Abstract
Atmospheric pressure non-equilibrium plasmas are efficacious in killing both prokaryotic and eukaryotic cells. While the mechanism of plasma induced cell death has been thoroughly studied in prokaryotes, detailed investigation of plasma mediated eukaryotic cell death is still pending. When plasma is generated, four major components that interact with cells are produced: electric fields, radiation, charged particles, and neutral gas species. The goal of this study was to determine which of the plasma components are responsible for plasma-induced cell death by isolating and removing each from treatment. The C3H10T1/2 murine mesenchyme stem cell line was treated in six well plates, stained with Propidium Iodide to determine viability, and analyzed by image cytometry. Our results show that plasma-generated charges and reactive oxygen species are the primary contributors to cell death.
Keywords: cell death, mesenchymal stem cell, nanosecond pulsed dielectric barrier discharge plasma medicine, reactive oxygen species
Graphical Abstract
1. Introduction
Non-equilibrium plasma technologies are recently growing as unique, therapeutic tools for medical applications.[1,2] Studies have shown that plasma delivery can be tuned to induce cellular proliferation, differentiation, and apoptosis.[3–5] Plasma has been demonstrated to kill cancer cells in vitro and reduce tumor masses in vivo,[5,6] suggesting possible applications in cancer therapy. However, the fundamental mechanism by which plasma triggers cell death and tumor reduction is still poorly understood. Plasma treatment delivers four major components: electric fields, radiation, charges, and neutral gas species.[7–9] These elements are known to independently influence cell death. For example, Beebe et al have shown that nanosecond pulsed electric fields alone can induce cellular apoptosis in vitro and reduce tumor size in vivo.[10] UV radiation has been known for a long time to induce DNA damage leading to cell death.[11,12] Superoxide and hydrogen peroxide, examples of charged and neutral components of plasma generated species, are also well documented to induce apoptosis at certain concentrations.[13,14] These constituents are all present in plasma, which raises the question: what is the relative contribution of each component on observed cell death following treatment? Determination of the key components in plasma responsible for cell death and controlling their delivery can lead to optimized treatment for future clinical use. Furthermore, they may lead to treatments that can be tuned to selectively kill cancerous cells and decrease toxicity in non-cancerous cells. In this study, we used a dielectric barrier discharge (DBD) plasma which operates at atmospheric pressure and ambient temperatures.[15] The electric fields and Columbic forces are known to dominate in electromagnetic interactions for DBDs.[16] Therefore, the contribution of temperature and magnetic fields during plasma exposure is considered insignificant.
DBDs create plasma in direct contact with living cells by applying fast, high voltage pulses between a copper electrode covered with a quartz dielectric and the biological target.[17] Previously, DBD plasma treatment studies were carried out with microsecond-pulsed power supplies in atmospheric air, which resulted in the formation of streamers in the discharge.[4,9,18] The resulting plasma was in the non-uniform regime.[15] However, nanosecond pulsed dielectric barrier discharges (nspDBD) in air have been shown to generate a diffuse, uniform discharge without streamers at specific operating parameters: very high voltage (~30 kV), fast rise times (~2 ns), over small gap distances (~1 mm). Uniformity is lost if any one of these conditions are not met.[19] Not only do streamers lead to uneven treatment of cells, but the head of the streamer also generates high local electric fields which result in higher electron energies compared to those in the uniform regime.[20] The electron energy influences the plasma-generated species, so changes in the plasma regime can also influence plasma chemistry. For this study, nspDBD plasma was operated primarily in the uniform regime because it was easier for plasma components to be separated. The authors have previously shown the effects of the different plasma components for microsecond-pulsed DBDs on biological systems while other labs have studied the components of plasma with non-thermal atmospheric jets and other plasmas.[9,21,22] Although nanosecond and microsecond DBDs may seem similar, the energy of the two discharges is significantly different, and so a direct comparison is challenging.[9] However, to address the contribution of streamers and plasma filaments on cell viability, the non-uniform regime of nspDBD was also explored. This would be reflective of microsecond DBDs.
In the present study, we determined the relative contribution of major plasma components on the viability of C3H10T1/2 murine mesenchyme stem cells. We utilized a nanosecond pulsed dielectric barrier discharge (nspDBD) and exposed cells in 6-well tissue culture plates to various distinct plasma doses and treatment conditions that included or excluded specific plasma components. Our results indicate that the plasma generated charges and reactive oxygen species (ROS) are the major contributors for plasma induced cell death. Under these conditions, cell death seems to occur partially through direct cell lysis.
2. Materials and Methods
2.1. Cell Culture
Murine mesenchymal stem cells (CH310T1/2, ATCC: CCL-226) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) with 5% fetal bovine serum, 5% fetal calf serum, and 1% penicillin/streptomycin (Invitrogen, Life Science Technologies, USA) and incubated at 37°C with 5% CO2. Prior to seeding cells in 6-well plates, wells were coated with 0.15 mg/mL Rat tail Collagen I (BD Biosciences, USA) in 0.02N acetic acid (Fischer Scientific, USA) for 1 h and then washed three times with phosphate buffered saline (PBS) with calcium and magnesium. Type I collagen is a protein made up of three α-chains and is often used to coat surfaces to enhance cell adhesion.[23,24] Cells were seeded in 2 mL of media at concentrations of 2.0 × 105 or 1.0 × 105 cells/well and grown to confluence in one or two days, respectively, before plasma treatment. Before treatment, cell culture media was removed, cells were washed with PBS, and 1 mL of DMEM clear without serum added to each well. This media was removed right before plasma treatment and 1 mL of fresh DMEM clear without any serum was replaced immediately after.
2.2. Nanosecond Pulsed Dielectric Barrier Discharge Plasma Parameters
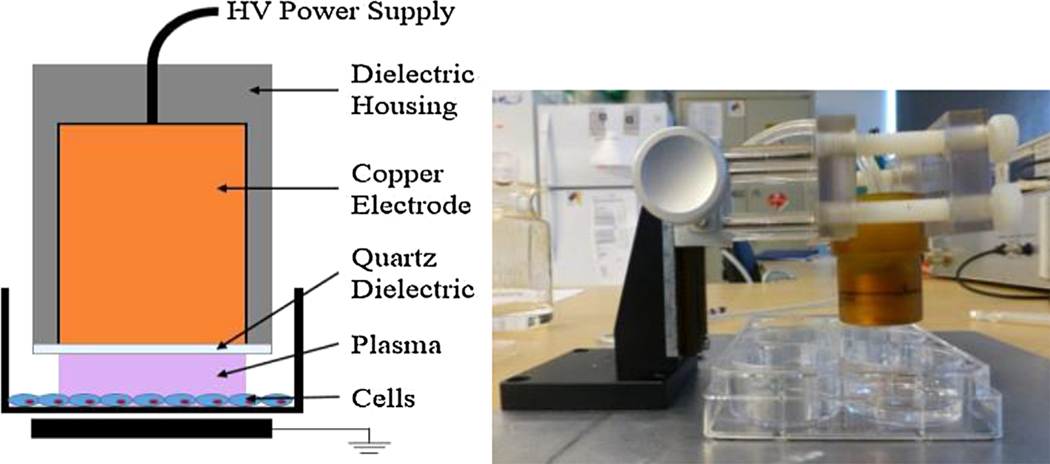
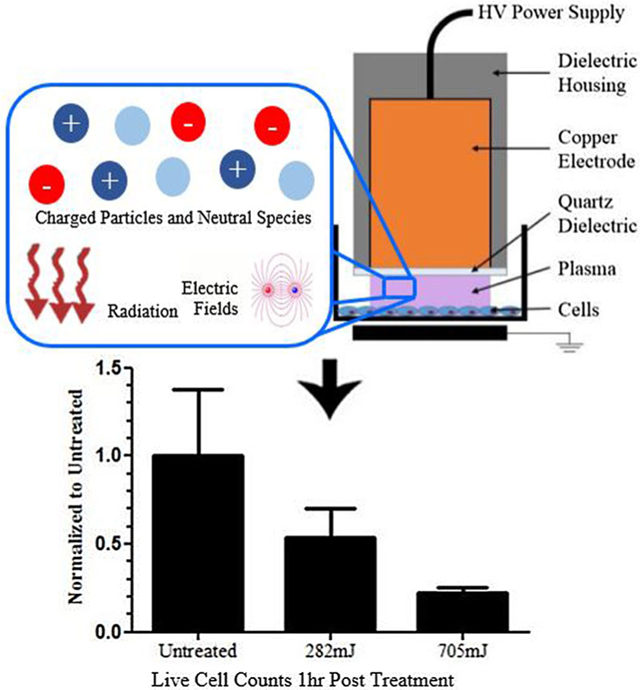
nspDBD plasma was produced by applying a positive voltage pulse of approximately 29 kV between the high voltage electrode and a grounded metal plate underneath the 6-well plate. Cells in the wells were at floating potential. The power supply (FID technology, FPG-20–05NM) generated 20 ns pulse widths with rise times of 2 ns. Frequency of the pulses was controlled with a function generation (TTi, TG5011 LXT) and treatment duration was fixed at 10 s. For uniform nspDBD plasma treatment, the gap distance was at 1 mm. Mock plasma treatment of negative control cells was performed in an identical manner, but without plasma being generated. These are indicated as untreated in all our graphs. A schematic of our electrode and experimental setup is shown in Figure 1.
Figure 1.
nspDBD plasma treatment (left) schematic and (right) device setup.
Accurate measurements of voltage at nanosecond times are possible when using techniques such as back current shunt. However, this method works when a relatively long transmission line, longer than the pulse duration, is used (in this case ~10 m). In these experiments, we have used a short high voltage (HV) cable (~2 m long), and therefore could only use a HV probe (Tektronix, P6015A) in conjunction with a current probe (CM-10-L, 2 ns usable rise time, Ion Physics Corporation) connected to a 1 GHz oscilloscope (DPO-4104B, Tektronix). Delay times from both voltage and current probes have been measured and accounted for using Tektronix AFG-3252 Arbitrary/Function Generator (signal rise/fall time of less than 2.5 ns and a typical jitter (RMS) less than 20 ps with a delay time resolution of 10 ps).
The energy per pulse of a single uniform nspDBD plasma discharge in a 6-well plate was calculated after measuring voltage and current, using the method and instruments stated previously, and then subtracting displacement current. This was found to be 0.9 mJ/pulse. However, in some treatment conditions, a grounded metal mesh barrier was added between the cells and the high voltage electrode; the energy per pulse measured for this condition was 1.9 mJ/pulse. The energy per pulse was also measured and calculated for the non-uniform nspDBD regime of plasma (0.6 mJ/pulse) where gap distance was increased to 2 mm. In order to keep the dose (total plasma energy delivered to the cells) the same during treatment, the frequency was adjusted based on the energy per pulse for each condition. Plasma treatment parameters are listed in Table 1.
Table 1.
Operating parameters for nspDBD plasma treatment.
| Parameter | Value |
|---|---|
| Excitation | Nanosecond pulsed |
| Voltage | 29 kV |
| Rise time | 2 ns |
| Pulse width | 20 ns |
| Treatment time | 10 s |
| Total doses | 47, 141, 282, 705 mJ |
| Uniform | |
| Gap distance | 1 mm |
| Energy per pulse (in wells) | 0.9 mJ/pulse |
| Frequency | 5, 15, 30, 75 Hz |
| Energy per pulse (on copper mesh) | 1.9 mJ/pulse |
| Frequency (for high doses) | 15, 37 Hz |
| Non-Uniform | |
| Gap distance | 2 mm |
| Energy per pulse (in wells) | 0.6 mJ/pulse |
| Frequency (for high doses) | 50, 128 Hz |
2.3. Isolation of Plasma Components
Several treatment conditions, utilizing various barriers and gases, were designed to remove specific plasma components during exposure to cells.
2.3.1. Direct Uniform nspDBD-Effect of All Components in Uniform Discharge
Direct uniform nspDBD in air, containing all the plasma effectors, served as our baseline for plasma induced cell death and as the control for all experiments.
2.3.2. Electrode Dipped in Media-Effect of Global Electric Field
To generate electric field without producing plasma, we did not remove the media prior to treatment. The electrode was dipped into the media and operated as before: 29 kV at 30 and 75 Hz (corresponding to the 282 and 705 mJ doses, respectively). Cells experienced electric fields of approximately 300 kV/cm.
2.3.3. Uniform nspDBD With Quartz Barrier-Effect of UV radiation
To determine the contribution of plasma-generated UV radiation on cell viability, a grounded copper mesh with a 0.5 mm-thick quartz barrier was positioned 1 mm away from the high voltage electrode and placed directly on top of the cell surface. Thus, while plasma was generated between the high voltage electrode and the copper mesh, the quartz barrier is only transparent to UV >200 nm; this consequently removes the gas species from treatment.
2.3.4. Uniform nspDBD With Mesh Barrier-Effect of Charges and Short-Lived Neutral Species
To study the contribution of charges and short-lived neutral species, we used a copper mesh without the quartz barrier.[8,25] Only long-lived neutral gas species and UV were delivered to the cell layer during treatment. To keep the total energy delivered to the cells the same, pulse frequencies were adjusted to 15 and 37 Hz as shown in Table 1.
2.3.5. Uniform Discharge in Oxygen-Effect of Oxygen Species
In order to examine the effect of oxygen species, the electrode was inserted into a sealed well. 99.999% pure oxygen (Airgas, NJ, USA) was flown at a rate of 5 SCFM for 10 s using 18 gauge inlet and outlet needles. The media was not degassed since it was removed immediately before treatment. Plasma treatment followed after oxygen was delivered and while gas was still flowing. The gas environment that plasma is created in can alter the energy per pulse. Therefore, the discharge energy in both air and oxygen was measured and compared to ensure that total energy delivered to cells during treatment was the same.
2.3.6. Uniform Discharge in Nitrogen-Effect of Nitrogen Species
To determine the effect of nitrogen species, the experimental setup was similar to the oxygen experiment, but oxygen gas was replaced with 99.999% pure nitrogen. Discharge energy was also measured in nitrogen and compared to that in air.
Treatment in these two gas conditions helped delineate the contribution of plasma generated oxygen and nitrogen species on cell viability respectively.
2.3.7. Non-Uniform nspDBD in Nitrogen-Effect of Streamers Part I
nspDBD treatment was ignited in a pure nitrogen environment as previously stated. To operate in the non-uniform regime, voltage and treatment time was kept at 29 kV and 10 s, respectively, but the electrode gap distance was increased to 2 mm. To maintain the same doses delivered to the cells, pulse frequencies were adjusted to 50 and 128 Hz based on our calculations tabulated in Table 1.
2.3.8. Non-Uniform nspDBD in Air-Effect of Streamers Part II
The non-uniform regime of plasma was produced in air by increasing gap distance to 2 mm. Taken together, these last two treatment conditions provided insight into the contribution of streamers on cell viability.
2.4. Visualization of Plasma Uniformity
Photographs of the plasma discharge were taken from underneath the well to determine the uniformity of plasma in air, oxygen, and nitrogen at 29 kV and 1 mm gap distances. The well rested on top of a clear plastic plate filled with water and sodium chloride (0.13 g/mL) which acted as our second, grounding electrode. Gap distance was increased to 2 mmunder air and nitrogen gas environments to visualize the discharge in the non-uniform regime of plasma. Images were captured with a Nikon D700 at the following settings: 25 600 ISO speed, f/4.5, 1/40 s exposure time, +5 step exposure bias, and 105 mm focal length.
2.5. Cell Count and Viability Measurement and Analysis
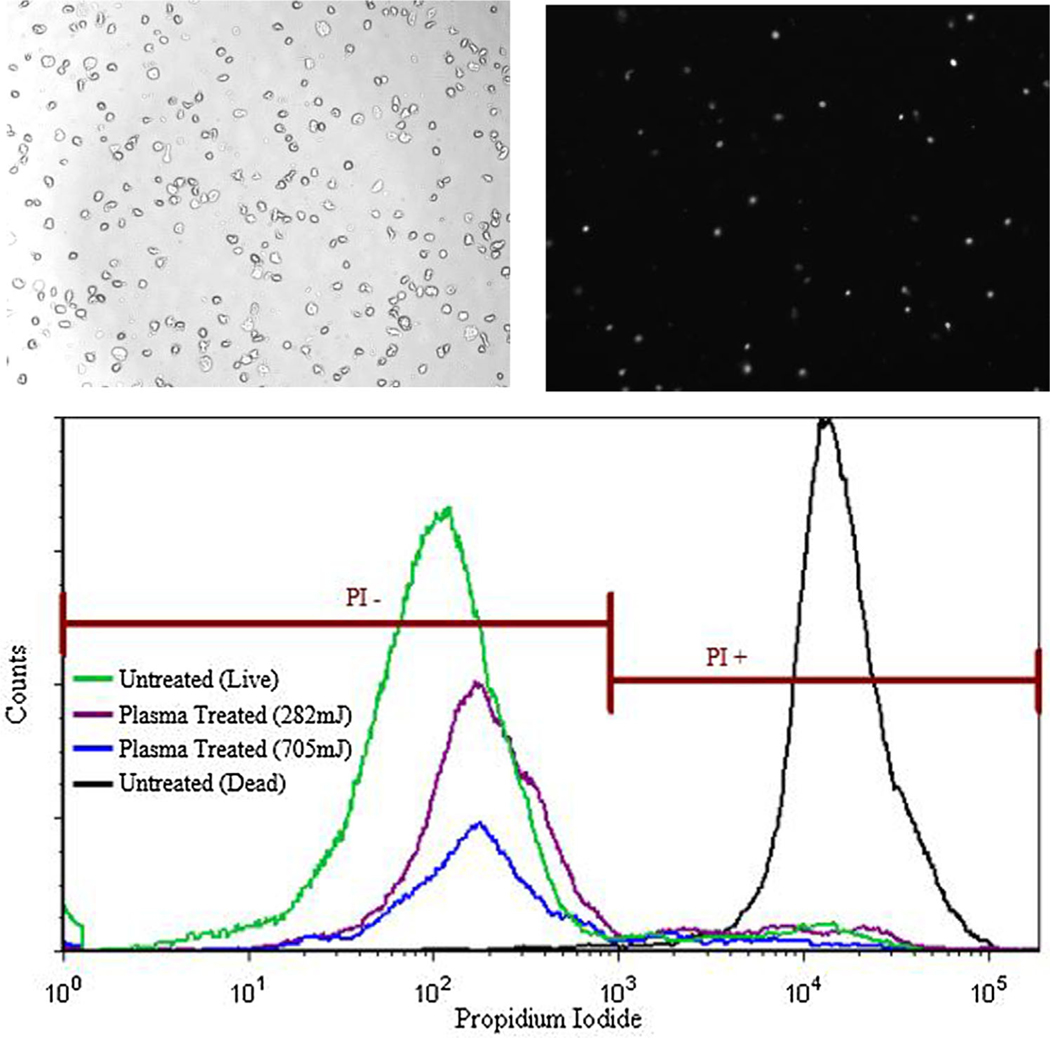
Cell viability following exposure to plasma was determined via fluorescence staining 1 h post plasma treatment with a working concentration of 100 μg/mL Propidium Iodide (Invitrogen, NY). Propidium Iodide (PI) is a DNA-binding fluorescent reagent that permeates damaged cell membranes. Data was collected with an image cytometer (Nexcelom Bioscience, USA) and further analysis including size gating was done on the FCS flow cytometry software (Nexcelom Bioscience) as seen in Figure 2.
Figure 2.
Bright-field and fluorescent images of cells stained with Propidium Iodide and analysis. (Top left) A bright-field image and (top right) a fluorescent image of cells stained with PI was taken, within the same field on the Nexcelom Cellometer 1 h post treatment. The number of cells in the bright-field and fluorescent images were counted. (Bottom) Further analysis and gating was done in the FCS Flow Cytometry Software. Untreated live cells and cells fixed with 4% paraformaldehyde (PFA) were also analyzed for each experiment to set the gates for positive and negative fluorescent signals.
2.6. BCA Assay
When cells are lysed, intracellular proteins are released into the supernatant. Hence, extracellular protein levels are a good indicator of damaged cells and can be quantified via a colorimetric assay. To determine if the observed cell loss was due to cell lysis, we performed the Bicinchoninic Acid (BCA) Assay (Thermo Scientific, USA). The released proteins react with Cu2+ reducing it to cuprous cation (Cu1+). BCA then reacts with Cu1+ and the chelation of two molecules of BCA with Cu1+ results in a purple color change with strong absorbance at 562 nm.[26] This assay was performed on the supernatant of plasma treated cells one hour post treatment and absorbance at 562 nm was measured with a spectrophotometer (Beckman, USA). Protein concentrations were quantified based on a standard curve.
2.7. Statistical Analysis
All experimental data points were from triplicate samples and plotted as the mean±standard deviation. A Student’s t-test was used to determine significance between data points. A value of p ≤ 0.05 was considered statistically significant and indicated with an asterisk (*) when comparing treated and untreated (mock treated) cells, and a pound sign (#) was used when comparing different treatment conditions within the same experiment. A plus sign (+) was used to indicate a value of p ≤ 0.005.
3. Results and Discussion
3.1. Dose-Dependent Effects of Direct nspDBD Plasma on Cell Viability
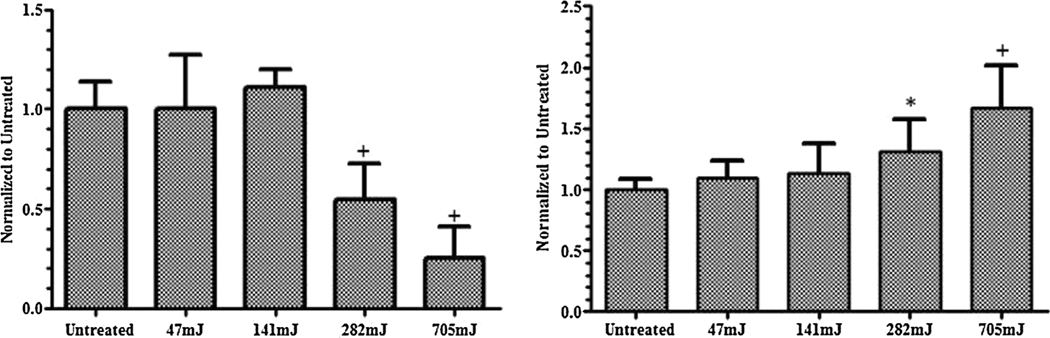
First, the dose-dependent effects of uniform nspDBD treatment in air directly in contact with the mesenchyme stem cell line CH310T1/2 were established. In this regime, cells were exposed to all components of plasma, i.e., electric field, radiation, charges, and neutral species. Pulse frequency for plasma treatment was 5, 15, 30, and 75 Hz corresponding to total treatment doses of 47, 141, 282, and 705 mJ. Cells were collected 1 h after treatment, stained with PI, and analyzed for viability. The results were normalized to the untreated group. Low plasma doses had minimal effect on cell viability, while higher doses decreased cell viability in a dose dependent manner (Figure 3). At 282 mJ the measured cell loss was 50% of the control and at 705 mJ it was 80%. Since changes in cell viability would be more apparent at these two doses, we selected them to examine the effects of individual plasma components on cell death. BCA assay showed an increase in measured extracellular protein, which corresponds with a loss of viability that was dose-dependent. Taken together, these results suggest that in the uniform nanosecond regime, one mechanism of plasma induced cell death is cell lysis.
Figure 3.
Cell viability following plasma treatment is dose dependent. (Left) Live cell concentration following treatment was normalized to untreated. While low dose treatment did not result in cell death, high dose treatment significantly reduced cell viability compared to cells untreated with plasma. (Right) Measurement of protein concentration released by the cell into the supernatant as a result of cell lysis was done via BCA assay. Results are normalized to the untreated cells.
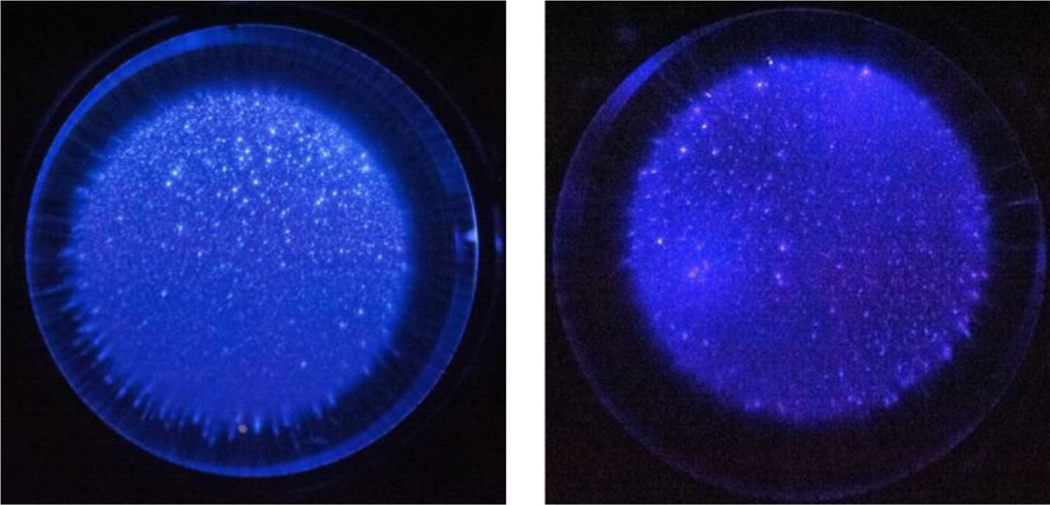
Images of the plasma were taken to confirm uniformity of the discharge (Figure 4). The difference in the intensity of the plasma discharge may be a result of the different emission wavelengths from the various gases. The camera may not be as sensitive in picking up the emissions from oxygen as opposed to nitrogen. However, the uniformity of the discharge can still be observed.
Figure 4.
Uniform nspDBD in (left) air, (middle) pure oxygen, and (right) pure nitrogen. Images were captured with a Nikon D700 at the following settings: 25600 ISO speed, f/4.5, 1/40 s exposure time, +5 step exposure bias, and 105 mm focal length. Plasma was generated by applying a high voltage pulse of 29 kV over a 1 mm gap distance. Differences in light intensity may be due to the different emission wavelengths from the gases.
3.2. Contribution of Plasma Components on Cell Death
3.2.1. Direct Uniform nspDBD-Effect of All Components in Uniform Discharge
As previously stated, direct uniform nspDBD induced 50% and 80% cell death following treatment. This condition served as the positive control for comparison in all other conditions. Mocktreated cells served as our negative control and are indicated as untreated on the graphs.
3.2.2. Electrode Dipped in Media-Effect of Global Electric Field
The dielectric strength for atmospheric air is approximately 30 kV/cm whereas the dielectric strength of water exceeds 700 kV/cm.[9] Since the electric field of nspDBD plasma (~300 kV/cm) did not exceed the dielectric strength of water, plasma was not generated when the electrode was dipped in media. Therefore, to study the effect of global electric field in the absence of plasma, the electrode was operated submerged in media over cells. Under this treatment condition, no effect on cell viability was observed (Figure 5). This indicates that the global electric field, generated from plasma, by itself does not play a role in cell killing.
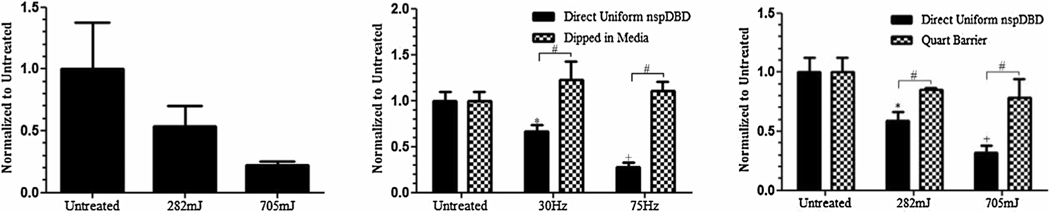
Figure 5.
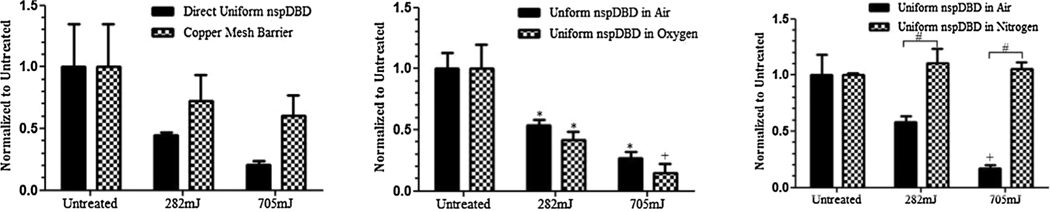
Plasma generated global electric fields and UV radiation alone are not the major contributors of cell death. (Left) Live cells that remained 1 h following nspDBD treatment in air were counted to determine cell viability and numbers. Data is expressed relative to the number of live cells in untreated wells and showed 50% and 80% cell death following 282 and 705 mJ treatments, respectively. This condition served as our positive control to the following treatments. (Middle) The electrode was dipped into the media and ran at 30 and 75 Hz to produce pulsed electric fields without the generation of plasma. Results showed no cell death following treatment. (Right) Copper mesh and quartz barrier inserted 1 mm from the high voltage electrode allowed for the penetration of UV radiation while removing charges and gas species generated from plasma. Cell death following this treatment was approximately 16% and 23% for the low and high doses, respectively.
Pulsed electric fields applied on cells have been shown to trigger electroporation, the process of inducing temporary permeability of cellular membranes.[27] It was shown that at pulse durations on the order of microseconds, electroporation occurs and cell death can be induced in HeLa cells at low electric fields (2.25 kV/cm).[28] Nanosecond electroporation, having pulse widths greater than 60 ns, has also been shown to induce death in tumor cells in vitro and tumor regression in vivo.[29–31] Additionally, Beebe et al showed that by decreasing pulse widths to 10–60 ns, there is insignificant poration effect on Human Jurkat cells even at high electric fields (300 kV/cm).[10,32] In our study, plasma discharge was generated with pulse widths of 20 ns and electric fields of ~300 kV/cm. Hence cell killing from electroporation is unlikely to occur, and we can rule out the effect of plasma generated electric fields as a factor causing cell death in our system.
3.2.3. Uniform nspDBD With Quartz Barrier-Effect of UV Radiation
In the presence of the quartz barrier, only UV radiation generated from plasma was applied to cells, and all other plasma components were suppressed. Treatment with UV radiation at 282 and 705 mJ reduced cell viability by 16% and 23%, respectively (Figure 5).
DBDs have long been used as sources of ultraviolet (UV) radiation from vacuum UV (110–180 nm) to UVA (320–400 nm).[23] UV radiation greater than 200 nm is known to be damaging to DNA,[12] and studies have shown that UV radiation can trigger and induce cell death through multiple independent pathways.[11,33] Caricchio et al have shown that high doses of irradiation (80 mJ/cm2) can induce cellular necrosis.[33] Furthermore, UV radiation has also been known to generate intracellular reactive oxygen species (ROS) including hydrogen peroxides and hydroxyl radicals that have been known to initiate cell damage and death.[34,35]Since we observed that UV treatment alone was not as effective in cell killing, it may play an ancillary role.
Results from these three conditions, taken together, show that for effective killing, plasma generated charges and gas species are required (Figure 5). However, possible synergistic and minor effects with other components on cell processes cannot be ignored.
3.2.4. Uniform nspDBD With Mesh Barrier-Effect of Long-Lived Neutral Species
In order to determine the contribution of charged and short-lived neutral species, a copper mesh barrier without the quartz barrier was used. Cells under this condition were exposed only to long-lived neutral species and UV. Our results showed that treatment with this barrier induced an average of 24% and 35% cell death at the 282 and 705 mJ dose, respectively (Figure 6). This is lower than our positive control, indicating that both charges and short-lived neutrals are very important for reduced cell viability.
Figure 6.
Contribution of charges and neutral gas species to cell killing. (Left) Treatment with a copper mesh barrier exposed cells only to long-lived neutral species in plasma and radiation. Live cell counts 1 h post treatment showed 24% and 35% cell death at low and high doses respectively, suggesting that charges and short-lived species are important for cell killing. (Middle) Uniform discharge in oxygen showed significant cell death at both doses, indicating that the oxygen species produced are the major contributors in plasma induced cell death. (Right) Cells only exposed to plasma generated nitrogen species showed no cell death for both doses, further emphasizing that for effective cell killing, the presence of oxygen is required.
The effect of charges and short-lived species from plasma on prokaryotes was published by Fridman et al in their experiments with Staphylococci, Streptococci, and Candida species.[25] They demonstrated that the time required to inactivate bacteria in a lawn culture with DBD was more than one order of magnitude longer when charged species from plasma treatment were removed. Their results closely resemble our observations in mammalian cells.
3.2.5. Uniform Discharge in Oxygen-Effect of Oxygen Species
To further delineate the chemical species affecting cell viability, the discharge was generated in pure oxygen so only oxygen species, such as OH, O2−, H2O2, are produced during treatment. Our results showed that 282 and 705 mJ dose treatments induced 58% and 85% cell death, only slightly higher than direct nspDBD treatment (Figure 6). However, there was no statistically significant difference between uniform treatment in air and treatment in oxygen. This clearly demonstrated that the oxygen species produced by plasma are the major contributors to plasma induced cell death.
Gas composition is known to affect discharge energy, which may in turn affect the total energy delivered to the cells during treatment. Therefore, it was crucial to verify whether the effect on cell viability was due to changes in treatment dose. Total discharge energy per pulse (including displacement current) in air and pure oxygen was measured and found to be 12.0 ± 0.3 and 12.1 ± 0.4 mJ/pulse, respectively. Since the discharge energies between air and oxygen were within error, the observed cell death was concluded to not be a result of changes in dose, but because of the species generated.
Computational modeling and optical emission spectroscopy of atmospheric plasma discharges in air have shown the production of various oxygen species.[26,36,37] ROS, such as hydrogen peroxide, can function as a signaling molecule to regulate cell processes,[14,34,38,39] but at high concentrations, they can become agents that lead to cell death.[40] Laurent et al have shown that exogenous hydrogen peroxide at low concentrations (0.02–0.13 μM) enhanced NIH 3T3 fibroblast proliferation while 0.25–2 μM of hydrogen peroxide resulted in cell death.[14] Thirunavukkarasu et al have also shown that treatment of hepatic stellate cells with superoxide, another plasma generated ROS, caused cell death.[13] Our experiment in oxygen suggests that the cell death we observe following plasma treatment is likely a result of one or more of these oxygen species.
nspDBD treatment of biological targets is usually carried out in air which is composed primarily of nitrogen and oxygen. Therefore, in addition to determining the effect of plasma generated oxygen species, the contribution of nitrogen species on cell death is also important to study. To further isolate and remove the plasma generated chemical species in air, treatment of cells was performed in a pure nitrogen environment.
3.2.6. Uniform Discharge in Nitrogen-Effect of Nitrogen Species
Following discharge in ultra-high purity nitrogen gas, no cell death was observed for either of the doses (Figure 6). These results clearly show that nitrogen species alone, such as electronically excited N2 and N+ ions,[9,41] do not contribute to plasma induced cell death.
The total discharge energy per pulse (including displacement current) pure nitrogen was also measured and found to be 10.4 ± 0.3 mJ/pulse. Although the discharge energy in nitrogen was slightly lower than that in air, we did not observe cell death in nitrogen, even for the high dose treatment. This re-emphasizes the importance of the presence of oxygen for plasma induced cell death.
Our discharge environment may be in pure nitrogen gas, but it has been proposed that some oxygen species, such as hydroxyl radicals, can be generated from the humidity in the atmosphere or from residual liquid remaining on cells during treatment.[24] Therefore it is possible that there are some small concentrations of ROS in this condition. However, our results showed insignificant cell death in this condition, which suggests that even if ROS was being generated through the proposed mechanism, the levels are low enough that their contribution to cell death is trivial.
This condition only considered the contribution of pure nitrogen species but did not take into account the effect of reactive nitrogen species (RNS) produced by plasma. The production of RNS, such as nitric oxide (NO) or peroxynitrite (ONOO−), require the presence of oxygen, so their contribution cannot be ruled out. However, these results reaffirmed that the presence of oxygen is required for effective cell killing.
It is important to note that electrons and ions are still present when cells are plasma treated in the presence of nitrogen. Since no effect on viability was observed, it strongly indicates that charges alone are not enough to induce cell death. However, a catalytic role in facilitating cell death with the oxygen species cannot be ruled out.
3.2.7. Non-Uniform nspDBD in Nitrogen and Air-Effect of Streamers
Since treatment in the pure nitrogen condition did not affect cell viability, this condition was used to assess the contribution of streamers in non-uniform plasma discharges. Any effect on cell viability following non-uniform plasma treatment in nitrogen would then be a direct result of the streamers generated in this regime. As stated in Table 1 the frequency of pulses was adjusted to 50 and 128 Hz for 282 and 705 mJ dose treatments respectively. To confirm the presence of streamers, images of the non-uniform plasma were taken. Comparing the images of uniform discharge in Figure 4 and non-uniform discharge in Figure 7, streamers are only observed in the non-uniform regime.
Figure 7.
Non-Uniform nspDBD in (left) nitrogen and (right) air. In the non-uniform regime of plasma, streamer structures are clearly observed. Images were captured at the same settings as those of the uniform discharge. In order to generate plasma in the non-uniform regime, the voltage was set at 29 kV, and the gap distance was increased from 1 to 2 mm.
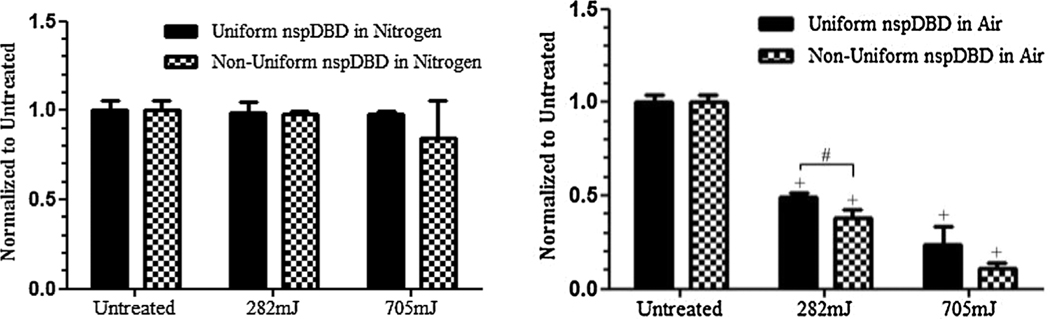
Following both uniform and non-uniform plasma treatment in pure nitrogen, no significant effect on cell viability was observed. This suggests that the streamers and corresponding local electric fields do not play a role in cell killing. However, when both regimes of nspDBD were performed in air, non-uniform treatment resulted in 62% and 89% cell death for 282 and 705 mJ dose treatments respectively (Figure 8). While only the effect at 282 mJ is statistically significant, cell death in both cases is higher than uniform nspDBD treatment at the same doses. There are two possible explanations for this effect:
Figure 8.
Comparison of uniform and non-uniform regimes of nspDBD treatment. (Left) Following non-uniform treatment under pure nitrogen conditions, results showed that the streamers and corresponding local electric fields from the streamer head did not significantly induce cell death. (Right) However when the discharges were done in air, analysis showed non-uniform treatment resulted in 62% and 89% cell death; this is greater than uniform nspDBD for both doses. The difference between uniform and non-uniform treatment was statistically significant for the 282 mJ dose treatment but not for the higher 705 mJ dose treatment.
streamers may play a synergistic role with charges and oxygen species in affecting cell killing;
the high local electric fields from the streamer heads altered the concentration of chemical species generated.
Further investigation and comparison of the species generated in the uniform and non-uniform regime of plasma is therefore required.
In summary, cell death following plasma treatment is a result of plasma generated oxygen species with contribution from charges. Our results from the various treatment conditions are compiled in Table 2.
Table 2.
Summary of conditions used to isolate and remove plasma components. The percent cell death following exposure at 282 and 705 mJ are shown in the two columns on the right.
| Effectors present | Effectors absent | 282 mJ [%] | 705 mJ [%] | |
|---|---|---|---|---|
| 1. Direct uniform nspDBD in air | Global E-field, radiation, charges, neutrals | Streamers | 50 | 80 |
| 2. Electrode dipped in media | Global E-field | Radiation, charges, neutrals, streamers | 0 | 0 |
| 3. Uniform nspDBD w/quartz barrier | UV radiation | Global E-field, charges, neutrals, streamers | 16 | 23 |
| 4. Uniform nspDBD w/mesh barrier | Long-lived neutrals, radiation | Global E-field, charges, short-lived neutrals, streamers | 24 | 35 |
| 5. Uniform nspDBD in oxygen | ROS, charges, global E-field, radiation | Other neutral species, streamers | 58 | 85 |
| 6. Uniform nspDBD in nitrogen | Nitrogen species, charges, global E-field, radiation | Other neutral species, streamers | 0 | 0 |
| 7. Non-uniform nspDBD in nitrogen | Streamers, nitrogen species, charges, global E-Field, radiation | Other neutral species | 2 | 16 |
| 8. Non-uniform nspDBD in air | Streamers, neutrals, charges, global E-field, radiation | None | 62 | 89 |
4. Conclusion
It was shown that the cell viability of C3H10T1/2 murine mesenchyme stem cells is reduced in a dose dependent manner with nspDBD plasma treatment. Cell lysis was identified as one mechanism of cell death following nspDBD treatment. Oxygen species and charged particles are the major plasma components contributing to cell death. UV radiation was shown to play a minor role, and the roles of global electric field and nitrogen species have been ruled out. Additionally, in the non-uniform regime of plasma treatment, the streamers and the corresponding high local electric fields at the streamer heads alone were not important for cell killing.
As the field of plasma medicine continues to progress with great promise in the area of cancer treatment, it is crucial to understand the components of plasma that induce cell death. Future studies include comparing effects of plasma components on various cancerous and non-cancerous cell types. Intracellular biochemistry and cell cycle analysis following treatment will be studied. Additionally, while we observed cell death through direct cell lysis, cell death from apoptosis must also be investigated. Understanding these fundamental plasma-cell interactions will enhance the tunability of plasma devices and lead to further optimization of plasma for cancer treatment while reducing toxicity to surrounding, non-cancerous cell types.
Acknowledgements:
We would like to thank the following Drexel undergraduate cooperating education program students for their assistance with this work: Harpreet Singh, Eric Dluhy, Andrew Kim, Jascha Brettschneider, and Daniel Terlecky. This work was supported in part by NIH Grants 1 R01 EB 013011-01 (Freeman).
Contributor Information
Abraham Lin, Drexel Plasma Institute, Drexel University, 200 Federal Street Suite 500, Camden 08103, New Jersey.
Natalie Chernets, Dr., Department of Orthopedic Surgery, Thomas Jefferson University, 1015 Walnut Street, Philadelphia 19107, Pennsylvania
Justine Han, Drexel Plasma Institute, Drexel University, 200 Federal Street Suite 500, Camden 08103, New Jersey.
Yordano Alicea, Drexel Plasma Institute, Drexel University, 200 Federal Street Suite 500, Camden 08103, New Jersey.
Danil Dobrynin, Dr., Drexel Plasma Institute, Drexel University, 200 Federal Street Suite 500, Camden 08103, New Jersey
Gregory Fridman, Dr., Drexel Plasma Institute, Drexel University, 200 Federal Street Suite 500, Camden 08103, New Jersey
Theresa A. Freeman, Dr., Department of Orthopedic Surgery, Thomas Jefferson University, 1015 Walnut Street, Philadelphia 19107, Pennsylvania
Alexander Fridman, Prof., Drexel Plasma Institute, Drexel University, 200 Federal Street Suite 500, Camden 08103, New Jersey
Vandana Miller, Dr., Drexel Plasma Institute, Drexel University, 200 Federal Street Suite 500, Camden 08103, New Jersey
References
- [1].Lademann J, Richter H, Schanzer S, Patzelt A, Thiede G, Kramer A, Weltmann KD, Hartmann B, Lange-Asschenfeldt B, Skin Pharmacol. Physiol. 2012, 25, 100–106. [DOI] [PubMed] [Google Scholar]
- [2].Dong X, Chen M, Wang Y, Yu Q, Clin. Plasma Med. 2014, 2, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kalghatgi S, Friedman G, Fridman A, Clyne AM, Ann. Biomed. Eng. 2010, 38, 748–757. [DOI] [PubMed] [Google Scholar]
- [4].Steinbeck MJ, Chernets N, Zhang J, Kurpad DS, Fridman G, Fridman A, Freeman TA, PloS ONE 2013, 8, e82143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ma Y, Ha CS, Hwang SW, Lee HJ, Kim GC, Lee K-W, Song K, PloS ONE 2014, 9, e91947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vandamme M, Robert E, Pesnel S, Barbosa E, Dozias S, Sobilo J, Lerondel S, Le Pape A, Pouvesle JM, Plasma Process. Polym. 2010, 7, 264–273. [Google Scholar]
- [7].Fridman A, Plasma Chemistry. Cambridge University Press, New York, New York, USA: 2008. [Google Scholar]
- [8].Dobrynin D, Fridman G, Friedman G, Fridman A, New J. Phys. 2009, 11, 115020. [Google Scholar]
- [9].Kalghatgi S, Kelly CM, Cerchar E, Torabi B, Alekseev O, Fridman A, Friedman G, Azizkhan-Clifford J, PloS ONE 2011, 6, e16270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beebe SJ, Fox PM, Rec LJ, Somers K, Stark RH, Schoenbach KH, IEEE Trans. Plasma Sci. 2002, 30, 286–292. [Google Scholar]
- [11].Locke BR, Shih K-Y, Plasma Sources Sci. Technol. 20, 034006. [Google Scholar]
- [12].de Gruijl FR, van Kranen HJ, Mullenders LH, J Photochem. Photobiol. B 2001, 63, 19–27. [DOI] [PubMed] [Google Scholar]
- [13].Thirunavukkarasu C, Watkins S, Harvey SA, Gandhi CR, Hepatol J. 2004, 41, 567–575. [DOI] [PubMed] [Google Scholar]
- [14].Laurent A, Nicco C, Chéreau C, Goulvestre C, Alexandre J, Alves A, Lévy E, Goldwasser F, Panis Y, Soubrane O, Cancer Res. 2005, 65, 948–956. [PubMed] [Google Scholar]
- [15].Fridman A, Chirokov AA, Gutsol A, Phys J. D 2005, 38p, R1. [Google Scholar]
- [16].Radu I, Bartnikas R, Czeremuszkin G, Wertheimer MR, IEEE Trans. Plasma Sci. 2003, 31, 411. [Google Scholar]
- [17].Fridman G, Shereshevsky A, Jost MM, Brooks AD, Fridman A, Gutsol A, Vasilets V, Friedman G, Plasma Chem. Plasma Process. 2007, 27, 163–176. [Google Scholar]
- [18].Volotskova O, Hawley TS, Stepp MA, Keidar M, Sci. Rep. 2012, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu C, Dobrynin D, Fridman A, J Phys. D 2014, 47, 252003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Babaeva NY, Kushner MJ, J Phys. D 2010, 43, 185206. [Google Scholar]
- [21].Fridman G, Brooks AD, Balasubramanian M, Fridman A, Gutsol A, Vasilets VN, Ayan H, Friedman G, Plasma Proc. Polym. 2007, 4, 370–375. [Google Scholar]
- [22].Herrmann HW, Henins I, Park J, Selwyn G, Phys. Plasmas (1994-present) 1994, 6, 2284–2289. [Google Scholar]
- [23].Reyes CD, Petrie TA, Burns KL, Schwartz Z, Garćıa AJ, Biomaterials 2007, 283228–283235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Geissler U, Hempel U, Wolf C, Scharnweber D, Worch H, Wenzel KW, J Biomed. Mat. Res. 2000, 51, 752–760. [DOI] [PubMed] [Google Scholar]
- [25].Dobrynin D, Friedman G, Fridman A, Starikovskiy A, New J. Phys. 2011, 13, 103033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Smith P, Krohn RI, Hermanson G, Mallia A, Gartner F, Provenzano M, Fujimoto E, Goeke N, Olson B, Klenk D, Anal. Biochem. 1985, 150, 76–85. [DOI] [PubMed] [Google Scholar]
- [27].Dev SB, Rabussay DP, Widera G, Hofmann GA, IEEE Trans. Plasma Sci. 2000, 28, 206–223. [Google Scholar]
- [28].Zhou W, Xiong Z, Liu Y, Yao C, Li C, Cancer Res J. Ther. 2012, 8, 80. [DOI] [PubMed] [Google Scholar]
- [29].Chen X, Kolb JF, Swanson RJ, Schoenbach KH, Beebe SJ, Pigm. Cell Melanoma Res. 2010, 23, 554–563. [DOI] [PubMed] [Google Scholar]
- [30].Ren W, Beebe SJ, Apoptosis 2011, 16, 382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hall EH, Schoenbach KH, Beebe SJ, Apoptosis 2007, 12, 17–1721. [DOI] [PubMed] [Google Scholar]
- [32].Beebe SJ, Fox PM, Rec LJ, Willis ELK, Schoenbach KH, FASEB J. 2003, 17, 1493–1495. [DOI] [PubMed] [Google Scholar]
- [33].Chanput W, Mes JJ, Savelkoul HF, Wichers HJ, Food Func. 2013, 4, 266–276. [DOI] [PubMed] [Google Scholar]
- [34].Sheikh MS, Antinore MJ, Huang Y, Fornace AJ Jr, Oncogene 1998, 17, 2555–2563. [DOI] [PubMed] [Google Scholar]
- [35].Cadet J, Sage E, Douki T, Mutat. Res./Fund. Mol. M. 2005, 571, 3–17. [DOI] [PubMed] [Google Scholar]
- [36].Kerr JF, Wyllie AH, Currie AR, Br. J. Cancer 1972, 26, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chernets N, Zhang J, Steinbeck MJ, Kurpad DS, Koyama E, Friedman G, Freeman TA, Tiss. Eng. A 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rehemtulla A, Hamilton CA, Chinnaiyan AM, Dixit VM, J Biol. Chem. 1997, 272, 25783–25786. [DOI] [PubMed] [Google Scholar]
- [39].Graves DB, Clinical Plasma Med. 2014, 2, 38–49. [Google Scholar]
- [40].Sarsour EH, Chaudhuri L, Kalen AL, Goswami PC, Antioxid. Redox Signal. 2009, 11, 2985–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Seepersad Y, Pekker M, Shneider MN, Fridman A, Dobrynin D, Phys J. D 2013, 46, 355201. [Google Scholar]