Abstract
INTRODUCTION:
In the treatment of upper GI endoscopy-negative patients with heartburn and epigastric pain or burning, antacids, antireflux agents, and mucosal protective agents are widely used, alone or as add-on treatment, to increase response to proton-pump inhibitors, which are not indicated in infancy and pregnancy and account for significant cost expenditure.
METHODS:
In this randomized, controlled, double-blind, double-dummy, multicenter trial assessing the efficacy and safety of mucosal protective agent Poliprotect (neoBianacid, Sansepolcro, Italy) vs omeprazole in the relief of heartburn and epigastric pain/burning, 275 endoscopy-negative outpatients were given a 4-week treatment with omeprazole (20 mg q.d.) or Poliprotect (5 times a day for the initial 2 weeks and on demand thereafter), followed by an open-label 4-week treatment period with Poliprotect on-demand. Gut microbiota change was assessed.
RESULTS:
A 2-week treatment with Poliprotect proved noninferior to omeprazole for symptom relief (between-group difference in the change in visual analog scale symptom score: [mean, 95% confidence interval] −5.4, −9.9 to −0.1; −6.2, −10.8 to −1.6; intention-to-treat and per-protocol populations, respectively). Poliprotect's benefit remained unaltered after shifting to on-demand intake, with no gut microbiota variation. The initial benefit of omeprazole was maintained against significantly higher use of rescue medicine sachets (mean, 95% confidence interval: Poliprotect 3.9, 2.8–5.0; omeprazole 8.2, 4.8–11.6) and associated with an increased abundance of oral cavity genera in the intestinal microbiota. No relevant adverse events were reported in either treatment arm.
DISCUSSION:
Poliprotect proved noninferior to standard-dose omeprazole in symptomatic patients with heartburn/epigastric burning without erosive esophagitis and gastroduodenal lesions. Gut microbiota was not affected by Poliprotect treatment. The study is registered in Clinicaltrial.gov (NCT03238534) and the EudraCT database (2015-005216-15).
KEYWORDS: dyspepsia, epigastric pain syndrome, gut microbiota, medical device made of natural substances, mucosal protective agent, nonerosive reflux disease, omeprazole
INTRODUCTION
Heartburn and the epigastric pain syndrome (EPS) subtype of functional dyspepsia (FD) are highly prevalent symptomatic conditions in endoscopy-negative patients that impair patients' quality of life (1). As stated, based on questionnaires and history taking alone, it can be clinically impossible to distinguish the 2 conditions (2,3); according to sound evidence (4,5), proton-pump inhibitors (PPI) are the recommended treatment of choice (2,6–8) for both conditions, although they may interact with co-administered drugs (9), are not indicated in infancy and pregnancy, and may account for significant cost expenditure (10).
Mucosal protective agents (MPA) adhere to the gastroesophageal epithelium, reinforce the mucosal barrier, and protect the epithelium from acid and nonacid luminal components. MPA improve gastroesophageal reflux (11) and FD symptoms (12) when added to a standard PPI treatment. So far, no controlled studies have assessed the benefit of MPA as monotherapy for heartburn and epigastric pain/burning in endoscopy-negative patients. In animal and ex vivo human studies, MPA Poliprotect (neoBianacid, Sansepolcro, Italy) significantly decreased ethanol-induced and indomethacin-induced gastric mucosa lesions and damage to esophageal mucosal integrity induced by acid-pepsin-bile solution, as assessed by transepithelial electrical resistance and the ulcerogenic index, respectively. It also maintained 36% mucoadhesivity for at least 2 hours and counteracted the oxidative stress induced by 2,2'-azobis(2-amidinopropane) dihydrochloride, in vitro (13 and unpublished proprietary data from the product's technical dossier). Postmarketing surveillance, consisting of large-scale, validated observational surveys, confirmed its effectiveness in relieving heartburn and/or epigastric pain or burning and did not report any serious adverse events (AE) (14).
The aim of the study was to compare the clinical efficacy and safety of Poliprotect with standard-dose omeprazole in the treatment of endoscopy-negative patients with heartburn and/or epigastric pain or burning. As an exploratory aim, changes in the gut microbiota after Poliprotect and PPI treatment were assessed.
MATERIALS AND METHODS
Study design
This randomized, controlled, double-blind, double-dummy, noninferiority trial, conducted in 13 Italian hospitals, aimed to evaluate the efficacy and safety of Poliprotect (neoBianacid, 1.55 g; Aboca, Sansepolcro, Italy) vs PPI (omeprazole 20 mg; Doc Generici Srl, Milano, Italy) in the relief of heartburn and/or epigastric pain or burning. The study design is reported in the Supplementary Digital Content (see Supplementary Digital Figure S1, http://links.lww.com/AJG/C969). The product, a CE-marked medical device marketed in 15 European countries, is a 100% natural product composed of Poliprotect (a polysaccharide fraction from Aloe vera, Malva sylvestris, and Althea officinalis; minerals limestone and nahcolite) and a flavonoid fraction from Glycyrrhiza glabra and Matricaria recutita.
Study population
Male and female patients aged 18–70 years (inclusive) were selected.
All patients were interviewed at the first visit with an identical printed questionnaire containing the key questions to detect the presence, the frequency, and the onset of symptoms matching the Montreal definition of heartburn and of the Rome III EPS.
The main inclusion criteria were symptoms of heartburn and/or epigastric pain/burning (EPS; Rome III criteria) (15); a negative upper endoscopy, to be performed during the screening period if not performed in the past 3 years; a visual analog scale (VAS) score ≥30 and ≤70 mm (VAS related to heartburn/epigastric pain or burning) for at least 6 of the 14 days preceding the screening visit; and willingness not to make diet and lifestyle changes during the trial.
The main exclusion criteria were esophagitis (Los Angeles A–D) or Barrett's esophagus; active gastric or duodenal ulcer; previous gastric or major gastrointestinal (GI) surgery; heartburn/epigastric pain/burning that has not previously responded to antacid or PPI treatment; current intake of any drugs that could affect symptoms; clinically significant disease of any body system; and pregnant, breastfeeding, or fertile women without contraception.
Detailed and comprehensive selection criteria are reported in the Supplementary Digital Content (see Supplementary Table S1, http://links.lww.com/AJG/C969).
The study protocol, approved by the competent authorities and the ethics committees of each participating center, was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and all relevant regulations. Written informed consent was obtained from all participants.
Blinding
Before randomizing a patient, the investigator or designee had to contact the centralized treatment allocation system and provide some information (such as patient number, date of birth, and date of visit). To keep the patients blind to the randomized treatment, a double-dummy method was used. Blinding, packaging, and distribution of investigational kits were managed by a qualified contract research organization. The Poliprotect and its placebo were supplied in hydrolytic class III amber glass bottles, sealed with aluminum caps. The PPI and its placebo were supplied in high-density polyethylene bottles, sealed with child-proof closures. All of the participants in the study (i.e., patients, investigators, research nurses, the study coordinator, personnel involved in monitoring study, and data entry personnel) were blinded to the identity of the study treatment and had no access to the patient codes.
Procedures
After a 2-week screening/washout period (V-1 to V0), subjects who met the selection criteria were randomized, ensuring a 1:1 ratio, into either the PPI group (PPI verum + Poliprotect placebo) or the Poliprotect group (PPI placebo + Poliprotect verum) for a double-blind 4-week period (V0–V2). During this randomized, double-blind 4-week period, the Poliprotect treatment schedule was different in the first 2 weeks (V0–V1) compared with the following 2 weeks (V1–V2). In the first 2 weeks, 1 tablet of Poliprotect had to be taken 5 times a day (30 minutes after breakfast, lunch, and dinner; midafternoon; and before going to bed), whereas in the following 2 weeks, Poliprotect intake was on demand (up to 8 times/d), defined as the product intake necessary to reach the healthy state of V1. Throughout the double-blind period, the PPI had to be taken once a day, 30 minutes before breakfast. For the remaining 4-week period (V2–V3), the blinding was removed, and all patients were administered Poliprotect verum only, on demand. Magaldrate oral gel (Riopan gel, 80 mg/mL, Takeda Italia S.p.A.) was allowed as rescue medication if needed (1 sachet at a time, at any time of day) throughout the study treatment periods. Patients were recommended to record their VAS symptom score, concomitant medications, investigational products (IP), and rescue therapy intake in once-daily paper diaries in the evenings. The Gastrointestinal Quality of Life Index (GIQLI), Gastrointestinal Symptom Rating Scale (GSRS), and Overall Treatment Evaluation (OTE; starting from V1) questionnaires were administered at each visit (16–18).
Outcomes
The primary efficacy end point was the comparison between the 2 groups for the severity of heartburn and/or epigastric pain or burning from baseline to V1, as measured by means of a 100 mm VAS (from no symptoms to overwhelming symptoms) reported in the patients' daily diaries.
Secondary efficacy end points were the comparison between groups of the VAS score for the severity of heartburn and/or epigastric pain or burning at day 1, day 3, and day 7; comparison between groups of the VAS score for the severity of heartburn and/or epigastric pain or burning from week 2 onward; the quantity, number of days of use, and proportion of patients using rescue medication; the on-demand intake of Poliprotect; the change in GIQLI and GSRS score at each visit vs baseline; and OTE scores at each visit.
The results of the safety assessment, except for laboratory test results, are shown in the Supplementary Digital Content (see Supplementary Digital Table S5, http://links.lww.com/AJG/C969).
Data assessment and statistical analysis
Sample sizes of 110 per group achieve 85% power to detect noninferiority (one-sided, 2-sample t test; significance level 0.025), using a noninferiority margin of −11 for the difference between groups in the absolute change in mean VAS score from baseline to day 13, assuming an expected SD of 27 for the change in VAS score in the standard therapy group (19).
According to the approved protocol, where the noninferiority margin is defined as a percentage of the standard mean (i.e., the mean change previously reported for the standard therapy group) and the alternative hypothesis is that the means for the 2 treatments differ by no more than 25% of the standard mean, the value of −11 was pre-established as the noninferiority margin, corresponding to 25% of the mean change in VAS score (i.e., 44 units) observed in a similar population after a 2-week course of PPI treatment, with the same PPI, dose, and dosage (19). A noninferiority margin of −11 was considered clinically acceptable by the investigators and was determined according to the technical recommendation (20) that the test treatment should retain at least a certain and clinically relevant amount of the previously shown superiority of the active comparator over placebo (21–23).
With an estimated 25% of patients not being evaluable for the primary end point for any reason, the total sample size was 276 patients. The intention-to-treat (ITT) population included all randomized patients who received at least 1 dose of the IP and was identical to the safety analysis population. The per-protocol (PP) population included all ITT subjects who completed the double-blind treatment period without major protocol deviations. The primary efficacy analysis was performed in both the ITT and PP populations. Secondary efficacy analyses were conducted in the ITT population. The methods of microbiota analysis used on the available fecal samples at both V0 and V2 are detailed in the Supplementary Digital Content (see Supplementary Digital Methods, http://links.lww.com/AJG/C969).
In analyzing the VAS score as a continuous variable, the average score for the 5 days before the 2 consecutive reference visits has been considered, with no imputation of missing values. For early time points (day 1, 3, and 7), the VAS score values for the day immediately before were used in cases of missing data. In determining the response rate (categorical variable), responder patients were defined as those patients with at least a 50% improvement in VAS symptoms. Patients with a missing VAS score in each of the 5 days before any of the 2 reference visits were considered nonresponders. Patients with missing questionnaire data were excluded from the analysis of the corresponding period.
The OTE score, which rates symptom change on a 15-point scale (−7 to −1 = worse; 0 = no change; +1 to +7 = better), was recoded as negative if scores were in the worse or no change range, and otherwise as positive, as previously reported (18).
Compliance with treatments was calculated as the ratio of the amount of IP taken (from the daily diary record) to what was expected to be taken according to the treatment schedule.
All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
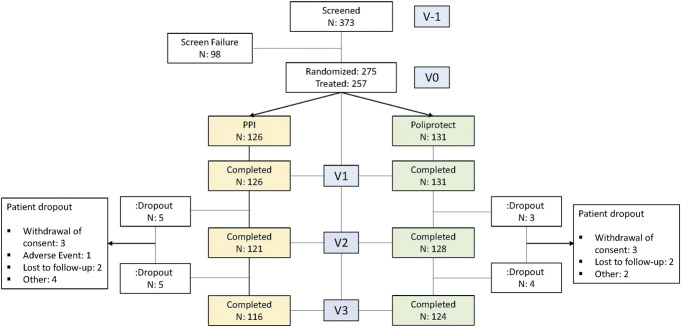
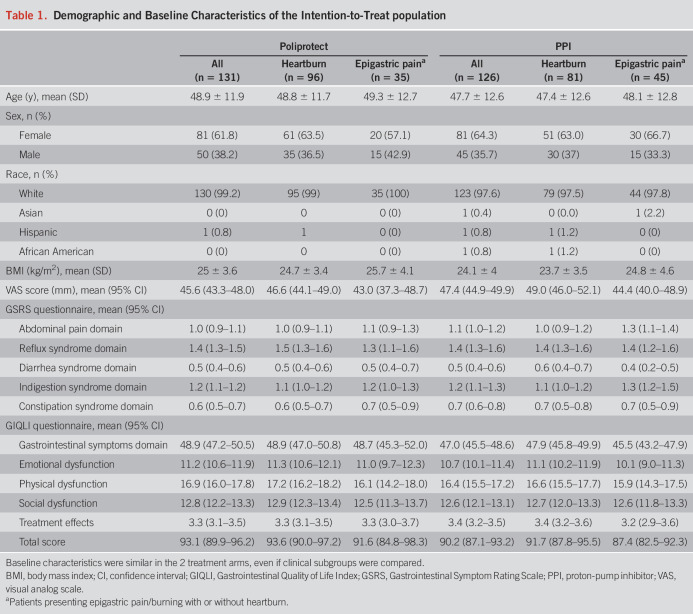
Between October 15, 2017, and September 3, 2021, 373 patients were screened and 98 did not meet the inclusion criteria. Thus, 275 patients were enrolled and randomly assigned to treatments. Of these, 257 were treated and 17 were dropouts (Figure 1). A summary of the patients' demographic and clinical characteristics and a list of screening failures are detailed in Table 1 and in the Supplementary Digital Content (see Supplementary Digital Table S7, http://links.lww.com/AJG/C969), respectively. Eighteen randomized people did not take even 1 treatment dose: the COVID pandemic and related environmental difficulties contributed to this decision. Compliance was equal to or greater than 90% in both treatment groups (see Supplementary Digital Table S3, http://links.lww.com/AJG/C969).
Figure 1.
Patient distribution. PPI, proton-pump inhibitor.
Table 1.
Demographic and Baseline Characteristics of the Intention-to-Treat population
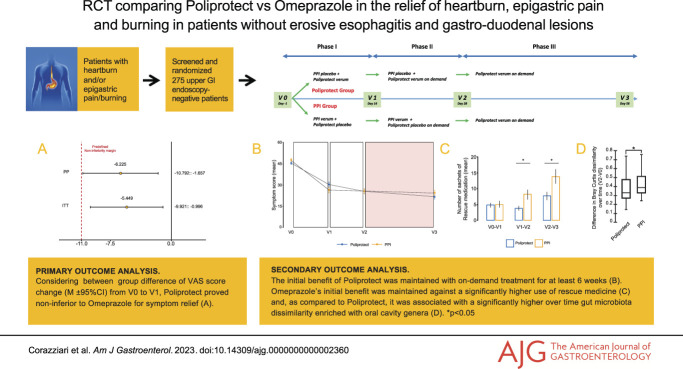
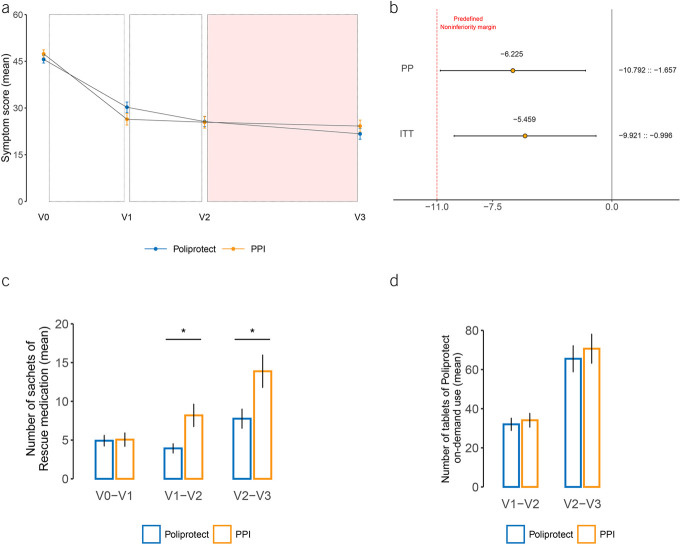
In both analyses, the lower limit of the 2-sided 95% confidence interval (CI) for the difference between groups in the change in VAS symptom score from V0 to V1 lies above the pre-established noninferiority margin of −11 (−5.4, 95% CI −9.9 to −0.1; −6.2, 95% CI −10.8 to −1.6; ITT and PP populations, respectively) (Figure 2b).
Figure 2.
Mean absolute symptom score values, results of primary analysis, and use of rescue medication and Poliprotect on demand. (a) Mean absolute values of symptom severity score, as measured by means of a 100 mm visual analog scale (VAS; from no symptoms equal to 0 mm to overwhelming symptoms equal to 100 mm). From V2 to V3, depicted with a colorful background, the comparison treatment (PPI) and blinding were both removed, and all patients were administered Poliprotect verum only, on demand. The I bars represent standard error. (b) Difference in absolute change in the VAS symptom severity score from baseline to day 13 (V0–V1) between the omeprazole group and the Poliprotect group and relative 95% confidence interval, supporting the hypothesis that Poliprotect was noninferior to omeprazole. In both the per-protocol and intention-to-treat populations, the inferiority hypothesis is rejected by means of the unilateral unpaired Student t test shifted by −11 (minus the noninferiority threshold) on the change in the symptom score between baseline and visit 1 (P = 0.020 and P = 0.008 for noninferiority, respectively). (c) Number of sachets of rescue medication used during the indicated study periods. (d) Number of tablets of Poliprotect (verum or placebo) used on demand. *P < 0.05. PPI, proton-pump inhibitor.
Mean VAS scores were not significantly different between groups at baseline or at any subsequent time points in the study (V0, 45.1 [42.7–47.5] and 47.2 [44.7–49.8], P = 0.31; V1, 30.2 [26.9–33.5] and 26.4 [22.7–30.0], P = 0.12; V2, 25.7 [22.4–28.8] and 25.4 [21.7–29.2], P = 0.94; V3, 21.7 [18.3–25.2] and 24.2 [20.4–28], P = 0.34; mean [95% CI]; Poliprotect and PPI group, respectively) (Figure 2a and see Supplementary Digital Table S2, http://links.lww.com/AJG/C969). From V1 to V2, the VAS score decreased more in the Poliprotect group (−3.89) than in the PPI group (−0.76) (Figure 2a), albeit the difference was not significant (−3.13, 95% CI −6.74 to 0.47; P = 0.09), even after excluding patients taking rescue medication (P = 0.06) (data not shown).
Likewise, the proportions of responders were not significantly different between groups (V1: 46/131 [35.1%] and 56/126 [44.4%], P = 0.16; V2: 60/128 [46.8%] and 56/121 [46.2%], P = 1; V3: 70/124 [56.4%] and 61/116 [52.5%], P = 0.64; Poliprotect and PPI group, respectively).
The number of rescue medication sachets used was significantly lower in the Poliprotect group than the omeprazole group in the V1–V2 (P = 0.019) and V2–V3 (P = 0.032) periods (Figure 2c and see Supplementary Digital Table S4, http://links.lww.com/AJG/C969), despite no difference in the VAS score and comparable on-demand intake of Poliprotect during both V1–V2 (tablets/d: 2.11 [1.90] and 2.23 [2.05]; mean [SD], Poliprotect and PPI group, respectively; P = 0.604) and V2–V3 (tablets/d: 2.11 [1.97] and 2.36 [2.14]; mean [SD], Poliprotect and PPI group, respectively; P = 0.543) (Figure 2d), even when comparing subgroups of patients who had taken and patients who had not taken rescue medication (data not shown).
The number of days of use of rescue medication (data not shown) was significantly lower in the Poliprotect group in the V1–V2 period (P = 0.013) and remained lower in the V2–V3 period, although not significantly (P = 0.156).
There were no significant differences between groups in the VAS score 1, 3, and 7 days after treatment start (see Supplementary Digital Table S2, http://links.lww.com/AJG/C969). All GSRS and GIQLI domain scores showed a trend of progressive improvement from V0 to V3 in both treatment groups, except for the GSRS reflux domain in the omeprazole group (see Supplementary Digital Figure S2, http://links.lww.com/AJG/C969). Domain score changes did not differ between groups at any time points, except for a significant difference favoring the omeprazole group for the GSRS reflux syndrome domain at V1 (P = 0.017), the constipation syndrome domain at V1 and V3 (P = 0.05 and P = 0.03, respectively), and the GIQLI gastrointestinal symptom domain at V1 (P = 0.004) (see Supplementary Digital Table S2, http://links.lww.com/AJG/C969).
The proportion of omeprazole-treated patients reporting a positive OTE score decreased in the V1–V2 period to stabilize thereafter, whereas it remained stable in the V1–V2 period to increase thereafter in the Poliprotect group (see Supplementary Digital Table S2 and Figure S2, http://links.lww.com/AJG/C969).
A total of 178 AE were recorded, with 79 (37.4%) in the Poliprotect arm and 99 (38.1%) in the PPI arm, none of which was serious; 4 of them, all occurring in the PPI group, were considered as related to the IP (see Supplementary Digital Table S5, http://links.lww.com/AJG/C969). None of the assessed laboratory test items was altered or significantly different between groups (data not shown).
Microbiota results
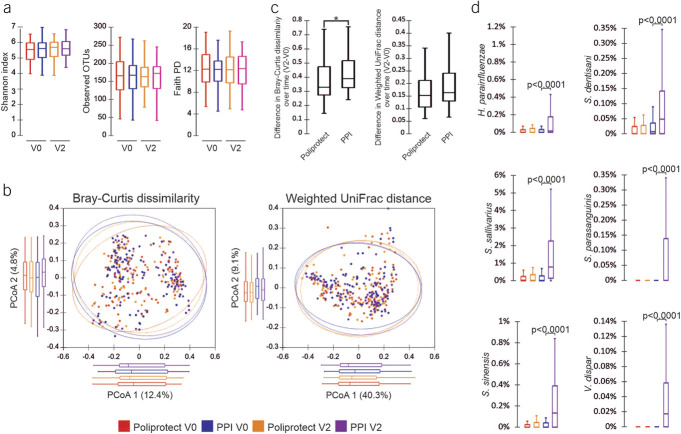
No significant within-group changes over time or between-group differences or partitions at both V0 and V2 were evidenced by alpha or beta diversity analysis, respectively (Figure 3). As compared to Poliprotect, a significantly higher degree of variability from V0 to V2 was found in the PPI group about the Bray-Curtis dissimilarity distance: in particular, a significant enrichment of the species Streptococcus salivarius and Streptococcus sinensis and a significant increase over time in the relative abundance of Haemophilus parainfluenzae, Streptococcus dentisani, Streptococcus parasanguinis, and Veillonella dispar (P < 0.0001 for each) were observed in the PPI group (Figure 3 and see Supplementary Digital Figure S4, http://links.lww.com/AJG/C969).
Figure 3.
Analysis of microbiome. (a) Color-coded box plots showing α-diversity estimators, measured for each group at different time points. (b) PCoA plot of bacterial β-diversity based on Bray-Curtis dissimilarity and weighted UniFrac distance according to individual health status. For each group, the 95% confidence interval has been drawn. Numbers between parentheses represent the percentage of the total variance explained by the principal coordinates. (c) Box plots showing the distribution of differences in the interindividual distances over time (V2–V0) for both considered beta measures. (d) Color-coded box plots showing the distribution of bacterial species that were significantly enriched at V2 with respect to V0. A P value ≤ 0.05 was considered statistically significant. PPI, proton-pump inhibitor.
Post hoc analyses
In a post hoc analysis performed on the 2 subpopulations of patients presenting with heartburn only (N = 177) and those with epigastric pain/burning with or without heartburn (N = 80), no significant differences were found between the subgroups for baseline characteristics, including symptom severity score (Table 1), or postbaseline mean VAS score (see Supplementary Digital Table S6, http://links.lww.com/AJG/C969). Similarly, the proportions of responders, defined as those patients with at least 50% symptom improvement according to the VAS, were not significantly different at any visit, comparing the subgroups of patients presenting with heartburn only and those with epigastric pain/burning (see Supplementary Digital Table S6, http://links.lww.com/AJG/C969).
DISCUSSION
A 2-week treatment with Poliprotect tablets, 5 times daily, provides noninferior efficacy compared with omeprazole 20 mg once daily for the relief of heartburn and epigastric pain or burning in adult symptomatic patients without erosive esophagitis and gastroduodenal lesions, likely including a mix of nonerosive reflux disease (NERD), functional esophageal disorders, and FD. To the best of our knowledge, only 2 randomized clinical trials (RCT) have reported that add-on treatment with irsogladine-based and hyaluronic acid-based MPA significantly enhanced the positive effect of PPI in patients with NERD (11,24). No RCT has compared the efficacy of an MPA vs a PPI in the treatment of heartburn and epigastric pain or burning.
Heartburn, epigastric pain, and epigastric burning are 3 slightly different and often interchangeably subjective sensory representations of pain. The general clinical use of these symptoms to identify and differentiate patients with reflux-like symptoms from the dyspeptic EPS subtype is not supported by several studies (25–27) or by a recent international expert consensus (2). In this RCT, all but 17 of the 80 patients with EPS complained of both epigastric pain/burning and heartburn and did not significantly differ from the patients presenting only heartburn in baseline characteristics, including VAS score, postbaseline mean VAS score, or response rate (VAS), further confirming that patients with EPS are not clinically distinguishable from endoscopy-negative patients with heartburn (26–29). Patient-reported outcome instruments like Reflux Disease Questionnaire and gastroesophageal reflux disease questionnaire are useful to capture GERD symptoms, but not epigastric pain and burning as well. There are no validated Italian patient-reported outcome instruments for dyspeptic symptoms. The Italian GSRS instrument has not been validated for test-retest reliability, and its internal consistency is unsatisfactory for the abdominal pain domain and barely satisfactory for the reflux domain (30). VAS score is a well-standardized tool for assessing pain, and it has been widely used to assess painful upper GI symptoms. We have thus used VAS for the primary pain end point and GSRS for the secondary ones, capturing the GI symptoms that often accompany reflux and dyspeptic symptoms.
The clinical benefit of Poliprotect and omeprazole, as assessed by the VAS score, is evident as early as the first day of treatment and increases over the 2-week period. Thereafter, the favorable effect of Poliprotect continues to increase, albeit slightly, even after switching from 5 times daily to on-demand intake, with a lesser mean daily consumption (on average 2–3 tablets/d). This effect was not affected by rescue medicine use, which was relatively stable. It would therefore seem that the initial benefit of Poliprotect can be maintained with on-demand treatment at lower daily consumption for at least 6 weeks. It would seem that, consistent with previous experience with PPI in NERD (31,32), the initial benefit obtained with daily Poliprotect in the treatment of symptomatic endoscopy-negative patients with heartburn and epigastric pain/burning can be maintained with on-demand therapy.
Based on the abovementioned preclinical studies, the clinical benefit of MPA relies on providing the mucosa with a complex, mucus-like, adherent, antioxidant, pH-buffering matrix, thus limiting the stimulation of acid, bile, and other luminal sensitizers on the gastroesophageal epithelium. The Rome III criteria for EPS were used in the protocol, which was approved before the publication of Rome IV. Nonetheless, as the term bothersome is the only addition to the Rome III criteria for EPS in Rome IV, and as we included only patients with a VAS score >30, the patients in this trial met the Rome IV criteria for EPS. The results of this trial can be reasonably extrapolated to the general population with moderate symptoms of heartburn and/or epigastric pain/burning because the patients were recruited in open-access outpatient clinics and, in addition, many of them were referred by general practitioners who had previously been instructed to send the trial centers any unselected patients presenting with heartburn and/or epigastric pain/burning. As patients reporting an initial VAS score >70 were not included, the effect of Poliprotect seems to benefit patients with moderate symptom severity, but this cannot be extrapolated to patients with high symptom severity. Unlike with Poliprotect, the initial benefit of omeprazole is maintained against significantly higher usage of rescue medicine compared with the Poliprotect arm and despite daily omeprazole intake, confirming that PPI cannot effectively control even moderate symptoms in a subgroup of endoscopy-negative patients with heartburn and patients with EPS (33–35).
Several reasons may account for the greater demand for rescue therapy in at least some of the patients on PPI: sensitization of the esophagus to weakly acidic reflux by preceding acid exposure and/or because impaired epithelial integrity was supported by studies on persistent symptoms despite PPI and also, partial gastric acid suppression may have still allowed a few symptomatic acid refluxes to occur (36–38). An esophageal pH impedance test would have been helpful for clarifying this; however, this invasive diagnostic investigation is rarely performed in general practice, and it would have hindered the recruitment rate in the outpatient clinic population.
Limitation of this trial is that a specific gastroesophageal reflux disease phenotype for which Poliprotect is optimal cannot be inferred from this study. Instead, this is potentially an option for the symptomatic foregut patient without erosive esophagitis and gastroduodenal lesions in whom a PPI would typically be considered. An esophageal pH impedance test would have been helpful for clarifying whether the treatment response might have been related to the presence of gastroesophageal reflux, which frequently occurs in patients with EPS and in endoscopy-negative patients with heartburn. According to the outcome of a pH impedance test, endoscopy-negative patients may present with abnormal acid or nonacid reflux (NERD), symptomatic normal acid or nonacid reflux (reflux hypersensitivity), or normal asymptomatic reflux (functional heartburn). Increased gastroesophageal acid reflux is associated with the presence of epigastric pain in dyspeptic heartburn-negative patients (26) and is detected in up to 50% of patients with FD complaining of epigastric burning without predominant typical reflux symptoms (27). It is thus conceivable that patients with acid reflux benefited from PPI treatment. Similarly, in the absence of a pH impedance test, we cannot say which patients benefited most from Poliprotect treatment: those with or those without gastroesophageal reflux. Nonetheless, we can argue that patients with either acid or nonacid gastroesophageal reflux might have benefited from the combined effect of epithelial barrier protection, antacids, and the antioxidant properties of Poliprotect. Such interpretation might explain the significantly lesser use of rescue medicine in the Poliprotect group than in the omeprazole group. In any case, only further properly designed RCTs can verify the comparative efficacy of Poliprotect and PPI in patients with endoscopy-negative gastroesophageal reflux disease-like symptoms according to phenotype based on esophageal pH impedance monitoring.
Heartburn and epigastric pain/burning often overlap with other dyspeptic and intestinal symptoms, and the GSRS questionnaire confirmed this association. It is notable that the improvement in heartburn and epigastric pain/burning obtained with PPI and Poliprotect was accompanied by a parallel improvement in the associated dyspeptic and intestinal symptoms. This finding confirms a previous observation in patients with gastroesophageal reflux disease with overlapping dyspepsia and irritable bowel syndrome-like symptoms treated with PPI (39) and also shows that the improvement in heartburn and epigastric pain/burning obtained with Poliprotect is associated with a parallel improvement in the accompanying dyspeptic and intestinal symptoms during 6 weeks of on-demand treatment with 2–3 tablets/d.
During the last unblinded 4 weeks of the study, the 2 groups did not differ in Poliprotect consumption and symptomatic benefit, which, in the omeprazole arm, was obtained alongside a significant increase in antacid rescue medicine as compared to the Poliprotect arm. This finding is in line with the notion that PPI suspension can be followed by symptoms worsening due to the gastric acid rebound effect (40).
The 4-week omeprazole treatment is comparable with most previous controlled trials with PPI in patients with NERD and FD because prolonging the treatment does not add symptomatic benefits (5,41).
Within the abovementioned limits of the Italian GSRS questionnaire, omeprazole performed better than Poliprotect in the reflux and constipation GSRS domains and in the gastrointestinal symptom GIQLI domain in the first 2 weeks of treatment, and not differently in the following 2 weeks. It would therefore seem that PPI also have an initial and temporary favorable effect on regurgitation that, unlike with the VAS, is captured by the reflux domain. The favorable and temporary effect of PPI on the constipation domain can be interpreted as the result of a possible increase in intestinal fermentation and/or in absorption alterations, both of which affect stool consistency and intestinal transit and have been related to the hyposecretory effects of PPI and the consequent increase in bacterial colonization in the small bowel (42,43).
Helicobacter pylori status was not assessed because the symptomatic response to PPI is not affected by H. pylori in these patients, whereas the modest positive response to H. pylori eradication in patients with FD is only apparent after months (44,45).
The study did not assess patients for hiatal hernia, NERD, reflux hypersensitivity and functional heartburn subtypes, eosinophilic esophagitis, or previous antacid or PPI use. Patients not previously responding to PPI and antacids were excluded from the trial to limit the inclusion of patients in whom symptoms are not caused by reflux or caused by nonacid reflux, who might have biased the study against PPI or Poliprotect (which acts also as an antacid). Nonetheless, because we did not exclude PPI-naive and antacid-naive patients, it was still possible that some patients not responding to PPI and some patients not responding to “antacid” Poliprotect were included in the trial. However, several considerations make unlikely that this may have given the Poliprotect treatment, or omeprazole treatment as well, an unfair advantage. First, given the high number of patients randomized to one or the other of the 2 treatments, randomization guarantees that the likely small number of PPI-naive and antacid-naive patients in this sample was equally distributed between the 2 treatment arms. Moreover, this study and previously reported data do not support the presence of a randomization bias, affecting the comparative outcome of this trial. Indeed, (i) PPI-treated patients performed better than the Poliprotect-treated ones at V1, both as assessed by VAS symptom score and GSRS reflux domain score and (ii) the 48% proportion of responders in the subgroup with heartburn only (see Supplementary Digital Table S6, http://links.lww.com/AJG/C969), after 4 weeks of PPI treatment, is comparable to the expected 49% response rate to PPI reported in a meta-analysis of 12 studies in patients with heartburn in the setting of normal endoscopy (46). In addition, because we did not exclude also antacid-naive patients, the observed symptomatic benefit of Poliprotect could have been limited by the presence of patients not responding to antacids.
In the PPI arm, the initial benefit of the treatment was progressively lost in the following 2 weeks, as evidenced by a parallel increase in rescue medicine use and a decrease in positive OTE by more than 10% from V1 to V2 (see Supplementary Digital Figure S2 and Table S2, http://links.lww.com/AJG/C969). The trend in improvement throughout the treatment period was continuously progressive in the Poliprotect arm for all efficacy variables, whereas it was not maintained in the omeprazole arm for the GSRS reflux domain, the GIQLI emotional dysfunction domain, or the positive OTE score.
We confirmed (47,48) that PPI treatment is associated with an increased abundance of oral cavity genera in the intestinal microbiota. Significant changes of microbiome composition after 4 weeks of 40 mg of omeprazole, twice daily, in healthy volunteers have been previously reported (49). Our data show for the first time that a microbiota change can take place after 4 weeks even with the 20-mg daily dose of omeprazole in a patient population. Unlike the hyposecretive action of PPI, the buffering activity of Poliprotect, exerted by the bicarbonate minerals embedded in the complex vegetable matrix adhered to the epithelial lining, does not affect the amount of intraluminal acid secretion and hence the microbiota composition.
In conclusion, starting from the first day of treatment, Poliprotect proved noninferior to omeprazole in the relief of heartburn and epigastric pain and burning in the initial 2 weeks, and even better on demand (on average 2–3 tablets/d) compared with omeprazole in the subsequent 2 weeks. In addition, Poliprotect on demand counteracted the predictable worsening of symptoms that follows the suspension of PPI treatment. Furthermore, the MPA is a 100% natural product and therefore biodegradable by definition, with no impact on the environment (50), and in the present RCT, it showed high safety without affecting the gut microbiota.
In large-scale surveys, aimed at the postmarketing surveillance of Poliprotect, 3,471 physicians and 848 patients did not report any serious AE. In addition, physicians largely reported good tolerability in the Poliprotect-treated population, which included pregnant women and children (14). Postmarket vigilance data reported an incidence rate <1/10,000 of nonserious gastrointestinal and skin adverse effects of Poliprotect and no serious adverse effects. Based on the results of this trial and considering such a high safety level, it is conceivable that Poliprotect might be used as first-line treatment for heartburn and EPS, and to substitute PPI in those conditions in which they are contraindicated. However, further RCT are required to assess whether Poliprotect has the potential to be indicated on a cost-benefit and cost-effective basis for the treatment of heartburn and epigastric pain and burning in endoscopy-negative patients as an alternative to PPI.
CONFLICTS OF INTEREST
Guarantor of the article: Enrico Stefano Corazziari, MD.
Specific author contributions: E.S.C.: contributed for conceptualization, literature search, figures, study design, data collection, data analysis, writing first draft, and had access and verified data. A.G., L.D.'A., V.D.'O., O.R., S.P., B.A., M.C., A.R., G.B., C.C., A.D.S., M.N., M.C.B., E.R., P.I., and D.B.: contributed equally for resources, investigation, data collection, writing-review & editing. G.R., M.M., S.S.: contributed for microbiota analysis and interpretation, writing & editing.
Financial support: This study received funding from Aboca S.p.A., the manufacturer of the medical device (i.e., the test product; brand name: neoBianacid). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Potential competing interests: E.S.C. and B.A.: perform consultancies for Aboca S.p.A.
Study Highlights.
WHAT IS KNOWN
✓ Mucosal protective agents (MPA) are widely used in the treatment of gastroesophageal reflux and functional dyspepsia but no study has so far compared their effectiveness with the reference standard Proton Pump Inhibitors (PPI) for these conditions.
WHAT IS NEW HERE
✓ The results of this RCT indicate that Poliprotect, an MPA made of natural substances, could be a valuable alternative to PPI in the treatment of heartburn, epigastric pain and burning in NonErosive Reflux Disease (NERD) and Epigastric Pain Syndrome (EPS) patients.
✓ Of clinical interest is also the evidence that NERD and EPS patients are not clinically distinguishable and benefit from the same treatment.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the clinical support of Pier Alberto Testoni, MD, Vita-Salute San Raffaele University, Milan, Italy; Giuseppe Frieri, MD, Università L'Aquila, L'Aquila, Italy; Giovanni Brandimarte, MD, Ospedale Cristo Re, Rome, Italy; Giuseppe Ventriglia, MD, Italian College of General Practitioners and Primary Care, Florence, Italy; Vincenzina Mora, MA, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Silvia Pecere, MD, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Martina De Siena, MD, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Alessia Leonetti, MA, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Caterina Fanali, MA, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Francesca D'Aversa, MD, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Irene Mignini, MD, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Giulia Wlderk, MA, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Maria Antonia Pirro, MA, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Daniele Napolitano, MA, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Michele Cacioni, MD, University of Perugia, Perugia, Italy; Alessandro Tullio, MD, Campus Bio Medico University, Rome, Italy; Caterina Mengoli, MD, University of Pavia, Pavia, Italy; Nicola Aronico, MD, University of Pavia, Pavia, Italy; Mariangela Delliponti, MA, University of Pavia, Pavia, Italy; Marilia Carabotti, MD, Ospedale Universitario Sant'Andrea, Rome, Italy; Sabrina Testoni, MD, IRCCS San Raffaele Scientific Institute, Milan, Italy; Maria Carlotta Sacchi, MD, Sapienza University of Rome, Rome, Italy; Claudio Cassieri, MD, ASL Roma2, Rome, Italy; and Kostantinos Efthymakis, MD, “G. D'Annunzio” University of Chieti-Pescara, Chieti, Italy. The authors thank BMR Genomics and IQVIA CRO for their contribution in microbiota DNA sequencing and study management, respectively.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C969
Contributor Information
Antonio Gasbarrini, Email: antonio.gasbarrini@unicatt.it.
Lucia D'Alba, Email: dalbalucia135@gmail.com.
Valeria D'Ovidio, Email: vale_do@yahoo.it.
Oliviero Riggio, Email: oliviero.riggio@uniroma1.it.
Sandro Passaretti, Email: passaretti.sandro@hsr.it.
Bruno Annibale, Email: bruno.annibale@uniroma1.it.
Michele Cicala, Email: m.cicala@unicampus.it.
Alessandro Repici, Email: alessandro.repici@hunimed.eu.
Gabrio Bassotti, Email: gabassot@tin.it.
Carolina Ciacci, Email: cciacci@unisa.it.
Antonio Di Sabatino, Email: a.disabatino@smatteo.pv.it.
Matteo Neri, Email: mneri@unich.it.
Maria Consiglia Bragazzi, Email: mariaconsiglia.braga@uniroma1.it.
Emanuela Ribichini, Email: emanuela.ribichini@gmail.com.
Giulia Radocchia, Email: giulia.radocchia@uniroma1.it.
Paola Iovino, Email: piovino@unisa.it.
Massimiliano Marazzato, Email: m.marazzato79@gmail.com.
Serena Schippa, Email: serena.schippa@uniroma1.it.
Danilo Badiali, Email: danilo.badiali@gmail.com.
REFERENCES
- 1.Sperber AD, Freud T, Aziz I, et al. Greater overlap of Rome IV disorders of gut-brain interactions leads to increased disease severity and poorer quality of life. Clin Gastroenterol Hepatol 2022;20:e945–e956. [DOI] [PubMed] [Google Scholar]
- 2.Wauters L, Dickman R, Drug V, et al. United European gastroenterology (UEG) and European society for neurogastroenterology and motility (ESNM) consensus on functional dyspepsia. United Eur Gastroenterol J 2021;9:307–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geeraerts A, Van Houtte B, Clevers E, et al. Gastroesophageal reflux disease-functional dyspepsia overlap: Do birds of a feather flock together? Am J Gastroenterol 2020;115:1167–82. [DOI] [PubMed] [Google Scholar]
- 4.Sigterman KE, van Pinxteren B, Bonis PA, et al. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2013;2013:Cd002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto-Sanchez MI, Yuan Y, Hassan A, et al. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev 2017;11:Cd011194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs KH, Babic B, Breithaupt W, et al. EAES recommendations for the management of gastroesophageal reflux disease. Surg Endosc 2014;28:1753–73. [DOI] [PubMed] [Google Scholar]
- 7.Katz PO, Dunbar KB, Schnoll-Sussman FH, et al. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2022;117:27–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moayyedi P, Lacy BE, Andrews CN, et al. ACG and CAG clinical guideline: Management of dyspepsia. Am J Gastroenterol 2017;112:988–1013. [DOI] [PubMed] [Google Scholar]
- 9.Wedemeyer RS, Blume H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: An update. Drug Saf 2014;37:201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: A review of cost-effectiveness and risk [corrected]. Am J Gastroenterol 2009;104(Suppl 2):S27–32. [DOI] [PubMed] [Google Scholar]
- 11.Savarino V, Pace F, Scarpignato C. Randomised clinical trial: Mucosal protection combined with acid suppression in the treatment of non-erosive reflux disease–efficacy of esoxx, a hyaluronic acid-chondroitin sulphate based bioadhesive formulation. Aliment Pharmacol Ther 2017;45:631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko SJ, Park J, Kim MJ, et al. Effects of the herbal medicine rikkunshito, for functional dyspepsia: A systematic review and meta-analysis. J Gastroenterol Hepatol 2021;36:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liguori G, Baldi F, Di Simone MP. Mo1175–topical protection of esophageal mucosa: In vitro evaluation of a medical device made of natural substances in comparison with sodium alginate. Gastroenterology 2018;154. [Google Scholar]
- 14.Cioeta R, Muti P, Rigoni M, et al. Effectiveness and tolerability of Poliprotect, a natural mucosal protective agent for gastroesophageal reflux disease and dyspepsia: Surveys from patients, physicians, and pharmacists. Front Drug Saf Regul 2022;2:969831. [Google Scholar]
- 15.Tack JTN, Camilleri M, et al. Functional gastroduodenal disorders. In: Drossman DACE, Delvaux M, Talley NJ, et al. (eds). Rome III. The functional gastrointestinal disorders. 3rd edn. Degnon Ass: McLean, VA, 2006, pp 419–86. [Google Scholar]
- 16.Eypasch E, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal quality of life index: Development, validation and application of a new instrument. Br J Surg 1995;82:216–22. [DOI] [PubMed] [Google Scholar]
- 17.Svedlund J, Sjödin I, Dotevall G. GSRS: A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci 1988;33:129–34. [DOI] [PubMed] [Google Scholar]
- 18.Shaw M, Dent J, Beebe T, et al. The reflux disease questionnaire: A measure for assessment of treatment response in clinical trials. Health Qual Life Outcomes 2008;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pouchain D, Bigard MA, Liard F, et al. Gaviscon® vs. omeprazole in symptomatic treatment of moderate gastroesophageal reflux. A direct comparative randomised trial. BMC Gastroenterol 2012;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Agostino RB, Sr, Massaro JM, Sullivan LM. Non-inferiority trials: Design concepts and issues–the encounters of academic consultants in statistics. Stat Med 2003;22:169–86. [DOI] [PubMed] [Google Scholar]
- 21.Richter JE, Peura D, Benjamin SB, et al. Efficacy of omeprazole for the treatment of symptomatic acid reflux disease without esophagitis. Arch Intern Med 2000;160:1810–6. [DOI] [PubMed] [Google Scholar]
- 22.Lind T, Havelund T, Carlsson R, et al. Heartburn without oesophagitis: Efficacy of omeprazole therapy and features determining therapeutic response. Scand J Gastroenterol 1997;32:974–9. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher J, Derakhshan MH, Jones GR, et al. BMI is superior to symptoms in predicting response to proton pump inhibitor: Randomised trial in patients with upper gastrointestinal symptoms and normal endoscopy. Gut 2011;60:442–8. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki T, Matsushima M, Masui A, et al. Irsogladine maleate and rabeprazole in non-erosive reflux disease: A double-blind, placebo-controlled study. World J Gastroenterol 2015;21:5023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dent J, Vakil N, Jones R, et al. Accuracy of the diagnosis of GORD by questionnaire, physicians and a trial of proton pump inhibitor treatment: The diamond study. Gut 2010;59:714–21. [DOI] [PubMed] [Google Scholar]
- 26.Tack J, Caenepeel P, Arts J, et al. Prevalence of acid reflux in functional dyspepsia and its association with symptom profile. Gut 2005;54:1370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao YL, Peng S, Tao J, et al. Prevalence and symptom pattern of pathologic esophageal acid reflux in patients with functional dyspepsia based on the Rome III criteria. Am J Gastroenterol 2010;105:2626–31. [DOI] [PubMed] [Google Scholar]
- 28.Talley NJ, Ford AC. Functional dyspepsia. N Engl J Med 2015;373:1853–63. [DOI] [PubMed] [Google Scholar]
- 29.Pleyer C, Bittner H, Locke GR, III, et al. Overdiagnosis of gastro-esophageal reflux disease and underdiagnosis of functional dyspepsia in a USA community. Neurogastroenterol Motil 2014;26:1163–71. [DOI] [PubMed] [Google Scholar]
- 30.Kulich KR, Calabrese C, Pacini F, et al. Psychometric validation of the Italian translation of the gastrointestinal symptom-rating scale and quality of life in reflux and dyspepsia questionnaire in patients with gastro-oesophageal reflux disease. Clin Drug Investig 2004;24:205–15. [DOI] [PubMed] [Google Scholar]
- 31.Pace F, Tonini M, Pallotta S, et al. Systematic review: Maintenance treatment of gastro-oesophageal reflux disease with proton pump inhibitors taken on-demand. Aliment Pharmacol Ther 2007;26:195–204. [DOI] [PubMed] [Google Scholar]
- 32.Khan Z, Alastal Y, Khan MA, et al. On-demand therapy with proton pump inhibitors for maintenance treatment of nonerosive reflux disease or mild erosive esophagitis: A systematic review and meta-analysis. Gastroenterol Res Pract 2018;2018:6417526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bytzer P, van Zanten SV, Mattsson H, et al. Partial symptom-response to proton pump inhibitors in patients with non-erosive reflux disease or reflux oesophagitis–a post hoc analysis of 5796 patients. Aliment Pharmacol Ther 2012;36:635–43. [DOI] [PubMed] [Google Scholar]
- 34.Moayyedi P, Soo S, Deeks J, et al. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev 2006:CD001960. [DOI] [PubMed] [Google Scholar]
- 35.de Leone A, Tonini M, Dominici P, et al. The proton pump inhibitor test for gastroesophageal reflux disease: Optimal cut-off value and duration. Dig Liver Dis 2010;42:785–90. [DOI] [PubMed] [Google Scholar]
- 36.Zerbib F, Duriez A, Roman S, et al. Determinants of gastro-oesophageal reflux perception in patients with persistent symptoms despite proton pump inhibitors. Gut 2008;57:156–60. [DOI] [PubMed] [Google Scholar]
- 37.Savarino E, Zentilin P, Savarino V. NERD: An umbrella term including heterogeneous subpopulations. Nat Rev Gastroenterol Hepatol 2013;10:371–80. [DOI] [PubMed] [Google Scholar]
- 38.Ribolsi M, Frazzoni M, Marabotto E, et al. Novel impedance-pH parameters are associated with proton pump inhibitor response in patients with inconclusive diagnosis of gastro-oesophageal reflux disease according to Lyon Consensus. Aliment Pharmacol Ther 2021;54:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mönnikes H, Schwan T, van Rensburg C, et al. Randomised clinical trial: Sustained response to PPI treatment of symptoms resembling functional dyspepsia and irritable bowel syndrome in patients suffering from an overlap with erosive gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2012;35:1279–89. [DOI] [PubMed] [Google Scholar]
- 40.Targownik LE, Fisher DA, Saini SD. AGA clinical practice update on de-prescribing of proton pump inhibitors: Expert review. Gastroenterology 2022;162:1334–42. [DOI] [PubMed] [Google Scholar]
- 41.Zhang JX, Ji MY, Song J, et al. Proton pump inhibitor for non-erosive reflux disease: A meta-analysis. World J Gastroenterol 2013;19:8408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su T, Lai S, Lee A, et al. Meta-analysis: Proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. J Gastroenterol 2018;53:27–36. [DOI] [PubMed] [Google Scholar]
- 43.Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: A meta-analysis. Clin Gastroenterol Hepatol 2013;11:483–90. [DOI] [PubMed] [Google Scholar]
- 44.Du LJ, Chen BR, Kim JJ, et al. Helicobacter pylori eradication therapy for functional dyspepsia: Systematic review and meta-analysis. World J Gastroenterol 2016;22:3486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho YK, Choi MG, Lim CH, et al. Diagnostic value of the PPI test for detection of GERD in Korean patients and factors associated with PPI responsiveness. Scand J Gastroenterol 2010;45:533–9. [DOI] [PubMed] [Google Scholar]
- 46.Weijnborg PW, Cremonini P, Smout AJ, Bredenoord AJ. PPI therapy is equally effective in well-defined non-erosive reflux disease and in reflux esophagitis: A meta-analysis. 2012;24:747–57.e350. [DOI] [PubMed] [Google Scholar]
- 47.Shi YC, Cai ST, Tian YP, et al. Effects of proton pump inhibitors on the gastrointestinal microbiota in gastroesophageal reflux disease. Genomics Proteomics Bioinformatics 2019;17:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65:740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freedberg DE, Toussaint NC, Chen SP, et al. Proton pump inhibitors alter specific taxa in the human gastrointestinal microbiome: A crossover trial. Gastroenterology 2015;149:883–5.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.EMA. Guideline on the environmental risk assessment of medicinal products for human use EMEA/CHMP/SWP/4447/00 Rev. 1 [pdf] 2018 (https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-environmental-risk-assessment-medicinal-products-human-use-revision-1_en.pdf). Accessed June 2022.