was determined based on a previous study conducted by Becher et al. [19] in which the original BPII 2.0 was translated from English to German and a similar design was employed with 64 participants. In our convenience sample, potential participants with an episode of a primary or recurrent patellar dislocation, evaluated for patellofemoral instability after nonoperative or operative treatment, were identified at the Department of Pediatric Orthopedics and the outpatient rehabilitation program, Karolinska University Hospital. All participants had a diagnosis of patellar dislocation with instability or recurrent patellar dislocation (S 83.0 or M 22.0 according to the ICD-10 [27]). The treating pediatric orthopedic surgeon confirmed the diagnosis based on the patient’s history, clinical examination, and imaging results (plain radiographs and magnetic resonance images). Demographic data collected between October 2020 and March 2022 included sex, date of birth, diagnosis, visit date, date of injury, and treatment. Children received an initial questionnaire package that included the Swedish BPII 2.0 and the Swedish KOOS-Child [23,24] and completed these 2 questionnaires during their initial clinic visit. They were then given a second questionnaire package to complete at home 1 week after this initial visit, as we anticipated no interventions or change in health status would occur during this timeframe. Participants were provided with a self-addressed stamped envelope and were instructed to mail the surveys back to one of the coauthors (MA), who registered the data in a database.
Measures
The BPII 2.0 contains 23 items and assesses 5 domains central to quality of life. These domains include: (1) symptoms and physical complaints (5 items), (2) work- and/or school-related concerns (4 items), (3) recreational/sport/activity (5 items), (4) lifestyle (5 items), and (5) social and emotional (4 items). Its validity, reliability, and responsiveness has been successfully demonstrated for the assessment of younger patients with lateral patellofemoral instability and following patellofemoral stabilization [18]. Patient responses to items are based on the current status and function of, and circumstances or beliefs related to, their affected knee during the past three months. To record their current knee status, patients placed a slash (/) on a 100-mm line to indicate a score ranging from 0 to 100. The lowest possible score on the BPII 2.0 is 0, indicating more symptoms and/or functional limitations and lower quality of life. The highest possible score is 100, indicating no symptoms and greater quality of life. Each item is equally weighted, and the total score is calculated as an average of all scores from all answered items, yielding a value from 0 to 100 [15].
The KOOS-Child is a self-administered, knee-specific questionnaire developed by one of the co-authors (MDI) and is used to evaluate knee function in children 7–16 years old with knee disorders. This instrument has been validated and assessed for reliability and responsiveness in a number of languages, including Swedish [23]. It contains 39 items. There are 5 subscales that are scored separately: pain (8 items), symptoms “knee problems” (7 items), difficulty during daily activities (ADL) (11 items), sport/play (7 items); and kneerelated quality of life (QoL) (6 items). Each question provides a score from 0 to 4, where 0 indicates no problem and 4 indicates severe problems. Raw scores are then transformed to a 0–100 scale, with 0 presenting extreme knee problems and 100 representing no knee problems within the 5 subscales [23,24]. The different dimensions were analyzed separately as the KOOS-Child subscales should not be combined into a single total score [28].
Statistics
Descriptive statistics were used to characterize the cohort. Floor and ceiling effects were examined using response frequency with a threshold set at > 15% maximum–minimum values, respectively [29]. To assess the test–retest reliability, defined as the consistency of responses under repeated application of the measure and similar circumstances, intraclass correlation coefficients (ICCs) and 95% confidence intervals (CIs) were calculated based on 2-way, random, single measures with absolute agreement. The threshold for interpreting a good agreement was established at ICC > 0.75, values between 0.50–0.75 indicate moderate agreement, and values greater than 0.90 indicate excellent reliability [30]. Given the similarity in constructs within the two measures, we hypothesized a moderate agreement between the Swedish BFII2.0 and Swedish KOOS-Child scores. To assess the internal consistency of the Swedish BPII 2.0 and KOOS-Child scores, the ICC was chosen for analysis, and 95% CIs were calculated based on a 2-way random, single measures with consistency model. While Cronbach’s alpha is often used to assess internal consistency, the ICC was selected to enable the calculation of 95% CIs. Cronbach’s alpha yields similar values to the ICC when the ICC is calculated using the 2-way random consistency model [31,32]. A value of 0.7 represents fair to good internal consistency [33]. Finally, Pearson correlation coefficients were used to assess concurrent validity of the Swedish BPII 2.0 subscales with KOOS-Child subscale scores, and 95% CIs for these correlations were estimated using the Fisher transformation method. Correlation coefficients of 0.10–0.39 were consider weak, 0.40–0.69 moderate, 0.70–0.89 good to strong, and 0.90–1.00 very strong [34]. A statistical significance level was set at a = 0.05 for the Pearson correlation. Statistical analyses were performed using IBM SPSS Statistics version 28 (IBM Corp, Armonk, NY, USA).
Ethics, consent to participate, data sharing, funding, and disclosures
Ethics approval for this cross-sectional study was obtained from Stockholm’s regional ethical review board (Dnr: 2019-05891). All participants and caregivers provided informed consent before participating. The datasets analyzed during the current study are not publicly available due to ethical concerns but are available from the corresponding author on reasonable request. Financial support for this study was not provided. Each author certifies that she or he has no commercial associations that might pose a conflict of interest in connection with the submitted article. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.21194
Results
Our study population consisted of 64 patients (18 males and 46 females) with a primary (n = 35) or recurrent (n = 29) patellar dislocation evaluated for patellofemoral instability after nonoperative or operative treatment. The average age of the patients was 13.8 years (range 10.0–16.3). The time after dislocation or surgery was 3–24 months. All 64 participants completed the Swedish BPII 2.0 questionnaire at baseline (t0), and 55 patients (86%) returned the Swedish BPII 2.0 questionnaire along with KOOS-Child at (t1), an average of 9 days later (range 5–22). The response rate was 100% for all questions in both BPII 2.0 questionnaires. Of these 55 participants, 54 answered all questions of the KOOS-Child at both t0 and t1. Thus, analyses involving KOOS-Child data or a combination of KOOS-Child and BPII 2.0 data included only these 54 participants. For analyses focusing solely on BPII 2.0 data, all 55 participants who responded to both questionnaires at t0 and t1 were included (Figure).
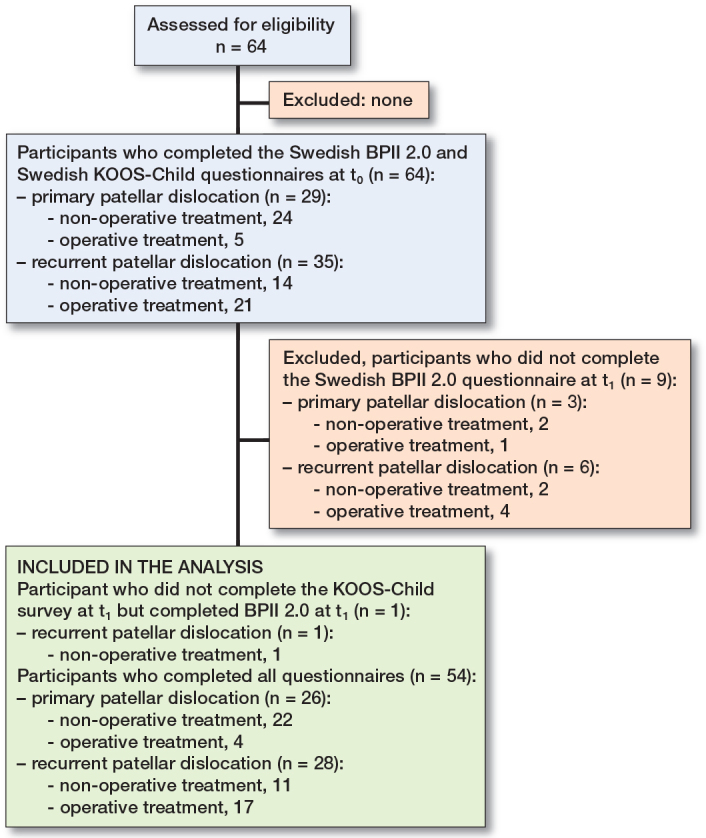
Flowchart of study participants with primary patellar dislocation and recurrent patellar dislocation evaluated for patellofemoral instability after nonoperative treatment or operative treatment.
To determine whether a floor or ceiling effect was present for the Swedish BPII 2.0 or its subscales, we examined the distribution of responses using the predetermined threshold of > 15% of respondents who answered the minimum or maximum value. Based on this threshold, there were no ceiling or floor effect for the total score or for its subscales (Table 1).
Table 1.
Total scores and subscale scores, differences over time, and test–retest reliability of the Swedish version of the Banff Patellar Instability Instrument (BPII) 2.0 and the Knee injury and Osteoarthritis Outcome Score for children (KOOS-Child)
| Mean (range) | Baseline (t0) Ceiling/floor effects a (%) | Internal consistency b (CI) | Mean (range) | Follow-up (t1) Ceiling/floor effects a (%) | Internal consistency b (CI) | Test–retest reliability c (CI) | |
|---|---|---|---|---|---|---|---|
| BPII 2.0 | |||||||
| Participants d | n = 55 | n = 55 | |||||
| Average total score | 67 (14–98) | 0/0 | 0.96 (0.95–0.97) | 67 (14–98) | 0/0 | 0.97 (0.95–0.98) | 0.97 (0.96–0.98) |
| Subscales | |||||||
| Symptoms and physical complaints | 72 (0–100) | 1.8/1.8 | 0.85 (0.78–0.91) | 73 (14–100) | 5.5/0 | 0.88 (0.82–0.92) | 0.93 (0.87–0.96) |
| Work- and/or school-related concerns | 76 (31–100) | 11/0 | 0.71 (0.56–0.82) | 73 (16–100) | 11/0 | 0.79 (0.68–0.87) | 0.94 (0.90–0.97) |
| Recreation/sport/activity | 58 (3–99) | 0/0 | 0.92 (0.88–0.95) | 59 (3–100) | 3.6/0 | 0.91 (0.86–0.94) | 0.96 (0.93–0.98) |
| Lifestyle | 72 (4–100) | 11/0 | 0.90 (0.85–0.93) | 73 (4–100) | 3.6/0 | 0.91 (0.86–0.94) | 0.95 (0.92–0.97) |
| Social and emotional | 57 (0–97) | 0/1.8 | 0.86 (0.78–0.91) | 59 (0–100) | 1.8/1.8 | 0.90 (0.85–0.94) | 0.94 (0.90–0.97) |
| KOOS-Child | |||||||
| Participants d | n = 54 | n = 54 | |||||
| Subscales | |||||||
| Symptoms | 86 (57–100) | 20/0 | 0.60 (0.40–0.74) | 86 (57–100) | 22/0 | 0.68 (0.53–0.80) | 0.86 (0.77–0.92) |
| Pain | 84 (31–100) | 13/0 | 0.88 (0.82–0.92) | 85 (31–100) | 19/0 | 0.88 (0.83–0.93) | 0.94 (0.90–0.97) |
| ADL | 93 (50–100) | 48/0 | 0.90 (0.85–0.93) | 93 (50–100) | 46/0 | 0.90 (0.86–0.94) | 0.93 (0.89–0.96) |
| Sport/Play | 69 (0–100) | 9.3/3.7 | 0.94 (0.92–0.96) | 69 (0–100) | 9.3/1.9 | 0.95 (0.92–0.97) | 0.98 (0.96–0.99) |
| QoL | 63 (4–100) | 1.9/0 | 0.90 (0.85–0.94) | 62 (4–100) | 1.9/0 | 0.89 (0.84–0.93) | 0.92 (0.86–0.95) |
t0 = first questionnaire at baseline.
t1 = second questionnaire, on average 9 (5–22) days later.
> 15% maximum–minimum values, respectively.
ICC = intraclass correlation coefficient 2-way, random, consistency model.
ICC = intraclass correlation coefficient 2-way, random, single measures with absolute agreement.
Patients with follow-up at t1.
ADL = difficulty during daily activities; CI = 95% confidence interval; QoL = knee-related quality of life.
The mean BPII 2.0 score for the 9 patients who completed the questionnaire only at t0 was 72 (range 34–100). Among the 55 patients who answered the questionnaire at both t0 and t1, the mean scores were 67 at t0 (range 14–89) and 67 at t1 (range 14–98), indicating little to no change in patient symptoms between the 2 test administrations. The internal consistency of the Swedish BPII 2.0 questionnaire at t0 and t1 was excellent (ICC t0 = 0.96, CI 0.95–0.97 and ICC t1 = 0.97, CI 0.95–0.98) with values ranging from 0.71 to 0.92 for the different subscores. The test–retest reliability of the Swedish BPII 2.0 total score was excellent, with an ICC of 0.97, CI 0.96–0.98. When examining the respective subscores for the Swedish BPII 2.0 at t0 and t1, there was no statistical difference over time and ICC ranged from 0.93–0.96 (Table 1).
The concurrent validity of the Swedish BPII 2.0 was significant, with moderate to strong correlations for all subscale scores as determined by the correlation between the Swedish BPII 2.0 and Swedish KOOS-Child subscale scores. The strongest correlation was observed between the subscale scores for “lifestyle” on the BPII 2.0 and “QoL” on KOOS-Child, with a correlation coefficient of rho = 0.88, CI 0.79–0.93 (Table 2).
Table 2.
Concurrent validity, Pearson correlations with 95% confidence interval between the subscores of the Swedish version of the Banff Patellar Instability Instrument (BPII) 2.0 and the Knee injury and Osteoarthritis Outcome Score for children (KOOS-Child) at baseline (t0) (N = 64)
| BPII 2.0 subscales | Symptoms | Pain | KOOS-Child subscales ADL | Sport/Recreation | QoL |
|---|---|---|---|---|---|
| Symptoms and physical complaints | 0.45 (0.21–0.64) | 0.63 (0.44–0.77) | 0.59 (0.38–0.74) | 0.65 (0.46–0.78) | 0.69 (0.52–0.81) |
| Work- and/or school-related concerns | 0.45 (0.21–0.64) | 0.63 (0.43–0.77) | 0.55 (0.34–0.71) | 0.77 (0.64–0.86) | 0.66 (0.48–0.79) |
| Recreation/sport/activity | 0.47 (0.23–0.66) | 0.69 (0.51–0.81) | 0.61 (0.41–0.76) | 0.76 (0.61–0.85) | 0.80 (0.68–0.88) |
| Lifestyle | 0.46 (0.22–0.65) | 0.67 (0.49–0.79) | 0.65 (0.47–0.78) | 0.75 (0.60–0.85) | 0.88 (0.79–0.93) |
| Social and emotional | 0.40 (0.14–0.60) | 0.58 (0.36–0.73) | 0.54 (0.32–0.71) | 0.67 (0.49–0.79) | 0.81 (0.70–0.89) |
ADL = difficulty during daily activities; QoL = knee-related quality of life.
All correlations significant (P < 0.001).
Discussion
Our study demonstrates that the BPII 2.0 was successfully adapted into Swedish and is the first validated, disease-specific, and reliable tool for use by Swedish-speaking children and adolescents with patellofemoral instability. We aimed to assess concurrent validity by comparing the Swedish BFII 2.0 scores with the Swedish KOOS-Child [23,24] scores. The test–retest reliability was excellent with an ICC of 0.97. These findings are in accordance with the validation of the original English BPII 2.0, which had an ICC of 0.97 [15] and the German BPII 2.0, which had an ICC of 0.89 [19].
The mean BPII 2.0 score was higher at t0 (67) in this cohort compared with previous translation studies evaluating recurrent patellar dislocation (range 30–55) [19-21]. This difference in mean BPII 2.0 score is probably related to our sample characteristics as this study included subjects with both first-time and recurrent LPD, treated both nonoperatively and operatively and not related to the translation process. However, the differences between t0 and t1 in the present study were minimal and comparable with scores in the German translation [19]. The broad range in BPII scores (14–98) found in our study and in previous translation studies [19-21] and the validation in adolescents by Lafave et al. [18] reveals that patellofemoral instability covers a broad range of clinical symptoms affecting quality of life and daily functional ability. The BPII 2.0 score is also affected by the variability in time from LPD or surgery in the cohort examined [18].
An important aspect of questionnaire interpretation is the presence of floor and ceiling effects. We used an established criteria (> 15%) of the sample answering the maximum or minimum value and found no floor or ceiling effects for the total score or its subscales. This is in accordance with a study conducted by Becher et al. [19] who used a similar study design and with previous translation studies [20-22], which did also report no floor or ceiling effects. The lack of floor or ceiling effects suggests this new translation of the BPII 2.0 may be useful in measuring improvement over time in patients with minor symptoms.
The internal consistency (ICC) of the Swedish BPII 2.0 was excellent over both administrations (t0 = 0.96 and t1 = 0.97) and was comparable to the Cronbach’s alpha values reported in the original English publication (0.91 at baseline, 0.93 at t0 and 0.95 at t1) [15] and the German translation [19]. This data suggests strong correlations among the items, with excellent reliability. All the Swedish BPII 2.0 questions were answered, indicating that the wording of the items was clear and suggesting that patients did not find the questionnaire too long or repetitive.
Correlations with the Swedish KOOS-Child subscales and the Swedish BPII 2.0 were calculated to assess the concurrent validity of the Swedish BPII 2.0. The Swedish BPII 2.0 is a disease-specific questionnaire designed to evaluate patellofemoral instability, while the KOOS-Child is a questionnaire designed to evaluate a range of knee disorders. The range of the correlations between subscores was moderate to high, indicating the KOOS-Child subscores measure different aspects of knee symptoms. In the German translation of BPII [19], the authors assessed concurrent validity by comparing the German BPII with the Kujula (0.58) and Norwich Patella scores (–0.47). These scores were moderately correlated, whereas the KOOS-Child subscores scores demonstrated higher correlations with the Swedish BPII.
In the Cross-Culture adaptation and translation of the BPII 2.0 into Swedish, two questions (6 and 9) required special attention. The word “pivoting” in item 6 does not translate directly into Swedish. The Swedish translation of BPII 2.0 used the same wording as the Swedish translation of “pivoting” in the Swedish KOOS-Child [23]. This may have led to higher correlations between the Swedish BPII and Swedish KOOS-Child.
Item 9 of the BPII asks, “Has the cost of your knee injury created financial hardship for you or your family?” Sweden is a country with state-funded medicine and surgery, and other treatment modalities (e.g., physiotherapy) are accessible and equitable for children with patellar instability. This item at t0 and t1 had an average score of 98 and 97, respectively, suggesting consistency in responses whereas other countries may have different medical systems affecting the score.
Limitations
First, there is a possibility that the present cohort is not comparable with previous BPII 2.0 studies because the children were asked to evaluate their symptoms up to 2 years after the patellar dislocation or surgery. Second, not everybody answered the second set of questionnaires, and those who did answer responded after 7–22 days. These 2 factors might have resulted in selection bias, and there is a possibility that children who responded beyond the 14-day window may have experienced a change in symptoms between t0 and t1. The mean BPII 2.0 score was similar for the 9 participants who only answered the questionnaire at baseline compared with the 55 participants who answered at both time points, suggesting these participants experienced similar subjective knee symptoms to those included in the analysis. The missing data from the 9 participants who did not answer the second set of questionnaires led to a smaller sample size. This smaller sample size may have impacted the robustness of the analysis and may affect the generalizability of this data to patients with different subjective symptoms of patellofemoral instability. Finally, it is possible that the Swedish children sampled for the initial comprehensibility study of the translated BPII did not reveal cultural issues with the translation even though none of the children in the study indicated challenges with understanding the questions.
Conclusion
BPII 2.0 was successfully translated into Swedish and showed excellent internal consistency as well as excellent test–retest reliability and is a reliable and valid questionnaire. The Swedish BPII 2.0 may be used as an important measure to aggregate data on a national and international level regarding patellofemoral instability to improve the quality of clinical care.
Supplementary data
The Swedish version of BPII 2.0 is available as supplementary data on the article page, doi: 10.2340/17453674.2023.21194
Supplementary Material
JvH: Study design, statistical analysis, interpretation of data, and manuscript preparation. MDI: Study design, statistical analysis, interpretation of data, and manuscript preparation. AH: Study design, data collection, interpretation of data, and manuscript preparation. MA: Study design, data collection, statistical analysis, interpretation of data, and manuscript preparation.
The authors thank Ebba Fridh, MD, for translating BPII from English to Swedish and Mia Karlberg, MD PhD, for translating from Swedish to English. The authors would like to thank the children and their families for participating in this study.
Handling co-editors: Ivan Hvid and Robin Christensen
References
- 1.Sanders T L, Pareek A, Hewett T E, Stuart M J, Dahm D L, Krych A J. Incidence of first-time lateral patellar dislocation: a 21-year population-based study. Sports Health 2018; 10: 146-51. doi: 10.1177/1941738117725055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askenberger M, Ekstrom W, Finnbogason T, Janarv P M. Occult intra-articular knee injuries in children with hemarthrosis. Am J Sports Med 2014; 42: 1600-6. doi: 10.1177/0363546514529639 [DOI] [PubMed] [Google Scholar]
- 3.Mostrom E B, Mikkelsen C, Weidenhielm L. Long-term follow-up of nonoperatively and operatively treated acute primary patellar dislocation in skeletally immature patients. ScientificWorldJournal 2014; 2014: 473281. doi: 10.1155/2014/473281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nwachukwu B U, So C, Schairer W W, Green D W, Dodwell E R. Surgical versus conservative management of acute patellar dislocation in children and adolescents: a systematic review. Knee Surg Sports Traumatol Arthrosc 2016; 24(3): 760-7. doi: 10.1007/s00167-015-3948-2 [DOI] [PubMed] [Google Scholar]
- 5.Sillanpaa P J, Maenpaa H M, Mattila V M, Visuri T, Pihlajamaki H. Arthroscopic surgery for primary traumatic patellar dislocation: a prospective, nonrandomized study comparing patients treated with and without acute arthroscopic stabilization with a median 7-year follow-up. Am J Sports Med 2008; 36: 2301-9. doi: 10.1177/0363546508322894 [DOI] [PubMed] [Google Scholar]
- 6.Vollnberg B, Koehlitz T, Jung T, Scheffler S, Hoburg A, Khandker D, et al. Prevalence of cartilage lesions and early osteoarthritis in patients with patellar dislocation. Eur Radiol 2012; 22: 2347-56. doi: 10.1007/s00330-012-2493-3 [DOI] [PubMed] [Google Scholar]
- 7.Sillanpaa P J, Mattila V M, Visuri T, Maenpaa H, Pihlajamaki H. Patellofemoral osteoarthritis in patients with operative treatment for patellar dislocation: a magnetic resonance-based analysis. Knee Surg Sports Traumatol Arthrosc 2011; 19: 230-5. doi: 10.1007/s00167-010-1285-z [DOI] [PubMed] [Google Scholar]
- 8.Mostrom E B, Lammentausta E, Finnbogason T, Weidenhielm L, Janarv P M, Tiderius C J. T2 mapping and post-contrast T1 (dGEMRIC) of the patellar cartilage: 12-year follow-up after patellar stabilizing surgery in childhood. Acta Radiol Open 2017; 6: 2058460117738808. doi: 10.1177/2058460117738808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arendt E A, Askenberger M, Agel J, Tompkins M A. Risk of redislocation after primary patellar dislocation: a clinical prediction model based on magnetic resonance imaging variables. Am J Sports Med 2018; 46(14): 3385-90. doi: 10.1177/0363546518803936 [DOI] [PubMed] [Google Scholar]
- 10.Palmu S, Kallio P E, Donell S T, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am 2008; 90: 463-70. 2018; 46(14): 3385-90. doi: 10.2106/jbjs.g.00072 [DOI] [PubMed] [Google Scholar]
- 11.Jaquith B P, Parikh S N. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation. J Pediatr Orthop 2017; 37: 484-90. doi: 10.1097/bpo.0000000000000674 [DOI] [PubMed] [Google Scholar]
- 12.Askenberger M, Bengtsson Mostrom E, Ekstrom W, Arendt E A, Hellsten A, Mikkelsen C, et al. Operative repair of medial patellofemoral ligament injury versus knee brace in children with an acute first-time traumatic patellar dislocation: a randomized controlled trial. Am J Sports Med 2018; 46(10): 2328-40. doi: 10.1177/0363546518770616 [DOI] [PubMed] [Google Scholar]
- 13.Kluzek S, Dean B, Wartolowska K A. Patient-reported outcome measures (PROMs) as proof of treatment efficacy. BMJ Evid Based Med 2022; 27: 153-5. doi: 10.1136/bmjebm-2020-111573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiemstra L A, Kerslake S, Lafave M R, Heard S M, Buchko G M, Mohtadi N G. Initial validity and reliability of the Banff Patella Instability Instrument. Am J Sports Med 2013; 41: 1629-35. doi: 10.1177/0363546513487981 [DOI] [PubMed] [Google Scholar]
- 15.Lafave M R, Hiemstra L, Kerslake S. Factor analysis and item reduction of the Banff Patella Instability Instrument (BPII): introduction of BPII 2.0. Am J Sports Med 2016; 44: 2081-6. doi: 10.1177/0363546516644605 [DOI] [PubMed] [Google Scholar]
- 16.Smith T O, Donell S T, Clark A, Chester R, Cross J, Kader D F, et al. The development, validation and internal consistency of the Norwich Patellar Instability (NPI) score. Knee Surg Sports Traumatol Arthrosc 2014; 22: 324-35. doi: 10.1007/s00167-012-2359-x [DOI] [PubMed] [Google Scholar]
- 17.Hiemstra L A, Page J L, Kerslake S. Patient-reported outcome measures for patellofemoral instability: a critical review. Curr Rev Musculoskelet Med 2019; 12(2): 124-37. doi: 10.1007/s12178-019-09537-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lafave M R, Hiemstra L A, Kerslake S. Validity, reliability, and responsiveness of the Banff Patellar Instability Instrument (BPII) in a adolescent population. J Pediatr Orthop 2018; 38: e629-e633. doi: 10.1097/BPO.0000000000001250 [DOI] [PubMed] [Google Scholar]
- 19.Becher C, Attal R, Balcarek P, Dirisamer F, Liebensteiner M, Pagenstert G, et al. Successful adaption of the Banff Patella Instability Instrument (BPII) 2.0 into German. Knee Surg Sports Traumatol Arthrosc 2018; 26: 2679-84. doi: 10.1007/s00167-017-4673-9 [DOI] [PubMed] [Google Scholar]
- 20.Van Sambeeck J D, Van de Groes S A, Koeter S. Dutch translation and validation of the Norwich Patellar Instability score and Banff Patella Instability Instrument in patients after surgery for patellar instability. Acta Orthop Belg 2020; 86: 470-81. PMID: [PubMed] [Google Scholar]
- 21.Galvao P, Marques D S, Gracitelli G C, Ferreira M C, Kubota M S, Franciozi C. Portuguese translation and cross-cultural adaption of the Banff Patella Instability Instrument. Rev Bras Ortop (Sao Paulo) 2021; 56: 747-60. doi: 10.1055/s-0040-1721840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhatomy S, Pontoh L A, Phatama K Y, Waskita H C, Al Mashur M I, Fiolin J, et al. The Banff Patellar Instability Instrument: validity and reliability of an Indonesian version. Eur J Orthop Surg Traumatol 2023; 33(3): 617-22. doi: 10.1007/s00590-022-03336-6 [DOI] [PubMed] [Google Scholar]
- 23.Örtqvist M, Roos E M, Brostrom E W, Janarv P M, Iversen M D. Development of the Knee Injury and Osteoarthritis Outcome Score for children (KOOS-Child): comprehensibility and content validity. Acta Orthop 2012; 83: 666-73. doi: 10.3109/17453674.2012.747921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Örtqvist M, Iversen M D, Janarv P M, Brostrom E W, Roos E M. Psychometric properties of the Knee injury and Osteoarthritis Outcome Score for Children (KOOS-Child) in children with knee disorders. Br J Sports Med 2014; 48: 1437-46. doi: 10.1136/bjsports-2013-093164 [DOI] [PubMed] [Google Scholar]
- 25.Gagnier J J, Lai J, Mokkink L B, Terwee C B. COSMIN reporting guideline for studies on measurement properties of patient-reported outcome measures. Qual Life Res 2021; 30: 2197-218. doi: 10.1007/s11136-021-02822-4 [DOI] [PubMed] [Google Scholar]
- 26.Mokkink L B, Prinsen C A, Patrick D L, Alonso J, Bouter L M, de Vet H C, et al. COSMIN Study design checklist for patient-reported outcome measurement instrument. COSMIN, 2019. (Cited 2023 September 29). Available from: https://www.cosmin.nl/wp-content/uploads/COSMIN-study-designing-checklist_final.pdf [Google Scholar]
- 27.Park H, Castano J, Avila P, Perez D, Berinsky H, Gambarte L, et al. An information retrieval approach to ICD-10 classification. Stud Health Technol Inform 2019; 264: 1564-5. doi: 10.3233/SHTI190536 [DOI] [PubMed] [Google Scholar]
- 28.A user’s guide to: The Knee injury and Osteoarthritis Outcome Score for children KOOS-Child. (Cited 2023 February 7). Available from: http://www.koos.nu/kooschildusersguide.pdf
- 29.Terwee C B, Bot S D, de Boer M R, van der Windt D A, Knol D L, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007; 60: 34-42. doi: 10.1016/j.jclinepi.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 30.Koo T K, Li M Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016; 15: 155-63. doi: 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bravo G, Potvin L. Estimating the reliability of continuous measures with Cronbach’s alpha or the intraclass correlation coefficient: toward the integration of two traditions. J Clin Epidemiol 1991; 44: 381-90. doi: 10.1016/0895-4356(91)90076-l [DOI] [PubMed] [Google Scholar]
- 32.IBM Support . Confidence interval for Cronbach’s alpha in SPSS. (Cited 2023 May 5). Available from: https://www.ibm.com/support/pages/confidence-interval-cronbachs-alpha-spss
- 33.Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ 2011; 2: 53-5. doi: 10.5116/ijme.4dfb.8dfd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schober P, Boer C, Schwarte L A. Correlation coefficients: appropriate use and interpretation. Anesth Analg 2018; 126: 1763-8. doi: 10.1213/ANE.0000000000002864 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


