Summary
T-cell large granular lymphocytic leukaemia (T-LGLL) is an incurable leukaemia characterised by clonal proliferation of abnormal cytotoxic T cells that can result in severe neutropenia, transfusion-dependent anaemia and pancytopenia requiring treatment. The most commonly used agents, methotrexate (MTX), cyclophosphamide (Cy) and cyclosporine primarily produce partial remissions (PRs), with few complete responses (CRs). We evaluated the clinical course and treatment response of 60 consecutive patients with T-LGLL to evaluate clinical outcomes and future potential treatment directions. Impaired overall survival was noted among male patients, patients with elevated lactate dehydrogenase, and those without rheumatoid arthritis. Cy was the most efficacious second-line agent, with a 70% overall response rate (ORR; three CR, four PR). All patients who failed frontline MTX responded to second-line Cy. In the relapsed or Cy-refractory setting, alemtuzumab (n = 4) and pentostatin (n = 3) had an ORR of 50% and 66%, respectively, while duvelisib induced a long-term response in one patient. In this large, retrospective analysis, our results suggest Cy is a highly effective therapy for second-line treatment in T-LGLL and should be considered a strong candidate for up-front therapy in select high-risk patients. Prospective studies evaluating pentostatin, alemtuzumab and novel agents, such as duvelisib, are needed for patients with relapsed/refractory T-LGLL.
Keywords: T cell, T-cell large granular lymphocytic leukaemia, therapeutics, overall response rate, leukaemia
Introduction
T-cell large granular lymphocytic leukaemia (T-LGLL) is an incurable mature T-cell leukaemia characterised by the abnormal clonal proliferation of CD3+/CD8+ memory effector T cells [cytotoxic T lymphocytes (CTLs)].1–3 While the cause of T-LGLL is not known, current models suggest that normal CTLs proliferate and expand in response to chronic antigen stimulation, as occurs in patients with autoimmune disease. Indeed, T-LGLL is often seen concomitantly in patients with autoimmune disease, in particular rheumatoid arthritis (RA), and clinical or laboratory manifestations of autoimmunity are highly prevalent in patients with T-LGLL.4,5 Over time, chronic inflammation, mediated in part by interleukin 15 (IL-15), leads to constitutive signal transducer and activator of transcription 3 (STAT3) activation, dysregulation of micro-RNAs and epigenetic changes that drive and sustain the proliferation of T-LGLL cells, independent of external stimuli. Somatic gain-of-function mutations in STAT3 are present in approximately 40% of patients with T-LGLL.6 There are multiple mechanisms leading to neutropenia and anaemia in T-LGLL, including: production of inflammatory cytokines by monoclonal T-LGLL cells, release of cytotoxic granules with direct marrow damage, and STAT3-mediated persistence of the T-LGLL clone due to resistance to Fas/Fas-Ligand mediated apoptosis. All of these processes work to perpetuate the T-LGLL clone and the resultant cytopenias seen in this disease.4,7–9
Patients with T-LGLL typically present in their 6th decade of life with asymptomatic relative or absolute lymphocytosis, with or without concomitant anaemia or neutropenia. As the disease progresses, patients develop progressive anaemia, often transfusion-dependent and/or severe neutropenia, with absolute neutrophil counts (ANCs) of <1000 K/μm. In patients with neutropenia, serious infectious complications can occur including sepsis and bacterial infections. The most severe manifestations of bone marrow failure associated with T-LGLL are pure red cell aplasia (PRCA), and pancytopenia that presents with overlap features with myelodysplasia (MDS) or aplastic anaemia (AA).10–13 However, in most patients T-LGLL is an indolent, treatable disease, with a median survival of 9 years.14
While asymptomatic patients with mild anaemia or neutropenia can be followed with observation, for patients who have transfusion-dependent anaemia or severe neutropenia, treatment is indicated. Current treatment strategies are predicated on the hypothesis that many if not all the clinical manifestations of T-LGLL are caused by pro-inflammatory cytokines secreted by neoplastic CTLs; therefore, immune suppression to decrease the proliferation and function of these CTLs is the current standard-of-care treatment.15,16 The most commonly used agents are methotrexate (MTX), cyclophosphamide (Cy) and cyclosporine (CsA).17,18 In retrospective series, median response rates to each of these agents varies widely. However, most patients who respond achieve only partial normalisation of blood counts (partial remission, PR), and complete responses (CRs) are <10% and typically not durable. This leads to prolonged treatment with immunosuppressive agents, and ultimately recurrence of cytopenias, transfusions and/or neutropenia with the potential for concomitant infections.19–21 In the largest prospective study conducted in T-LGLL to date, the Eastern Cooperative Oncology Group (ECOG) 5998 (E5998) study, patients were treated with MTX initially, and then switched to Cy if no response.22 In that study, response rates to MTX were modest [overall response rate (ORR) 38%]. However, 64% of the patients that failed MTX attained a response after switching to Cy. Cyclosporine is also used, typically as a third-line option in patients who failed Cy and MTX, with response rates ranging from 21% (4% CR) to 56% (28% CR).19,23 However, its use is limited by hypertension and renal dysfunction. These poor results with standard therapies in T-LGLL highlight the need to develop novel strategies, and better understand predictors of disease response in T-LGLL.
For patients with T-LGLL failing the three standard therapies, a handful of new T-cell-targeting agents have been explored in the past 10 years. Alemtuzumab, an anti-CD52 monoclonal antibody, has shown some efficacy, with a Phase 2 study showing a 74% response rate in 25 patients that had typical T-LGLL.24 Other therapies, including the purine analogue pentostatin, the histone deacetylase (HDAC) inhibitor romidepsin, and splenectomy have been explored with anecdotal reports of clinical efficacy. However, these agents have not been adequately explored in clinical trials.25,26
Given the rarity of T-LGLL, very few studies have assessed the outcomes of large (N ≥50) cohorts of patients with T-LGLL, particularly in patients with relapsed and refractory disease. Furthermore, many of these retrospective studies were conducted >10 years ago and did not include patients treated with these agents. Thus, well-characterised single-centre retrospective cohort studies remain important tools to identify potentially promising therapies for additional preclinical and clinical testing. In the present study, we conducted a large retrospective cohort study of all patients treated with T-LGLL at the Ohio State University James Comprehensive Cancer Center between 1995 and 2018. The aim of the present study was to outline the disease course and prognostic factors for patients with T-LGLL with a focus on patients who failed standard front-line therapies.
Patients and methods
Patients
This study was conducted at the Ohio State University JamesComprehensive Cancer Center (OSU-CCC). All patients with a diagnosis of T-LGLL seen at OSU-CCC after 1995 and diagnosed prior to 1 December 2018 were included in the analysis. Patients with a diagnosis of T-LGLL were identified from the OSU lymphoma database. Additionally, patients were identified utilising the OSU Information Warehouse by utilising the International Classification of Diseases (ICD)-9 and ICD-10 codes for T-LGLL.
Diagnosis
The diagnosis of T-LGLL was made based on 2016 World Health Organization (WHO) criteria. In order to meet inclusion criteria for this study, the presence of a monoclonal T-cell receptor (TCR) and a CD3+ CD8+ population on flow cytometry ≥500 cells/mm3 was required. A monoclonal TCR was positive if detected by TCR polymerase chain reaction (PCR) or by restriction of TCR-Vbeta noted on flow cytometry. For patients diagnosed with a clonal TCR by flow cytometry, a panel of 30 TCR-Vbeta rearrangements was used with positivity considered if one or more clone was detected in ≥10% of events as previously described.27 Patients with a diagnosis of natural killer LGL (NK-LGL) or lack of a clonal TCR were excluded from the study. All pathology was reviewed by a Board-Certified haemato-pathologist in the OSU Department of Pathology.
Follow-up and response assessment
All patients were followed from 1995 to 2018 in the T-cell lymphoma clinic at the OSU, staffed by a dedicated T-cell physician. The work-flow, diagnostic and treatment approach were thus standardised over time. On treatment, patients were typically seen in clinic every 2–3 months. Patients off treatment, or on observation, were typically followed every 6 months to 1 year. Treatment regimens varied by patient based upon the clinical scenario. However, in general, front-line patients were treated with standard agents (MTX, Cy, CsA) for 4 months in order to assess for a clinical response. MTX was administered orally weekly with a target dose of 20 mg/week. Cy was administered orally daily at a dose of 100 mg, and CsA was administered at 100 mg twice daily.
For the purpose of uniform assessments, response rates were determined using established criteria from the E5998 prospective clinical trial.22 These same response criteria are currently being used in another prospective clinical trial in T-LGLL (ClinicalTrials.gov Identifier: NCT03239392). Response was assessed after four cycles (months) of therapy, unless the clinical scenario required urgent change in therapy. A CR was defined as having attained a normal complete blood count with ANC of >1500 K/μl,3 lymphocyte count of <1500 K/μl, and a haemoglobin that was within normal range. A PR was defined as having an improvement in ANC, lymphocyte count and/or haemoglobin in the absence of a CR. For patients with neutropenia, a PR was defined as a ≥50% improvement in ANC over baseline, or ANC >500 K/μm for patients with severe neutropenia provided it was >50% increase from baseline. For transfusion-dependent patients, a PR was attained with a >50% decrease in transfusion requirements. For patients with symptomatic anaemia, an increase in haemoglobin by 10 g/l indicated a PR. No response was defined as having no change in haematological parameters.
Statistical analysis
Given the rarity of T-LGLL, most of the analysis is descriptive in nature. The median follow-up time was determined by using the Reverse Kaplan–Meier method. Univariate probabilities of overall survival (OS) were estimated using the Kaplan–Meier method using the Statistical Package for the Social Sciences (SPSS®), version 26.0 (SPSS Inc., IBM Corp., Armonk, NY, USA). The log-rank test was used to assess for differences between groups for OS. OS was measured from the date of diagnosis until the date of death from any cause. For each variable evaluated using univariate analysis, OS was taken at the date of diagnosis, to understand the impact of any particular risk factor on OS. Given that most patients with T-LGLL progress over a long period of time, progression-free survival (PFS) was taken from the date of diagnosis, until the date of first progression on therapy, death due to disease, or death due to any cause.
Results
Patients
A total of 60 patients with confirmed T-LGLL on pathological review were included in the analysis. Full patient characteristics are included in Table 1. The median (range) age was 64 (30–87) years and 52% of patients were women. The median (range) T-LGLL count at diagnosis was 1763 (513–14 782)/μl. A majority of patients (68%) presented with a cytopenia, while 25% of patients were incidentally diagnosed with T-LGLL on routine laboratory testing. Of the patients who presented with a cytopenia, 26% presented with neutropenia (ANC <1500 K/μl), 41% with anaemia (haemoglobin <120 g/l) and 32% with both. In all, 21% of patients had transfusion-dependent anaemia and 28% of patients presented with splenomegaly on abdominal imaging or clinical examination. Only four patients had a STAT3 mutation out of a total of 12 that were tested, although standard mutational testing began only in 2018. In all, 27% of patients had concomitant RA, while 37% of patients had concomitant autoimmune disease (including, but not limited to RA). A concomitant haematological disorder was present in 18% of patients. A flowchart outlining the patients, their stage of progression and the treatment they received is shown in Fig 1.
Table I.
Patient characteristics (n = 60).
| Characteristic | N (%) |
|---|---|
| Sex | |
| Male | 29 (48) |
| Female | 31 (52) |
| Presenting cytopenia | |
| Neutropenia (ANC <1500/mm3) | 11 (18) |
| Anaemia (haemoglobin <120 g/l) | 17 (28) |
| Both | 13 (22) |
| Neither* | 15 (25) |
| Unknown | 4 (7) |
| LGL count: CD3CD8+ (at diagnosis) | N = 52 |
| <1500 | 25 (48) |
| ≥1500 | 27 (52) |
| LDH at diagnosis (190 = u/l normal) | N = 50 |
| ≤190 | 28 (56) |
| >190 | 22 (44) |
| Splenomegaly | N = 58 |
| Yes | 16 (28) |
| No | 42 (72) |
| Associated autoimmune disease | |
| Rheumatoid arthritis | 16 (27) |
| Sjögren’s disease | 1 |
| Acquired Factor VIII deficiency | 1 |
| Anti-phospholipid antibody syndrome | 1 |
| Granulomatosis with polyangiitis | 1 |
| Interstitial pneumonitis | 1 |
| Multiple sclerosis | 1 |
| None | 38 (63) |
| Additional haematological malignancy | |
| Pure red cell aplasia | 3 (5) |
| Myelodysplasia | 2 (3) |
| Lymphoma | 5 (8) |
| Evolving myeloma | 1 (2) |
Noted as a lymphocytosis, or other incidental finding Anytime during T-LGLL course; some of these patients later required therapy. Not all patients receive bone marrow biopsy.
Fig 1.
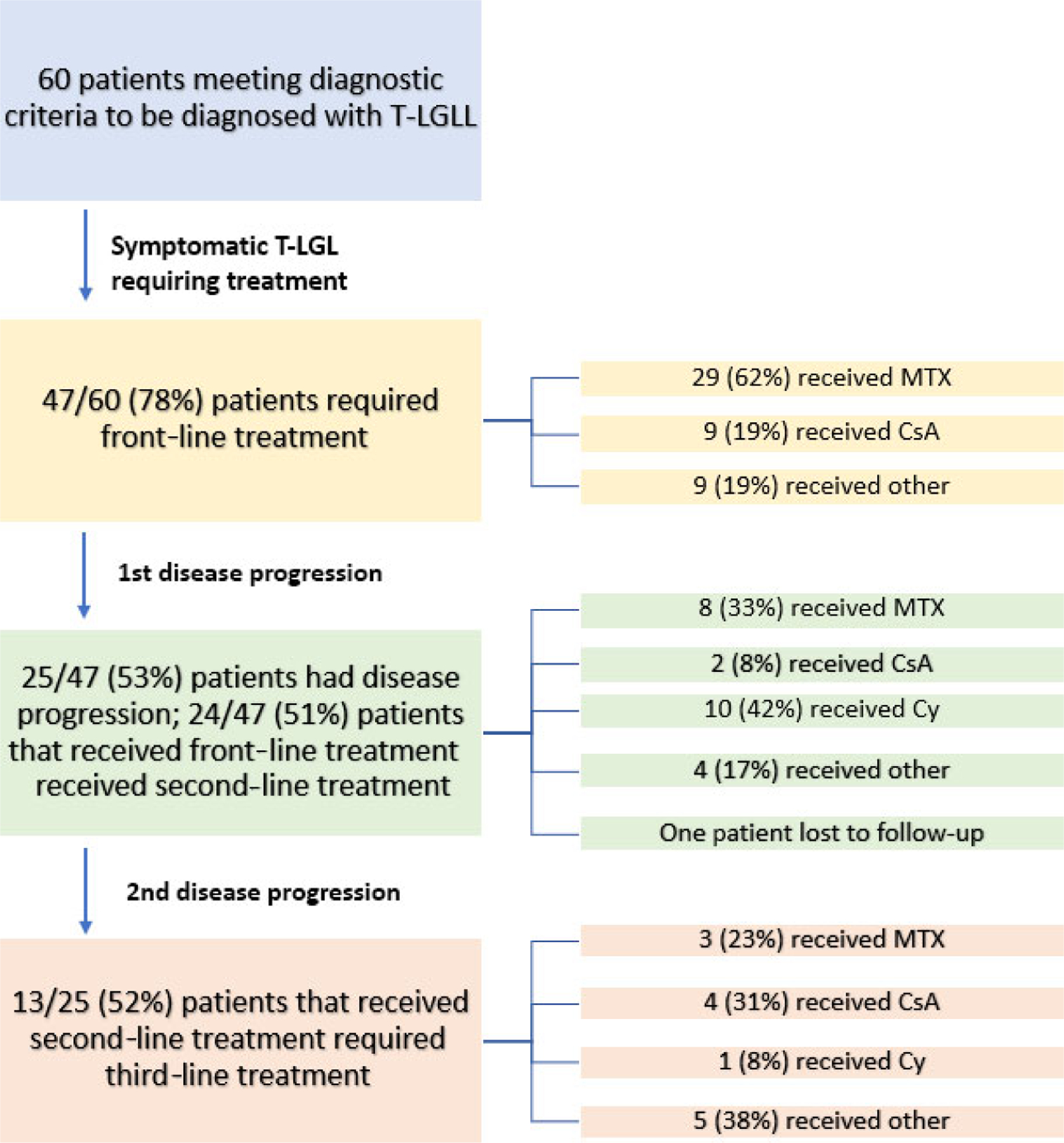
Flowchart of patient progress.
Front-line treatment of T-LGLL
A total of 78% (n = 47/60) of patients received first-line treatment for T-LGLL due to associated cytopenias; 13 patients (22%) received no treatment. Table 2 provides a summary of treatments used in the patients with T-LGLL. Of the patients requiring therapy, the most frequently used front-line treatment was MTX (29/47 patients, 62%), followed by CsA (nine of 47 patients, 19%). The ORR to front-line MTX was 41%; three of 29 (10%) with a CR and nine of 29 with a PR (31%). Of 43 evaluable patients, the ORR to any front-line therapy was 37% (30% PR, 7% CR). For patients who responded to frontline MTX, the median (range) time on treatment was 16·5 (7–47) months. No patients treated with front-line CsA had a response and no patients who were treated with front-line Cy had a response either. Of the patients that were treated with MTX as first-line therapy, there were two patients that had an initial PR and then later progressed to a CR at 1 year and 17 months, respectively. In all, 26% (12/47) of patients received steroids (prednisone) with their first-line treatment. The ORR for patients that did not receive steroids with first-line treatment was 37% (13/35: three CR, 10 PR). The ORR for patients that did receive steroids with their first-line treatment was 17% (2/12: no CR, two PR). There was a subjective change in the treatments used over time. From 1995 to 2004, most patients were treated front-line with MTX, and CsA was used as second-line. Cy was rarely used until after 2005. For patients that were diagnosed between 2005 and 2012, eight of 16 (50%) treated patients received MTX as front-line, six of 16 (37·5%) received CsA and two of 16 (12.5%) received other therapies. For patients diagnosed from 2013 or later, front-line therapies include: MTX, 18/27 (67%); CsA, two of 27 (7%); BNZ-1, four of 27 (15%) and the other three of 27 (11%) received other front-line therapies.19
Table II.
T-LGLL treatment.
| Treatment | N (%) |
|---|---|
| First-line treatment | N = 47 |
| Methotrexate | 29 (62) |
| Cyclosporine | 9 (19) |
| BNZ-1 | 4 (9) |
| Chemotherapy | 3 (6) |
| Alemtuzumab | 1 (2) |
| Chlorambucil | 1 (2) |
| Prednisone with first-line treatment | |
| Yes | 12 (26) |
| No | 35 (74) |
| First-line treatment response | |
| Methotrexate | 3 (10) CR, 9 (31) PR |
| Cyclosporine | 0 (0) CR, 2 (22) PR |
| Others | 0 (0) CR, 0 (0) PR |
| Second-line treatment | N = 24 |
| Cyclophosphamide | 10 (42) |
| Methotrexate | 8 (33) |
| Cyclosporine | 2 (8) |
| Pentostatin | 1 (4) |
| BNZ-1 | 1 (4) |
| Splenectomy | 2 (8) |
| Second-line treatment response | |
| Cyclophosphamide* | 3 (30) CR, 4 (40) PR |
| Methotrexate | 0 (0) CR, 4 (57) PR |
| Cyclosporine | 0 (0) CR, 0 (0) PR |
| Splenectomy | 0 (0) CR, 1 (50) PR |
| Others | 0 (0) CR, 1 (50) PR |
| Third-line treatment | N = 13 |
| Alemtuzumab | 3 (23) |
| Cyclosporine | 4 (31) |
| Methotrexate | 3 (23) |
| Cyclophosphamide | 1 (8) |
| Romidepsin | 1 (8) |
| Duvelisib | 1 (8) |
| Fourth-line or greater treatment | |
| Pentostatin | 2 |
| Cyclophosphamide | 2 |
| Tacrolimus | 1 |
| Methotrexate | 1 |
| Alemtuzumab | 1 |
| Romidepsin | 1 |
| Siltuximab | 1 |
| Duvelisib | 1 |
| Selinexor | 1 |
Seven of seven patients treated with Cy after methotrexate attained a response (three CR, four PR).
Treatment of relapsed T-LGLL
A total of 25 (42%) patients progressed on or after first-line therapy; with a median (range) time to first progression of 7 (2–57) months and 24/25 patients received a second-line treatment as one patient was lost to follow-up. Prior frontline treatments for this group of relapsed patients included: MTX (11, 44%) CsA (nine, 36%), and five (20%) other [EPOCH (etoposide phosphate, prednisone, vincristine, cyclophosphamide, and doxorubicin hydrochloride), CEOP (cyclophosphamide, etoposide phosphate, vincristine, prednisone), CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone), alemtuzumab and chlorambucil/prednisone]. The second-line treatments used in the patients with T-LGLL who failed front-line therapy are summarised in Table 2 and Fig 1. The most frequent second-line agent used was Cy (10; 42% of patients), followed by MTX (eight; 33%) and CsA (two; 8%). Two patients that received MTX as second-line treatment had a response, with the duration of treatment 28 months and 40 months, respectively. For patients that had a response to Cy as second-line treatment, the median (range) time on treatment was 12 (6–21) months. No patients had a response to CsA as a second-line treatment. Seven of the 10 patients who received Cy had received prior MTX. The ORR for Cy in the seven patients who received prior MTX was 100%: three CR (43%) and four PR (57%). All three patients that received Cy as second-line but received a treatment other than MTX as first-line had no response to the Cy. For all second-line therapies, the ORR in the 24 evaluable patients was 54% (nine PR, four CR). Two patients received a splenectomy with one attaining a CR. As a point of reference, while MTX, Cy and CsA were readily available throughout the treatment study period, novel therapies became available later including: alemtuzumab (2001), duvelisib (2014 on trial), BNZ-1 (2018 on trial), romidepsin (2009) and siltuximab (2014).
The median (range) time to second progression was 6 (1–38) months. A total of 13 patients received third-line or greater treatment. Of the 13 patients who received third-line therapy, six (46%) received prior MTX, three (23%) received prior Cy, two (15%) received prior CsA and two (15%) received other (one pentostatin and one splenectomy). There was significant heterogeneity in patients receiving treatment beyond second-line, although the most commonly used agent was CsA (four patients, 31%). Only two of the four had a response to CsA in this population (no CR, two PR). After second relapse, non-standard agents were more frequently used including agents such as: alemtuzumab (four patients), romidepsin (one), duvelisib (one), siltuximab (one), an anti-IL-6 monoclonal antibody, and pentostatin (two) (Table 2). The ORR across all agents was 54%. Two of three patients had a PR to pentostatin. Four patients received alemtuzumab, with two having a PR with no CR. Interestingly, duvelisib produced one PR for 3 years free of transfusion, although another patient had no response. The patients that received tacrolimus, romidepsin, siltuximab and selinexor all had no response. Table 3 and Fig 1 provide a summary of third-line or greater treatments, response and updated clinical status.
Table III.
Third-line treatment or greater.*
| Pt # | Sex | Age, years | Prior treatment | Treatment | Response | Status (most recent) |
|---|---|---|---|---|---|---|
| 8 | M | 59 | CsA/Pred. | CsA/Pred. | NR | Deceased |
| 12 | F | 47 | CsA | Duvelisib | PR | PR |
| 16 | M | 61 | MTX | Cy | PR | PR |
| 21 | M | 55 | Cy | CsA | NR | NR |
| 21 | CsA | Pentostatin | NR | NR | ||
| 24 | F | 41 | Cy | MTX | PR | PR |
| 33 | M | 51 | Splenectomy | MTX/Pred. | NR | CR |
| 33 | MTX/Pred. | Cy | NR | CR | ||
| 33 | Cy | Tacrolimus | NR | CR | ||
| 33 | Tacrolimus | Romidepsin | NR | CR | ||
| 33 | Romidepsin | Alemtuzumab | NR | CR | ||
| 33 | Alemtuzumab | Duvelisib | NR | CR | ||
| 33 | Duvelisib | Selinexor | NR | CR | ||
| 33 | Selinexor | Pentostatin | CR | CR | ||
| 47 | M | 51 | MTX | CsA/Pred. | PR | PR |
| 48 | F | 68 | MTX | Cy | PR | PR |
| 49 | M | 43 | Pentostatin | Romidepsin | NR | Deceased |
| 49 | Romidepsin | Siltuximab | NR | Deceased | ||
| 54 | M | 72 | MTX | Alemtuzumab | PR | PR |
| 55 | F | 66 | MTX | Cy | PR | PR |
| 64 | M | 70 | Cy/Pred. | Alemtuzumab | PR | PR |
| 67 | M | 74 | MTX | Alemtuzumab | SD | Deceased |
CsA, cyclosporine; MTX, methotrexate; Cy, cyclophosphamide; Pred; prednisone; NR, no response; PR, partial response; CR, complete response.
Some patients have multiple columns as they received greater than three lines of therapy.
Retreatment of T-LGLL
We also evaluated patients who were re-treated with a previously effective regimen. Overall, four patients were re-treated with an agent that had been used before and had produced a response, with all four patients responding (no CR, four PR). All four patients had previously had a PR with initial treatment. Two patients were re-treated with MTX with a response rate of 100% (no CR and two PR). The elapsed time between re-challenge for these patients was 50 and 67 weeks, respectively. Two patients were re-treated with CsA with a response rate of 100% (no CR and two PR).
Survival analysis
With a median follow-up of 28 months; the OS at 1, 3, 5 and 10 years was 94%, 82%, 72% and 66% (Fig 2). The PFS at 1, 3, 5 and 10 years was 65%, 40%, 35% and 10% (Fig 3). There was a non-statistically significantly different improvement in 5-year OS in patients with RA versus those without (88% vs. 66%; P = 0·138). Women had improved OS at 4 years when compared to men (87% vs. 66%; P = 0.081) that was approaching statistical significance, while there was a decrease in OS at 3 years for patients with lactate dehydrogenase (LDH) >190 u/l (72% vs. 86%; P = 0.139) that was also approaching significance. There was no impact of age of < or >65 years, cytopenia type, severity of neutropenia or anaemia at diagnosis, LGL count at diagnosis, lymphocyte count at diagnosis, concomitant haematological disorder or autoimmune disease, or response to front-line therapy on OS. OS analysis was limited by only 11 death events, of which only two were confirmed due to disease and several were lost to follow-up.
Fig 2.
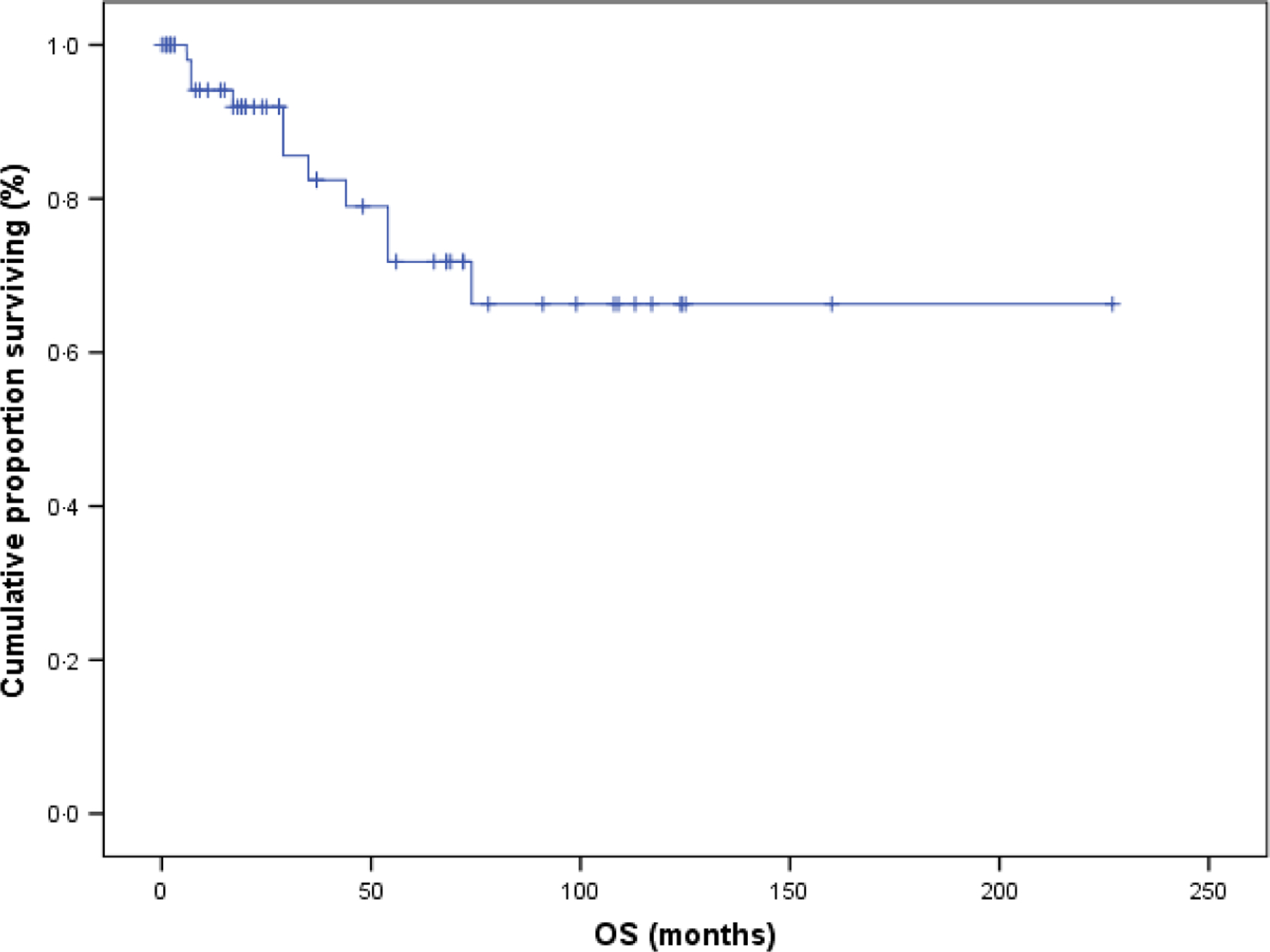
Overall survival (OS; n = 60). OS with a median follow-up time of 28 months, OS at 1, 3, 5 and 10 years was 94%, 82%, 72% and 66%.
Fig 3.
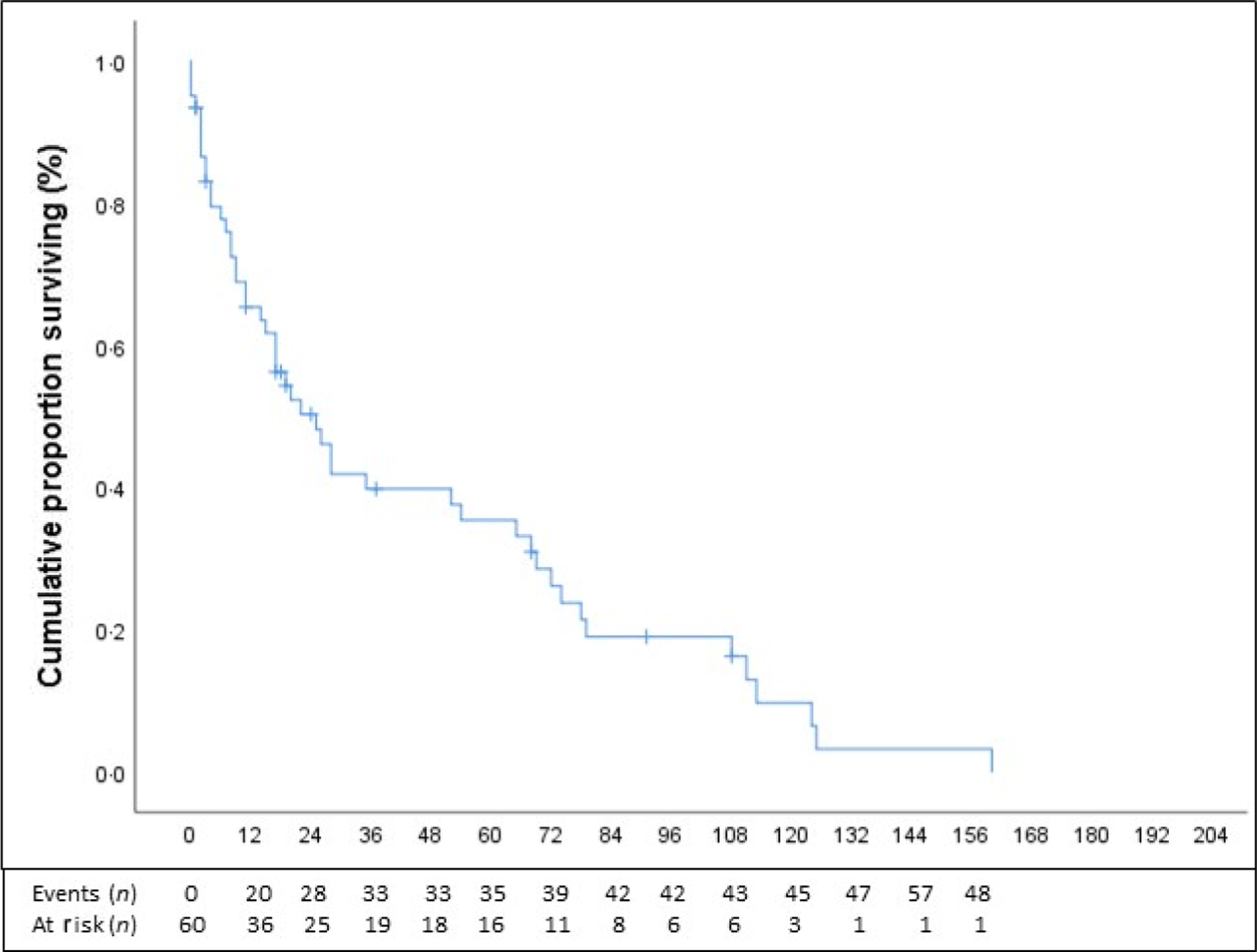
Progression-free survival (PFS; n = 60). PFS with number of events and number at risks (months) is presented in Fig 3. PFS was calculated from time of diagnosis, until first progression (on treatment) or death from any cause. PFS at 1, 2, 3 and 5 years was 65%, 50%, 40% and 35%, respectively.
Discussion
In the present study, we present a review of a large, single institution, cohort of patients with T-LGLL, with a focus on the outcomes of therapy in patients with relapsed/refractory disease. In our population, long-term OS remained favourable, with 72% and 66% of patients living 5 and 10 years, respectively, consistent with prior studies. However, this favourable long-term survival statistic belies the impact of T-LGLL on the quality of life of patients. The PFS at 1, 3, 5 and 10 years was only 65%, 40%, 35% and 10%, indicating that while patients remained alive with the disease, they required frequent, multiple therapies. Indeed, during the course of the study period, most (78%) of the patients required treatment. The ORR to first-line therapy was only 37%, and many patients required multiple lines of therapy or re-treatment, with a median (range) number of therapies of 3 (1–8). Additionally, disease remissions to first- and second-line therapy were relatively short, with median time to first and second progression of 7 and 6 months, respectively. While some patients attained longer-term remissions, the short median duration of remission and need for frequent treatment or re-treatment clearly demonstrates the urgent need to develop more effective and durable therapies in T-LGLL, and better predictors of response to therapy.
The currenttreatment strategy in T-LGLL is based upon the only reported prospective front-line clinical trial in T-LGLL, the E5998 study. That study evaluated the efficacy of MTX front-line in patients with T-LGLL. If patients failed to attain a response, they were switched to Cy. In the present study, the ORR of front-line MTX was 41%, which is similar to the E5998 experience. The ORR for Cy second-line in our study paralleled the ORR in the E5998 study, with 21% CR. Similarly, the French group published a registry analysis in 2009 that demonstrated an ORR in all comers of 55% to MTX, and 66% to Cy, and another French retrospective study of front-line Cy demonstrated ORR of 71% and CR rates of 47%.18,19 These results are summarised in Table 4. Importantly, in our present analysis, all patients who received prior MTX who were subsequently treated with Cy had a response (three CR, four PR). While relatively small numbers, the efficacy of Cy in our present analysis, particularly with favourable CR rates and response to MTX failures, combined with data from the French and E5998 studies is notable, and suggests that the use of Cy may be the preferred front-line agent for patients with T-LGLL. While MTX and CsA can result in remissions, the clinical observation has been that these agents do not frequently result in clearance of the T-LGLL clone, and thus may impact the cytopenias without affecting the neoplastic T cells driving the course of the disease. There is only anecdotal evidence that prolonged MTX treatment can lead to clearance of the T-LGLL clone. In the E5998 study, only one patient attained clearance of their T-LGLL clone, and in our present cohort, two patients who initially had an initial response (PR) later cleared their T-LGLL clone by 18 months after the start of treatment. As a more potent cytotoxic drug, Cy has the potential of inducing greater reductions of tumour burden. To test this hypothesis, there is an ongoing French National Study in which Cy is being compared front-line against MTX in a randomised, prospective clinical trial (ClinicalTrials.gov Identifier: NCT01976182).28 This study randomly assigned patients to Cy or MTX and evaluated the patients at 4 months for response. Responders continued through month 12, while non-responders received one of the drugs that they did not receive first-line, or CsA. In all, 96 patients were randomised in this trial with 80 being evaluated at month 4. The ORR was 52·6% and 36 patients ended up undergoing a second randomisation. The trial is still ongoing, though the initial results suggest that there may not be a substantial difference between Cy and MTX frontline, with CR rates less than20% with both agents.28 As final results from that study are awaited, the data from our cohort and others suggest that Cy can be considered as a front-line therapeutic option, or in patients who have received prior MTX.
Table IV.
Summary of major T-LGLL studies.
| Reference | Year | Prospective trial | Patients, n | Presenting anaemia, % | Presenting neutropenia, % | Autoimmune disease, % | Rheumatoid arthritis, % | Splenomegaly, % | Concomitant malignancy, % | Response rates (MTX, Cy, CsA), % | Alemtuzumab response, % | OS (5 years), % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present study | 2020 | No | 60 | 41 | 26 | 37 | 27 | 28 | 18 | 38, ND, 22 | 50 | 72 |
| Semenzato et al.3 | 1997 | No | 162 | 26 | ND | ND | 36 | 50 | ND | ND | ND | ND |
| Neben et al.29 | 2003 | No | 44 | 52 | 89 | ND | 20 | 35 | ND | ND | ND | ND |
| Bareau et al.19 | 2010 | No | 201 | 61 | 24 | ND | 17 | 24 | ND | 55, 66, 21 | ND | 89 |
| ECOG 5998 study22 | 2015 | Yes | 55 | 53 | 47 | ND | 7 | ND | ND | 38, 64 (2nd line) | ND | 69, 76 months* |
| Moignet et al.18 | 2014 | No | 45 | 53 | 24 | 11 | 2 | ND | ND | ND, 71, ND | ND | ND |
| Dumitriu et al.24 | 2016 | No | 25 | 44 | 72 | ND | ND | 2 | ND | ND | 56 | ND |
ND, no data.
Patients with anaemia had a median OS of 69 months, whereas patients with neutopenia had a median OS of 76 months.
Aside from the activity of Cy, our analysis provides additional insights on the up-front treatment of T-LGLL. Based on our results, patients with high LDH (>190 u/l), those who developed T-LGLL without a history of autoimmune disease or males had worse OS. In particular, at the 4 year mark, males had nearly 20% worse OS compared to females (87% vs. 66%; P = 0.081). While these data will need to be validated in a larger dataset and should be considered hypothesis generating, these findings suggest a population of patients with T-LGLL that may need more aggressive front-line therapy. We also evaluated the effect of concomitant prednisone use up-front on OS and ORR. A total of 26% of patients received prednisone with their first-line treatment. We did not find any benefit in OS for the patients that received concomitant prednisone, and while numbers were low, there was no significant difference in ORR. Thus, the use of steroids must be considered clinically on a case-by-case basis, but can be considered in patients with severe anaemia or neutropenia with infections as a bridge to more definitive therapy.
In the absence of a standard of care, of particular interest in tour cohort was the efficacy of novel or non-standard agents in patients with relapsed/refractory T-LGLL. Prior published studies had very limited discussion on newer novel agents, so we aimed to provide a summary of our institution’s experience to inform potential future directions. A total of 13 patients received third-line or greater treatment for T-LGLL. Table 3 provides a detailed outline of third-line or greater treatments with a description of clinical responses. It should be noted some patients received multiple therapies. The ORR across all agents was surprisingly high at 54%, although only one patient attained a CR. Two patients received pentostatin, one attained a CR, while the other patient had stabilisation of their disease. Intriguingly, the dual γ/δ PI3 kinase inhibitor, duvelisib, induced a long-term remission in one of two patients with transfusion-dependent anaemia, without the need for transfusions for 2 years. Alemtuzumab, the humanised monoclonal antibody directed against CD52, was used in four patients, two of which attained a response. These data, combined with a recent report from the National Cancer Institute (NCI) that provided a 74% ORR with intensive therapy with alemtuzumab, suggests that this agent may be a potent tool in patients with relapsed/refractory T-LGLL (Table 4).24 In that study, patients received a single course of alemtuzumab 10 mg intravenously daily over 7 days. While this approach has been studied in detail in that report, we have used 10–20 mg subcutaneously injected three-times a week for 8–10 weeks with good response, with minimal side-effects, which can be considered a potential alternative dosing strategy, particularly in elderly/infirm patients. This lower dose, subcutaneous approach may limit toxicities such as bone marrow suppression and cytomegalovirus (CMV) reactivation. The potential benefit of alemtuzumab must be balanced against the potential for serious infectious risk, particularly CMV/viral infections. CsA is typically used as the standard third-line treatment for patients with relapsed T-LGLL. In our analysis, albeit in low numbers, CsA had some efficacy in this population (two of four responders), but did not appear to be significantly better than other agents. Furthermore, the use of CsA is limited by renal dysfunction and hypertension, neurological toxicity and electrolyte imbalances, limiting its use, particularly in older patients with comorbidities, highlighting the need for newer, more efficacious agents for these patients. These data, while limited and hypothesis generating provide, a real-world experience at a large academic centre of patients with relapsed/refractory T-LGLL. Ourresults suggest that pentostatin or alemtuzumab can be considered an effective third-line treatment option in patients who are not candidates for CsA, particularly in patients with chronic renal disease or adverse reactions to CsA. Intriguingly, the PI3-kinase inhibitor, duvelisib, resulted in a long-term remission in a patient with transfusion-dependent anaemia, showing that this may be a good agent to explore in future clinical trials.
Given the rarity of T-LGLL, and the very limited prospective data, it is important to put the present data in the context of other large retrospective cohort studies in T-LGLL, as well as the prospective E5998 and NCI alemtuzumab studies. Table 4 provides a summary of major published prospective and retrospective studies in T-LGLL. Our study represents the third largest study of patients with T-LGLL to date. The OS was similar in our population compared to other studies. Of patients with a cytopenia, our cohort had 41% of patients presenting with anaemia, 26% presenting with neutropenia, and 32% with both. The rate of anaemia is very similar to other studies and the rate of neutropenia is similar to some studies but some inter-study variability in neutropenia rates is noted, probably due to low numbers. However, compared to other studies, our cohort had comparable rates of splenomegaly and RA. Response rates to Cy were remarkably similar compared to the French registry study, E5998 study and the Cy review from the French group, suggesting this is a true clinical effect of this agent.
This study has the limitations inherent to all retrospective, single-centre reviews. The study encompassed a long period of time, during which treatment strategies changed and new agents became available, and due to the long OS in patients even with severe T-LGLL, it is difficult to determine prognostic factors of T-LGLL. Additionally, analysis of clinical outcomes to treatment, particularly novel agents, must be interpreted with caution, given low patient numbers. It is difficult to correlate STAT3 mutations to treatment response, as there are low numbers of patients where this was tested and the inability to retrospectively test patients. On the other hand, this study provides the third largest dataset of patients with T-LGLL, using stringent modern consensus response criteria. Additionally, diagnostic criteria were stringent, based on updated WHO criteria, and this population excluded NK-LGL, allowing for a truly homogenous patient population. Clinical responses to first- and second-line treatment and patient characteristics are in concordance with other prospective and retrospective clinical studies, which increases the validity and potential generalisability of our observations.
Conclusions
We present treatment and outcome data from a large retrospective analysis of patients with T-LGLL. In our analysis, we found Cy to have significant activity in the relapsed/refractory setting. Its use should be considered up-front in patients with high-risk features such as severe anaemia or neutropenia, male sex, absence of autoimmune disease, or high LDH. In the relapsed/refractory setting, aside from Cy, alemtuzumab and pentostatin are potentially efficacious agents in this setting and can be considered before CsA. Additional prospective studies evaluating agents such as antagonists of IL-15 (BNZ-1), STAT3 inhibitors, or combinations of existing therapies are needed.
Funding sources
The project described was supported by Award Number Grant KL2TR002734 (Jonathan E. Brammer) from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Conflict of interest
The authors have no relevant conflicts of interest.
References
- 1.Loughran TP, Kadin ME, Starkebaum G, Abkowitz JL, Clark EA, Disteche C, et al. Leukemia of large granular lymphocytes: association with clonal chromosomal abnormalities and autoimmune neutropenia, thrombocytopenia, and hemolytic anemia. Ann Intern Med 1985;102:169–75. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenzato G, Zambello R, Starkebaum G, Oshimi K, Loughran TP Jr. The lymphoproliferative disease of granular lymphocytes: updated criteria for diagnosis. Blood 1997;89:256–60. [PubMed] [Google Scholar]
- 4.Lamy T, Moignet A, Loughran TP Jr. LGL leukemia: from pathogenesis to treatment. Blood 2017;129:1082–94. [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Epling-Burnette PK, Painter JS, Zou JX, Bai F, Wei S, et al. Antigen activation and impaired Fas-induced death-inducing signaling complex formation in T-large-granular lymphocyte leukemia. Blood 2008;111: 1610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koskela HL, Eldfors S, Ellonen P, van Adrichem AJ, Kuusanmäki H, Andersson EI, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med 2012;366:1905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston JA, Bacon CM, Finbloom DS, Rees RC, Kaplan D, Shibuya K, et al. Tyrosine phosphorylation and activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proc Natl Acad Sci USA 1995;92: 8705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra A, Liu S, Sams GH, Curphey DP, Santhanam R, Rush LJ, et al. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer Cell 2012;22:645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra A, Sullivan L, Caligiuri MA. Molecular pathways: interleukin-15 signaling in health and in cancer. Clin Cancer Res 2014;20:2044–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Go RS, Li CY, Tefferi A, Phyliky RL. Acquired pure red cell aplasia associated with lymphoproliferative disease of granular T lymphocytes. Blood 2001;98:483–5. [DOI] [PubMed] [Google Scholar]
- 11.Go RS, Tefferi A, Li CY, Lust JA, Phyliky RL. Lymphoproliferative disease of granular T lymphocytes presenting as aplastic anemia. Blood 2000;96:3644–6. [PubMed] [Google Scholar]
- 12.Risitano AM, Maciejewski JP, Muranski P, Wlodarski M, O’Keefe C, Sloand EM, et al. Large granular lymphocyte (LGL)-like clonal expansions in paroxysmal nocturnal hemoglobinuria (PNH) patients. Leukemia 2005;19:217–22. [DOI] [PubMed] [Google Scholar]
- 13.Maciejewski JP, O’Keefe C, Gondek L, Tiu R. Immune-mediated bone marrow failure syndromes of progenitor and stem cells: molecular analysis of cytotoxic T cell clones. Folia Histochem Cytobiol 2007; 45:5–14. [PubMed] [Google Scholar]
- 14.Shah MV, Hook CC, Call TG, Go RS. A population-based study of large granular lymphocyte leukemia. Blood Cancer J 2016;6:e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loughran TP Jr, Kidd PG, Starkebaum G. Treatment of large granular lymphocyte leukemia with oral low-dose methotrexate. Blood 1994;84: 2164–70. [PubMed] [Google Scholar]
- 16.Fujishima N, Sawada K, Hirokawa M, Oshimi K, Sugimoto K, Matsuda A, et al. Long-term responses and outcomes following immunosuppressive therapy in large granular lymphocyte leukemia-associated pure red cell aplasia: a Nationwide Cohort Study in Japan for the PRCA Collaborative Study Group. Haematologica 2008;93:1555–9. [DOI] [PubMed] [Google Scholar]
- 17.Lamy T, Loughran TP Jr. How I treat LGL leukemia. Blood 2011;117 (10):2764–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moignet A, Hasanali Z, Zambello R, Pavan L, Bareau B, Tournilhac O, et al. Cyclophosphamide as a first-line therapy in LGL leukemia. Leukemia 2014;28:1134–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bareau B, Rey J, Hamidou M, Donadieu J, Morcet J, Reman O, et al. Analysis of a French cohort of patients with large granular lymphocyte leukemia: a report on 229 cases. Haematologica 2010;95:1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan SR, Maciejewski JP. Diagnosis and therapy of neutropenia in large granular lymphocyte leukemia. Curr Opin Hematol 2009;16:27–34. [DOI] [PubMed] [Google Scholar]
- 21.Osuji N, Matutes E, Tjonnfjord G, Grech H, Del Giudice I, Wother-spoon A, et al. T-cell large granular lymphocyte leukemia: a report on the treatment of 29 patients and a review of the literature. Cancer 2006; 107:570–8. [DOI] [PubMed] [Google Scholar]
- 22.Loughran TP Jr, Zickl L, Olson TL, Wang V, Zhang D, Rajala HIM, et al. Immunosuppressive therapy of LGL leukemia: prospective multicenter phase II study by the Eastern Cooperative Oncology Group (E5998). Leukemia 2015;29:886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battiwalla M, Melenhorst J, Saunthararajah Y, Nakamura R, Molldrem J, Young NS, et al. HLA-DR4 predicts haematological response to cyclosporine in T-large granular lymphocyte lymphoproliferative disorders. Br J Haematol 2003;123:449–53. [DOI] [PubMed] [Google Scholar]
- 24.Dumitriu B, Ito S, Feng X, Stephens N, Yunce M, Kajigaya S, et al. Alemtuzumab in T-cell large granular lymphocytic leukaemia: interim results from a single-arm, open-label, phase 2 study. Lancet Haematol 2016;3: e22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fortune AF, Kelly K, Sargent J, O’brien D, Quinn F, Chadwick N, et al. Large granular lymphocyte leukemia: natural history and response to treatment. Leuk Lymphoma 2010;51:839–45. [DOI] [PubMed] [Google Scholar]
- 26.Subbiah V, Viny AD, Rosenblatt S, Pohlman B, Lichtin A, Maciejewski JP. Outcomes of splenectomy in T-cell large granular lymphocyte leukemia with splenomegaly and cytopenia. Exp Hematol 2008;36:1078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morice WG, Kimlinger T, Katzmann JA, Lust JA, Heimgartner PJ, Halling KC, et al. Flow cytometric assessment of TCR-Vbeta expression in the evaluation of peripheral blood involvement by T-cell lymphoproliferative disorders: a comparison with conventional T-cell immunophenotyping and molecular genetic techniques. Am J Clin Pathol 2004;121: 373–83. [DOI] [PubMed] [Google Scholar]
- 28.Lamy T, Pastoret C, Houot R, Ysebaert L, Hunault M, Damaj G, et al. Prospective, multicentric phase II randomized trial comparing the efficacy of methotrexate or cyclophosphamide in large granular lymphocytic leukemia: a French National Study. Report on the interim analysis. Blood 2019;134 (Suppl.):1545. [Google Scholar]
- 29.Neben MA, Morice WG, Tefferi A. Clinical features in T-cell vs. natural killer-cell variants of large granular lymphocyte leukemia. Eur J Haematol 2003;71(4):263–5. [DOI] [PubMed] [Google Scholar]


