Abstract
Transposon Tn5 was used to mutate Bradyrhizobium japonicum USDA 61N. From over 5000 clones containing Tn5, 12 were selected and purified using a chemical reaction to identify oxidase-deficient clones. Four classes of mutants were identified based on the alterations in cytochromes. Most of the mutants had alterations in more than one cytochrome. Southern hybridization analysis of restricted genomic DNA of a representative strain of each class demonstrated that each mutant had a single Tn5 insert. Thus a single Tn5 insert produced pleiotropic effects on cytochromes. One class, which was totally deficient in cytochromes aa3 and c, produced ineffective nodules on soybeans. Most of the strains representing the other classes produced effective nodules but exceptions were observed in each class. Bacteroids of the wild-type strain contained cytochrome aa3. Bacteroids from one class of mutants were totally devoid of cytochrome aa3. Several of these strains produced effective symbioses indicating that cytochrome aa3 is not required for an effective symbiosis in this DNA homology group II strain which normally has this terminal oxidase in bacteroids.
Full text
PDF

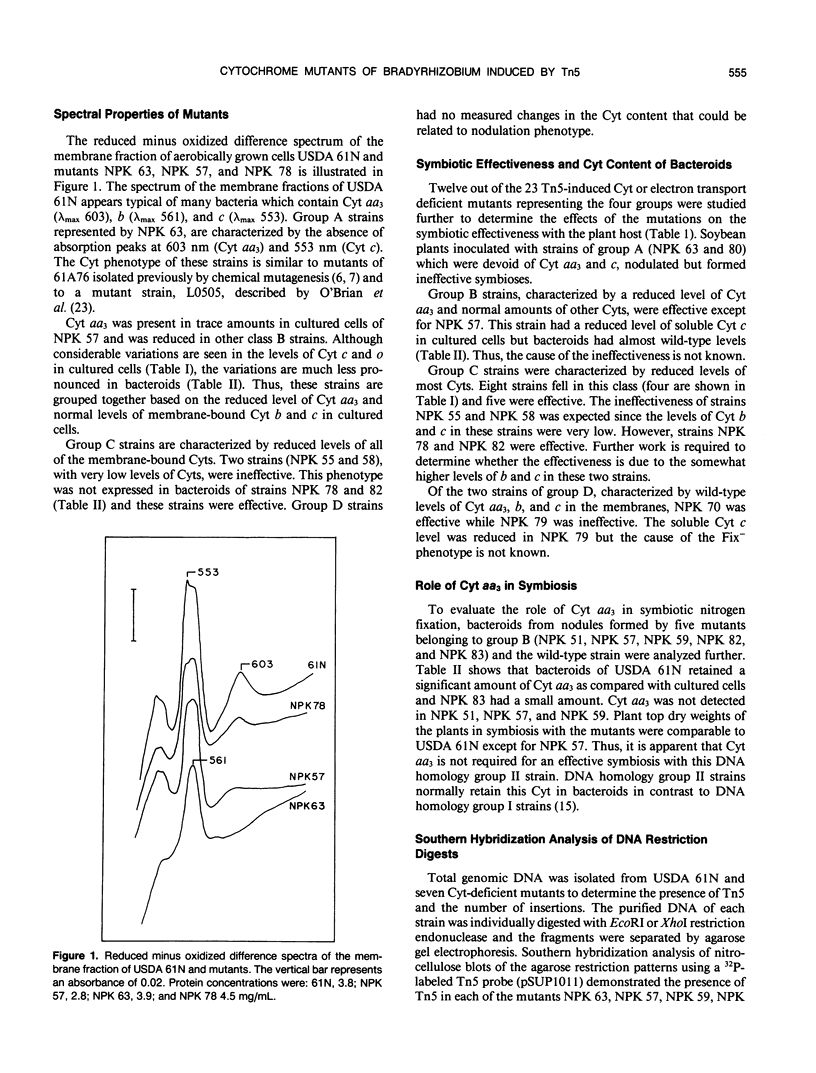
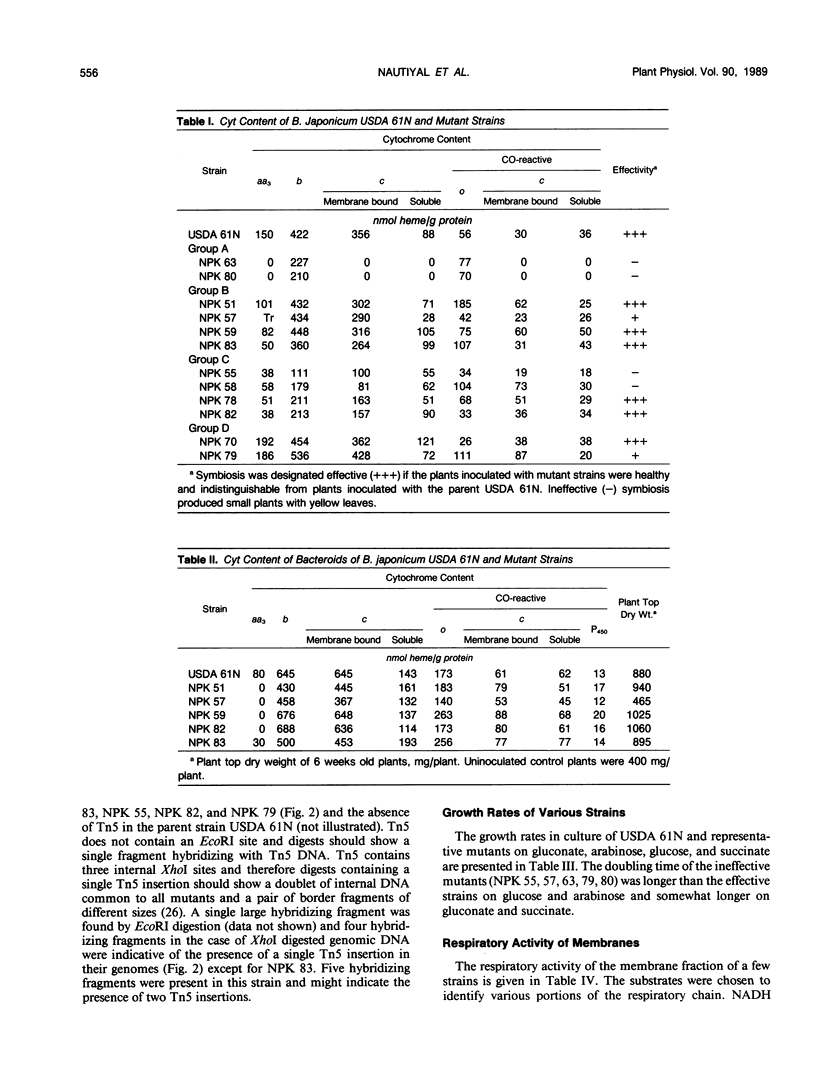
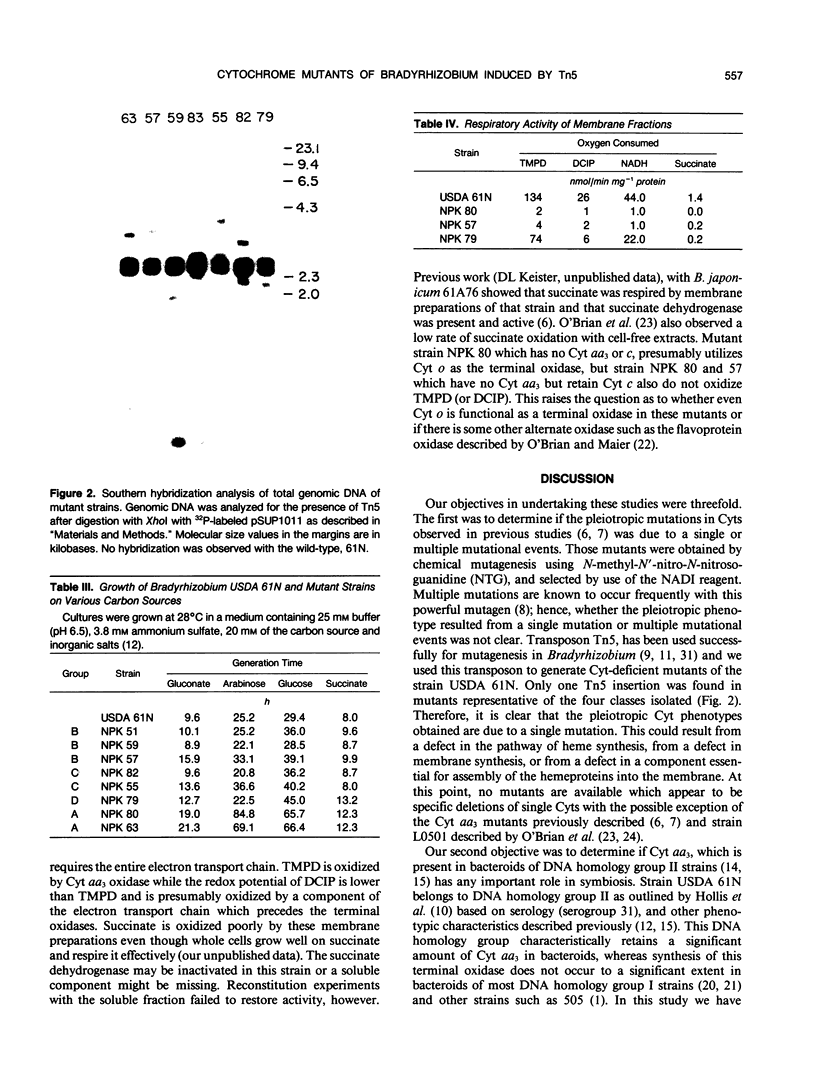


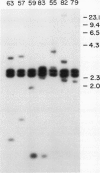
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A. Electron transport systems of Rhizobium japonicum. I. Haemoprotein P-450, other CO-reactive pigments, cytochromes and oxidases in bacteroids from N2-fixing root nodules. Biochim Biophys Acta. 1969 Jan 14;172(1):71–87. doi: 10.1016/0005-2728(69)90093-0. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. Electron transport systems of Rhizobium japonicum. II. Rhizobium haemoglobin, cytochromes and oxidases in free-living (cultured) cells. Biochim Biophys Acta. 1969 Jan 14;172(1):88–105. doi: 10.1016/0005-2728(69)90094-2. [DOI] [PubMed] [Google Scholar]
- Appleby C. A., Turner G. L., Macnicol P. K. Involvement of oxyleghaemoglobin and cytochrome P-450 in an efficient oxidative phosphorylation pathway which supports nitrogen fixation in Rhizobium. Biochim Biophys Acta. 1975 Jun 17;387(3):461–474. doi: 10.1016/0005-2728(75)90086-9. [DOI] [PubMed] [Google Scholar]
- Daniel R. M. The electron transport system of Acetobacter suboxydans with particular reference to cytochrome. Biochim Biophys Acta. 1970 Sep 1;216(2):328–341. doi: 10.1016/0005-2728(70)90224-0. [DOI] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Hom S. S., Uratsu S. L., Hoang F. Transposon Tn5-induced mutagenesis of Rhizobium japonicum yielding a wide variety of mutants. J Bacteriol. 1984 Jul;159(1):335–340. doi: 10.1128/jb.159.1.335-340.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber T. A., Agarwal A. K., Keister D. L. Extracellular polysaccharide composition, ex planta nitrogenase activity, and DNA homology in Rhizobium japonicum. J Bacteriol. 1984 Jun;158(3):1168–1171. doi: 10.1128/jb.158.3.1168-1171.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. L., Marsh S. S., El Mokadem M. T. Cytochromes of Rhizobium japonicum 61A76 Bacteroids from Soybean Nodules. Plant Physiol. 1983 Jan;71(1):194–196. doi: 10.1104/pp.71.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marrs B., Gest H. Genetic mutations affecting the respiratory electron-transport system of the photosynthetic bacterium Rhodopseudomonas capsulata. J Bacteriol. 1973 Jun;114(3):1045–1051. doi: 10.1128/jb.114.3.1045-1051.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Kirshbom P. M., Maier R. J. Tn5-induced cytochrome mutants of Bradyrhizobium japonicum: effects of the mutations on cells grown symbiotically and in culture. J Bacteriol. 1987 Mar;169(3):1089–1094. doi: 10.1128/jb.169.3.1089-1094.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Electron transport components involved in hydrogen oxidation in free-living Rhizobium japonicum. J Bacteriol. 1982 Oct;152(1):422–430. doi: 10.1128/jb.152.1.422-430.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Involvement of cytochromes and a flavoprotein in hydrogen oxidation in Rhizobium japonicum bacteroids. J Bacteriol. 1983 Aug;155(2):481–487. doi: 10.1128/jb.155.2.481-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'brian M. R., Maier R. J. Isolation of a cytochrome aa(3) gene from Bradyrhizobium japonicum. Proc Natl Acad Sci U S A. 1987 May;84(10):3219–3223. doi: 10.1073/pnas.84.10.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky M. J., Tully R. E., Cregan P. B., Keyser H. H. Genetic Diversity in Bradyrhizobium japonicum Serogroup 123 and Its Relation to Genotype-Specific Nodulation of Soybean. Appl Environ Microbiol. 1987 Nov;53(11):2624–2630. doi: 10.1128/aem.53.11.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Suicide plasmid vehicles for insertion mutagenesis in Rhizobium meliloti and related bacteria. J Bacteriol. 1983 Dec;156(3):1292–1300. doi: 10.1128/jb.156.3.1292-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wilson K. J., Anjaiah V., Nambiar P. T., Ausubel F. M. Isolation and characterization of symbiotic mutants of bradyrhizobium sp. (Arachis) strain NC92: mutants with host-specific defects in nodulation and nitrogen fixation. J Bacteriol. 1987 May;169(5):2177–2186. doi: 10.1128/jb.169.5.2177-2186.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]