Abstract
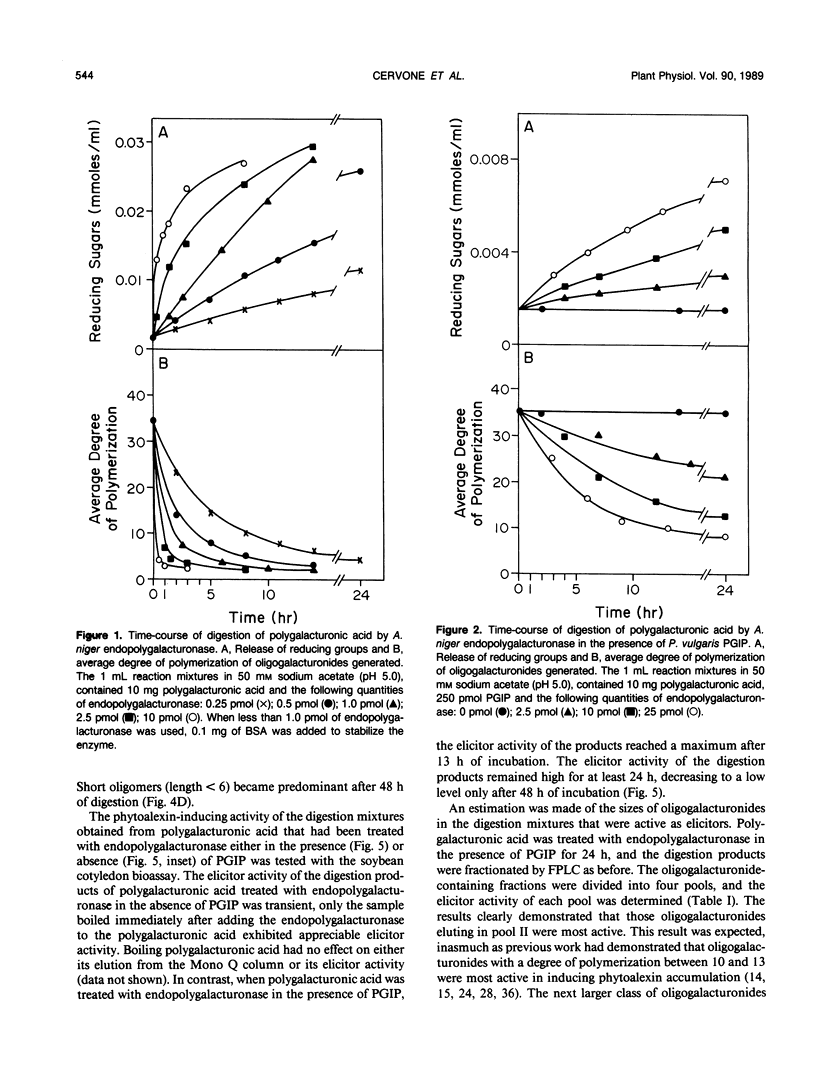
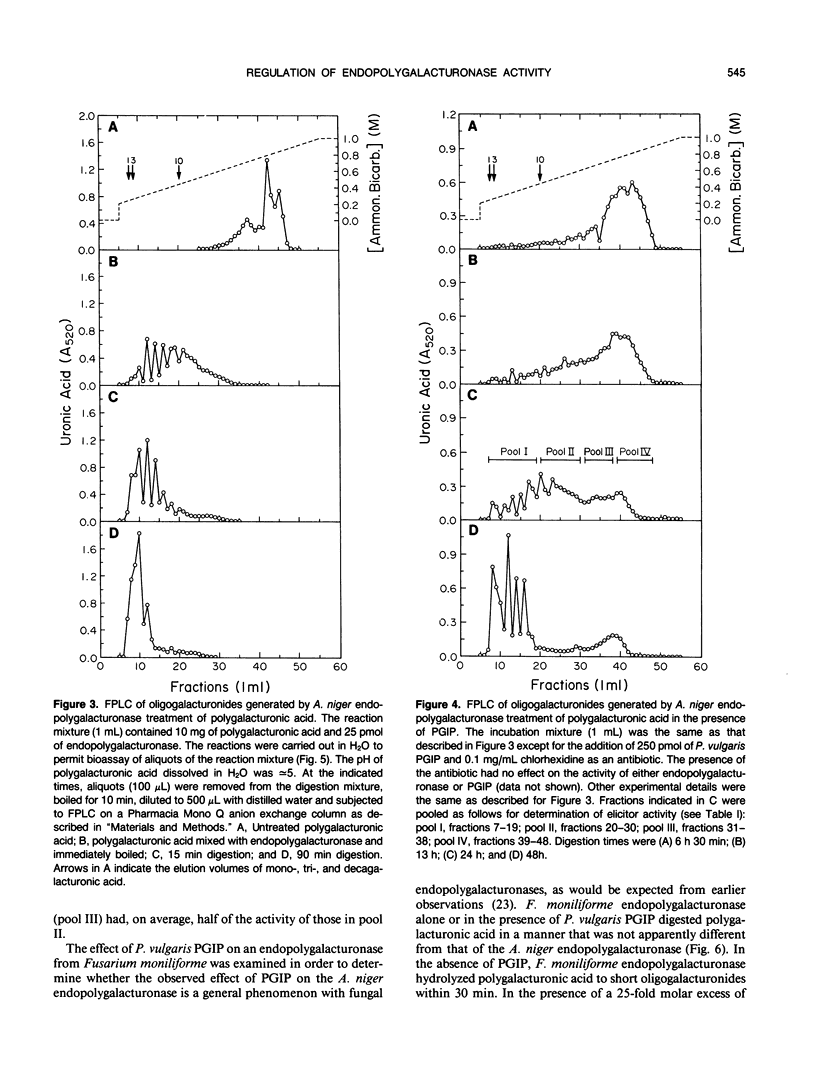
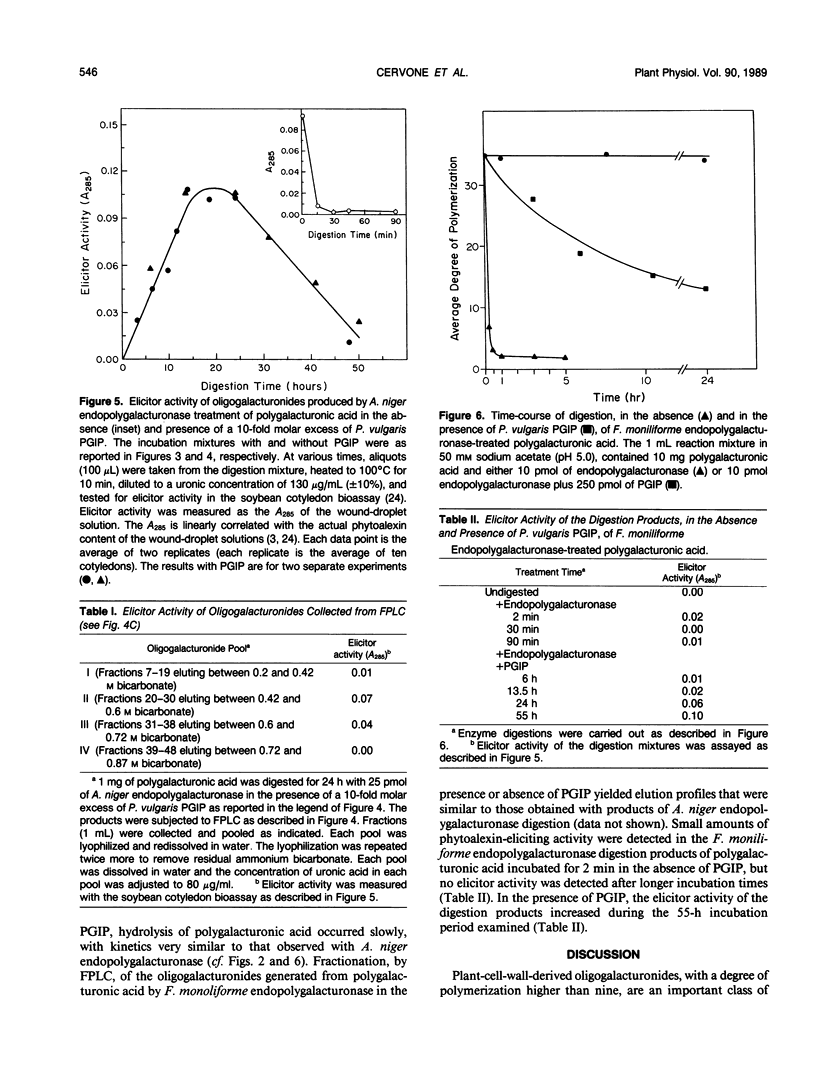
This paper describes the effect of a plant-derived polygalacturonase-inhibiting protein (PGIP) on the activity of endopolygalacturonases isolated from fungi. PGIP's effect on endopolygalacturonases is to enhance the production of oligogalacturonides that are active as elicitors of phytoalexin (antibiotic) accumulation and other defense reactions in plants. Only oligogalacturonides with a degree of polymerization higher than nine are able to elicit phytoalexin synthesis in soybean cotyledons. In the absence of PGIP, a 1-minute exposure of polygalacturonic acid to endopolygalacturonase resulted in the production of elicitor-active oligogalacturonides. However, the enzyme depolymerized essentially all of the polygalacturonic acid substrate to elicitor-inactive oligogalacturonides within 15 minutes. When the digestion of polygalacturonic acid was carried out with the same amount of enzyme but in the presence of excess PGIP, the rate of production of elicitor-active oligogalacturonides was dramatically altered. The amount of elicitor-active oligogalacturonide steadily increased for 24 hours. It was only after about 48 hours that the enzyme converted the polygalacturonic acid into short, elicitor-inactive oligomers. PGIP is a specific, reversible, saturable, high-affinity receptor for endopolygalacturonase. Formation of the PGIP-endopolygalacturonase complex results in increased concentrations of oligogalacturonides that activate plant defense responses. The interaction of the plant-derived PGIP with fungal endopolygalacturonases may be a mechanism by which plants convert endopolygalacturonase, a factor important for the virulence of pathogens, into a factor that elicits plant defense mechanisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albersheim P., Anderson A. J. Proteins from plant cell walls inhibit polygalacturonases secreted by plant pathogens. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1815–1819. doi: 10.1073/pnas.68.8.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers A. R., Ebel J., Finelli F., Berger N., Albersheim P. Host-Pathogen Interactions: IX. Quantitative Assays of Elicitor Activity and Characterization of the Elicitor Present in the Extracellular Medium of Cultures of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):751–759. doi: 10.1104/pp.57.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. D., Pearce G., Bryant J. E., Ryan C. A. Isolation and characterization of the proteinase inhibitor-inducing factor from tomato leaves. Identity and activity of poly- and oligogalacturonide fragments. J Biol Chem. 1984 Nov 10;259(21):13172–13177. [PubMed] [Google Scholar]
- Cervone F., De Lorenzo G., Degrà L., Salvi G. Elicitation of Necrosis in Vigna unguiculata Walp. by Homogeneous Aspergillus niger Endo-Polygalacturonase and by alpha-d-Galacturonate Oligomers. Plant Physiol. 1987 Nov;85(3):626–630. doi: 10.1104/pp.85.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K. R., Darvill A. G., Albersheim P., Dell A. Host-Pathogen Interactions : XXIX. Oligogalacturonides Released from Sodium Polypectate by Endopolygalacturonic Acid Lyase Are Elicitors of Phytoalexins in Soybean. Plant Physiol. 1986 Feb;80(2):568–577. doi: 10.1104/pp.80.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K. R., Lyon G. D., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XXV. Endopolygalacturonic Acid Lyase from Erwinia carotovora Elicits Phytoalexin Accumulation by Releasing Plant Cell Wall Fragments. Plant Physiol. 1984 Jan;74(1):52–60. doi: 10.1104/pp.74.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English P. D., Maglothin A., Keegstra K., Albersheim P. A Cell Wall-degrading Endopolygalacturonase Secreted by Colletotrichum lindemuthianum. Plant Physiol. 1972 Mar;49(3):293–298. doi: 10.1104/pp.49.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. L., Anderson A. J., Albersheim P. Host-Pathogen Interactions: VI. A Single Plant Protein Efficiently Inhibits Endopolygalacturonases Secreted by Colletotrichum Lindemuthianum and Aspergillus Niger. Plant Physiol. 1973 Mar;51(3):489–491. doi: 10.1104/pp.51.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. G., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XIX. THE ENDOGENOUS ELICITOR, A FRAGMENT OF A PLANT CELL WALL POLYSACCHARIDE THAT ELICITS PHYTOALEXIN ACCUMULATION IN SOYBEANS. Plant Physiol. 1981 Nov;68(5):1161–1169. doi: 10.1104/pp.68.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D. F., West C. A. Characteristics of galacturonic Acid oligomers as elicitors of casbene synthetase activity in castor bean seedlings. Plant Physiol. 1984 Apr;74(4):989–992. doi: 10.1104/pp.74.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr A. L., Albersheim P. Polysaccharide-degrading Enzymes are Unable to Attack Plant Cell Walls without Prior Action by a "Wall-modifying Enzyme". Plant Physiol. 1970 Jul;46(1):69–80. doi: 10.1104/pp.46.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp M., Rouster J., Fritig B., Darvill A., Albersheim P. Host-Pathogen Interactions : XXXII. A Fungal Glucan Preparation Protects Nicotianae against Infection by Viruses. Plant Physiol. 1989 May;90(1):208–216. doi: 10.1104/pp.90.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., West C. A. Polygalacturonase from Rhizopus stolonifer, an Elicitor of Casbene Synthetase Activity in Castor Bean (Ricinus communis L.) Seedlings. Plant Physiol. 1981 Apr;67(4):633–639. doi: 10.1104/pp.67.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothnagel E. A., McNeil M., Albersheim P., Dell A. Host-Pathogen Interactions : XXII. A Galacturonic Acid Oligosaccharide from Plant Cell Walls Elicits Phytoalexins. Plant Physiol. 1983 Apr;71(4):916–926. doi: 10.1104/pp.71.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M., Jin D., West C. A., Hadwiger L., Ryan C. A. Comparison of proteinase inhibitor-inducing activities and phytoalexin elicitor activities of a pure fungal endopolygalacturonase, pectic fragments, and chitosans. Plant Physiol. 1984 Nov;76(3):833–836. doi: 10.1104/pp.76.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]


