Abstract
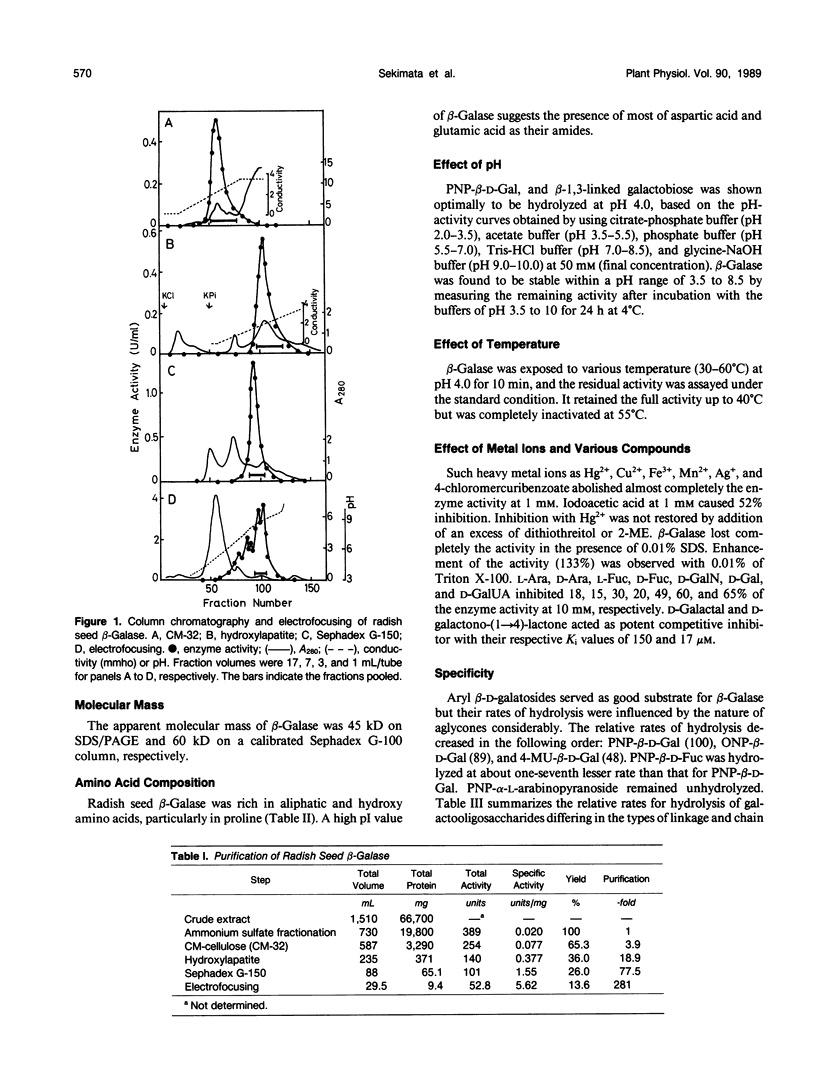
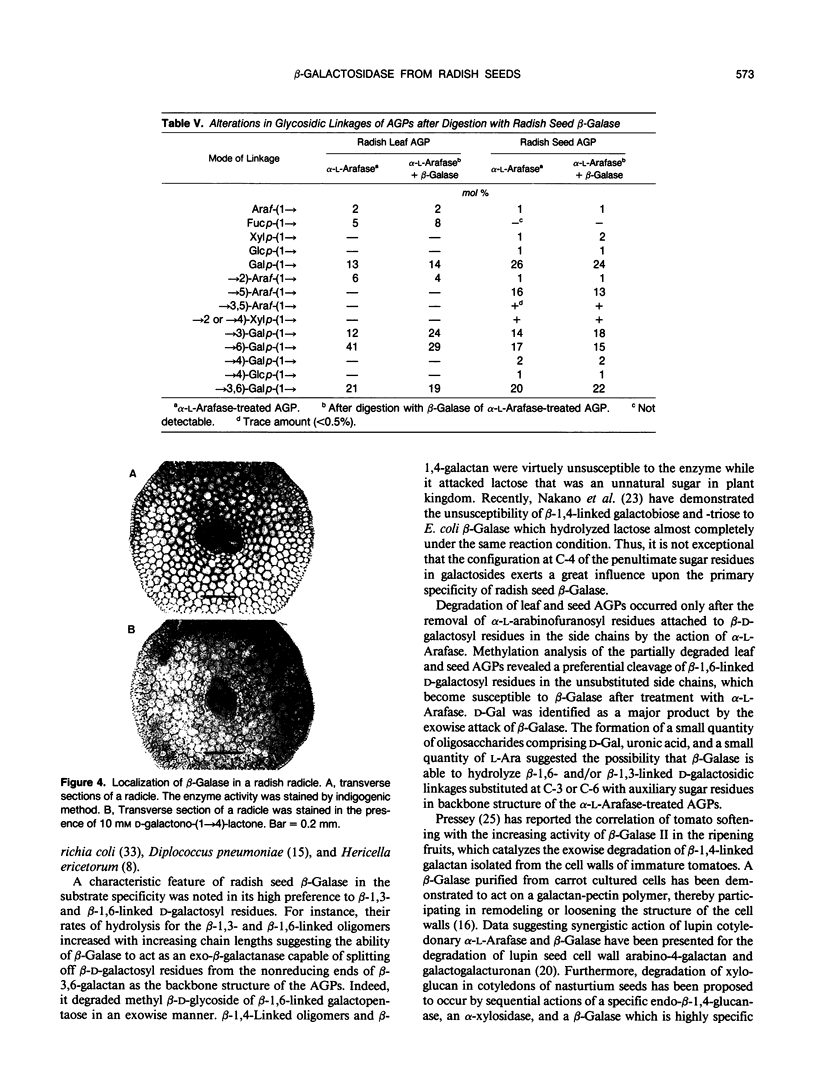
A basic β-galactosidase (β-Galase) has been purified 281-fold from imbibed radish (Raphanus sativus L.) seeds by conventional purification procedures. The purified enzyme is an electrophoretically homogeneous protein consisting of a single polypeptide with an apparent molecular mass of 45 kilodaltons and pl values of 8.6 to 8.8. The enzyme was maximally active at pH 4.0 on p-nitrophenyl β-d-galactoside and β-1,3-linked galactobiose. The enzyme activity was inhibited strongly by Hg2+ and 4-chloromercuribenzoate. d-Galactono-(1→4)-lactone and d-galactal acted as potent competitive inhibitors. Using galactooligosaccharides differing in the types of linkage as the substrates, it was demonstrated that radish seed β-Galase specifically split off β-1,3- and β-1,6-linked d-galactosyl residues from the nonreducing ends, and their rates of hydrolysis increased with increasing chain lengths. Radish seed and leaf arabino-3,6-galactan-proteins were resistant to the β-galase alone but could be partially degraded by the enzyme after the treatment with a fungal α-l-arabinofuranosidase leaving some oligosaccharides consisting of d-galactose, uronic acid, l-arabinose, and other minor sugar components besides d-galactose as the main product.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa M., Ogata S., Muramatsu T., Kobata A. Beta-galactosidases from jack bean meal and almond emulsin. Application for the enzymatic distinction of Galbeta1 leads to 4GlcNAc and Galbeta1 leads to 3GlcNAc linkages. J Biochem. 1974 Apr;75(4):707–714. doi: 10.1093/oxfordjournals.jbchem.a130443. [DOI] [PubMed] [Google Scholar]
- Bhalla P. L., Dalling M. J. Characteristics of a beta-Galactosidase Associated with the Stroma of Chloroplasts Prepared from Mesophyll Protoplasts of the Primary Leaf of Wheat. Plant Physiol. 1984 Sep;76(1):92–95. doi: 10.1104/pp.76.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury J. H., Jenkins G. A. Determination of the structures of trisaccharides by 13C-n.m.r. spectroscopy. Carbohydr Res. 1984 Mar 1;126(1):125–156. doi: 10.1016/0008-6215(84)85131-9. [DOI] [PubMed] [Google Scholar]
- Dey P. M., Del Campillo E. Biochemistry of the multiple forms of glycosidases in plants. Adv Enzymol Relat Areas Mol Biol. 1984;56:141–249. doi: 10.1002/9780470123027.ch3. [DOI] [PubMed] [Google Scholar]
- Edwards M., Bowman Y. J., Dea I. C., Reid J. S. A beta-D-galactosidase from nasturtium (Tropaeolum majus L.) cotyledons. Purification, properties, and demonstration that xyloglucan is the natural substrate. J Biol Chem. 1988 Mar 25;263(9):4333–4337. [PubMed] [Google Scholar]
- Edwards M., Dea I. C., Bulpin P. V., Reid J. S. Purification and properties of a novel xyloglucan-specific endo-(1----4)-beta-D-glucanase from germinated nasturtium seeds (Tropaeolum majus L.). J Biol Chem. 1986 Jul 15;261(20):9489–9494. [PubMed] [Google Scholar]
- HUGHES R. C., JEANLOZ R. W. THE EXTRACELLULAR GLYCOSIDASES OF DIPLOCOCCUS PNEUMONIAE. I. PURIFICATION AND PROPERTIES OF A NEURAMINIDASE AND A BETA-GALACTOSIDASE. ACTION ON THE ALPHA-1-ACID GLYCOPROTEIN OF HUMAN PLASMA. Biochemistry. 1964 Oct;3:1535–1543. doi: 10.1021/bi00898a025. [DOI] [PubMed] [Google Scholar]
- Holden D. W., Rohringer R. Peroxidases and glycosidases in intercellular fluids from noninoculated and rust-affected wheat leaves : isozyme assay on nitrocellulose blots from two-dimensional gels. Plant Physiol. 1985 Nov;79(3):820–824. doi: 10.1104/pp.79.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labavitch J. M., Freeman L. E., Albersheim P. Structure of plant cell walls. Purification and characterization of a beta-1,4-galactanase which degrades a structural component of the primary cell walls of dicots. J Biol Chem. 1976 Oct 10;251(19):5904–5910. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li S. C., Mazzotta M. Y., Chien S. F., Li Y. T. Isolation and characterization of jack bean beta-galactosidase. J Biol Chem. 1975 Sep 10;250(17):6786–6791. [PubMed] [Google Scholar]
- Matheson N. K., Saini H. S. alpha-L-Arabinofuranosidases and beta-D-galactosidases in germinating-lupin cotyledons. Carbohydr Res. 1977 Aug;57:103–116. doi: 10.1016/s0008-6215(00)81924-2. [DOI] [PubMed] [Google Scholar]
- Pressey R. beta-Galactosidases in Ripening Tomatoes. Plant Physiol. 1983 Jan;71(1):132–135. doi: 10.1104/pp.71.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Saunders J. A., Gillespie J. M. Localization and Substrate Specificity of Glycosidases in Vacuoles of Nicotiana rustica. Plant Physiol. 1984 Dec;76(4):885–888. doi: 10.1104/pp.76.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. B., Knox R. B. Quantitative cytochemistry of beta-galactosidase in normal and enzyme deficient (gal) pollen of Brassica campestris: application of the indigogenic method. Histochem J. 1984 Dec;16(12):1273–1296. doi: 10.1007/BF01003726. [DOI] [PubMed] [Google Scholar]
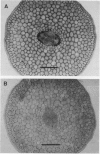
- Tsumuraya Y., Ogura K., Hashimoto Y., Mukoyama H., Yamamoto S. Arabinogalactan-Proteins from Primary and Mature Roots of Radish (Raphanus sativus L.). Plant Physiol. 1988 Jan;86(1):155–160. doi: 10.1104/pp.86.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka E., Sato M., Raiju M., Kaji A. Alpha-l-arabinofuranosidase from Rhodotorula flava. J Bacteriol. 1978 Mar;133(3):1073–1077. doi: 10.1128/jb.133.3.1073-1077.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLENFELS K., LEHMANN J., MALHOTRA O. P. [Research on lactose-splitting enzymes. VII. The specificity of beta-galactosidase from E. coli ML 309]. Biochem Z. 1960;333:209–225. [PubMed] [Google Scholar]