Abstract
Honey is an attractive natural product with various health benefits. A few honey-based commercial products have successfully been adopted in clinics to improve wound healing. However, screening of other potential sources of medical-grade honey, in particular, honeys from territories with high floral species diversity and high endemicity, is highly needed. The goal of this study was to characterise the physicochemical and antibacterial properties of New Caledonian honey samples (n = 33) and to elucidate the major mechanism of their antibacterial action. Inhibitory antibacterial activity of honeys against Staphylococcus aureus and Pseudomonas aeruginosa was determined with a minimum inhibitory concentration (MIC) assay. Enzymatic activity of glucose oxidase and the content of hydrogen peroxide (H2O2) in honey samples were analysed. Furthermore, total protein content of honeys together with their electrophoretic protein profiles were also determined in the study. The antibacterial efficacy of 24% of the tested honey samples was slightly superior to that of manuka honey with unique manuka factor 15+. The antibacterial activity of catalase-treated honey sample solutions was significantly reduced, suggesting that H2O2 is a key antibacterial compound of diluted honeys. However, the kinetic profiles of H2O2 production in most potent honeys at a MIC value of 6% was not uniform. Under the experimental conditions, we found that a H2O2 concentration of 150 μM in diluted honeys is a critical concentration for inhibiting the growth of S. aureus. In contrast, 150 μM H2O2 in artificial honey solution was not able to inhibit bacterial growth, suggesting a role of phytochemicals in the antibacterial activity of natural honey. In addition, the continuous generation of H2O2 in diluted honey demonstrated an ability to counteract additional bacteria in re-inoculation experiments. In conclusion, the tested New Caledonian honey samples showed strong antibacterial activity, primarily based on H2O2 action, and therefore represent a suitable source for medical-grade honey.
Introduction
Antimicrobial drug resistance represents a major burden on global health and jeopardises the effectiveness of currently used antimicrobials. It is therefore crucial to reduce and optimise the use of antibiotics and introduce antimicrobial stewardship programmes to tackle drug-resistant infections globally [1]. Although a recent systematic review and meta-analysis showed promising results achieved with such programmes [1], it seems that the problem of antimicrobial drug resistance is more complex and linked to climate change (e.g., higher concentrations of contaminants in the environment) [2]. In addition, the use of topical antibiotics is still subject of debate because of their overall efficiency and the development of bacterial resistance [3]. In the last decade, natural products have become attractive platform for developing new antimicrobials which might help to tackle the problem of bacterial resistance [4]. Indeed, natural products are diverse and rich sources of antimicrobial compounds. Marine [5], insect [6] and plant [7] products are widely studied for their multi-factorial antimicrobial activities and perspectives to identify novel antimicrobial drugs which are effective against multi drug-resistant microorganisms. However, most of these natural products and their isolated antimicrobial compounds are mainly implicated for topical therapy because their minimal inhibitory concentrations (MICs) are substantially higher than the possible systemic concentrations achieved in oral therapies [8]. Topical antimicrobials are often used for the elimination of wound infections, which are considered major factors responsible for the delay in wound healing.
Nowadays, natural products such as honey and essential oils are attractive antibacterial agents in the management of infected acute and chronic wounds [9, 10]. Honey has been used in clinical practice for decades and currently, several medical-grade honey-based products are available as medical devices worldwide [11]. Interestingly, honey can effectively be used together with other topically applied antiseptics (e.g., benzalkonium bromide, silver (I) nitrate or tea tree oil) for treating superficial bacterial infections [12, 13].
Manuka monofloral honey, made from the nectar of the manuka bush (Leptospermum scoparium), which is native to New Zealand and Australia, is unique among other honeys due to its higher content of methylglyoxal (MGO). This compound is the key component responsible for the non-peroxide antibacterial activity exerted by manuka honey [14]. High amounts of MGO are present in manuka honey, even more than 1,000 mg/kg, up to 100-fold higher compared to those found in most other honeys [15, 16]. Globally, manuka honey is the most frequently used honey in wound care.
Over the past decade, intense screenings of other honey types have been performed to identify new sources of medical-grade honey that would be attractive for the pharmaceutical industry [11]. In many cases, the antibacterial activity of newly described honey types is often compared to that of manuka honey, with different levels of MGO or different unique manuka factor (UMF) [17–25]. However, the mechanism of antibacterial action in all analysed non-manuka honeys is based on the action of hydrogen peroxide (H2O2). The generation of H2O2 in diluted honey is mainly mediated through the action of bee-derived enzyme glucose oxidase (GOX) [26]. However, the level of accumulated H2O2 may be significantly diverse among honeys, depending on several factors [27]. Interestingly, honeydew honeys contain significantly higher amounts of generated H2O2 compared to blossom honeys, probably due to the considerable presence of polyphenolic compounds [20, 25]. As stated above, H2O2 is produced mainly enzymatically and its amount produced in honey significantly correlates with honey minimum inhibitory concentration (MIC) [27]. On the other hand, some studies have reported a lack of correlation between the H2O2 level and overall antibacterial activity [25, 28]. Therefore, it is required to elucidate observed discrepancies by determining the critical concentration of accumulated H2O2 and identifying phytochemicals which can enhance honey antibacterial effect.
Additional screening the antibacterial potential of traditional botanical honey types such as acacia, linden, buckwheat, clover, sunflower and other honeys, despite their different geographical origins, did not very likely identify different mechanisms of antibacterial action. Therefore, it is important to focus on territories with high floral species diversity and high endemicity to identify honey samples with high antibacterial efficacy and possible with different mechanisms of antibacterial action. One of such territories is New Caledonia, which is characterised by a remarkably high species diversity as well as due to their high endemicity and an unusual abundance of archaic plant taxa [29]. New Caledonia is remarkable for its high rate of endemism; 75% (around 2500 species) of the vascular plant species are found nowhere else in the world [30, 31]. The flora of New Caledonia has links with Australia, New Guinea and New Zealand [30].
In this study, we characterised a representative collection of New Caledonian honey samples (n = 33) in terms of physicochemical and antibacterial properties. Furthermore, we analysed the protein content and enzymatic activity of GOX in honey samples. We also described the mechanism of the antibacterial action of the tested honeys at their MICs and elucidated the role of accumulated H2O2 in inhibiting the growth of Staphylococcus aureus (S. aureus).
Materials and methods
Honey samples
A total of 33 honey samples from local beekeepers from all regions of New Caledonia were evaluated. The samples were harvested in 2021 and identification of the floral source of each honey sample using mellisopalynological analysis according to Erdtman [32] was performed by CARI asbl, Louvain-La-Neuve, Belgium. Two different commercial manuka honeys, Comvita UMF15+ (Comvita NZ, Ltd., New Zealand) and Manuka Honey MGO 550+ (Manuka Health New Zealand, Ltd., New Zealand) were also evaluated. All collected samples were kept at 4°C in the dark.
As a negative control artificial honey (AH) was prepared by dissolving 39 g d-fructose, 31 g d-glucose, 8 g maltose, 3 g sucrose and 19 g distilled water, as described elsewhere [33].
Bacteria
The tested bacterial isolates Staphylococcus aureus CCM4223 (Czech Collection of Microorganisms, Brno, Czech Republic) and Pseudomonas aeruginosa CCM1960 (Czech Collection of Microorganisms, Brno, Czech Republic) were acquired from the Department of Medical Microbiology at the Slovak Medical University in Bratislava, Slovakia. Both bacteria were stored at -80°C in Muller-Hinton broth (MHB) /glycerol stocks. Bacterial working cultures were cultured on MHB agar then stored at 4°C and were refreshed from frozen stocks every two weeks.
Physicochemical and organoleptic characteristics of honey
All physicochemical analyses were performed at CARI asbl, Louvain-La-Neuve, Belgium. Analytical methods measuring pH, electric conductivity, hydroxymethylfurfural (HMF) and the fructose/glucose (F/G) ratio were performed according to "Harmonized Methods of the International Honey Commission" (2009) and the European Directive 2001/110/EC and humidity according to APAQ-W and German guidelines for honey (Leitsätze) [34, 35].
Aliquots of honey samples were heated to 50°C to dissolve sugar crystals, and the colour was determined by the spectrophotometric (Genesys 10 UV, Thermo Scientific, UK) measurement of the absorbance of a 50% honey solution (w/v) at 635 nm. The honeys were classified according to the Pfund scale after conversion of the absorbance values: mm Pfund = − 38.70 + 371.39 × Abs [36].
Determining the protein profile of honey samples and total protein content
For protein determination, 15 μL aliquots of diluted honey samples (50% w/w in distilled water) were loaded on 12% SDS-PAGE electrophoresis gels and separated using a Mini-Protean II electrophoresis cell (Bio-Rad, Hercules, CA, USA).
The total protein content (TPC) was measured using the Quick StartTM Bradford reagent (Bio-Rad, Hercules, CA, USA), according to the manufacturer’s instructions.
Determination of GOX enzymatic activity
The bee-derived GOX activity was determined with a Megazyme GOX assay kit (Megazyme International Ireland Ltd, Bray, Ireland), which is based on the oxidative catalysis of β-D-glucose to D-glucono-δ-lactone, with the concurrent release of H2O2. The resultant H2O2 reacts with p-hydroxybenzoic acid and 4-aminoantipyrine in the presence of peroxidase to form a quinoneimine dye complex, which has a strong absorbance at 510 nm. The enzyme activity was determined in freshly prepared and centrifuged (10,000 g, 5 min) 20% (w/v) honey solutions in a 96-well microplate, according to the manufacturer’s instructions.
Determination of the H2O2 concentration after 24h and H2O2 kinetics
The H2O2 content in the honey samples was determined using a Megazyme GOX assay kit (Megazyme International Ireland Ltd); based on the release of H2O2. As a standard, 9.8–312.5 μM diluted H2O2 was used. 40% (w/w) of the honey solutions in 0.1 M potassium phosphate buffer (pH 7.0) were prepared. After 24 h of incubation at 37°C, each honey sample and H2O2 standard were tested in duplicate in a 96-well microplate. Absorbance was measured at 510 nm using a Synergy HT microplate reader (BioTek Instruments, VT, USA).
In order to mimic the experimental conditions during determination of antibacterial activity of honey samples, H2O2 production was also measured in selected honey solutions which were diluted with MHB instead of phosphate buffer (as stated above) to a final honey concentration corresponded to their MIC values. The level of H2O2 was determined in honey solutions after incubation at 37°C at 0, 1, 2, 4, 6, 8 and 24 h using a Megazyme GOX assay kit.
Determining the antibacterial activity of honey
The honey samples were subjected to an antibacterial MIC assay to determine their antibacterial activity against S. aureus, following the modified method of Bucekova et al. [26] based on broth microdilution method described by the Clinical and Laboratory Standards Institute [37]. Bacteria were cultured in MHB at 37°C overnight. Bacterial cultures were suspended in phosphate-buffered saline (PBS), with a pH of 7.2, and the turbidity of the suspension was adjusted to 108 colony-forming units (CFU)/mL and diluted with MHB medium (pH 7.3 ± 0.1) to a final concentration of 106 CFU/mL. The final volume in each well of sterile 96-well polystyrene U-shaped plates (Sarstedt, Germany) was 100 μL, consisting of 90 μL of sterile MHB medium (as a positive control) or diluted honey sample and 10 μL of bacterial suspension. Each honey sample dilution was prepared from a 50% honey solution (w/w in MHB medium) by further dilution with the MHB medium, resulting in final concentrations of 40%, 35%, 30%, 25%, 20%, 18%, 16%, 14%, 12%, 10%, 8%, 6% and 4%. Wells containing 100 μL of sterile MHB medium was considered as a negative control. After 18 h of incubation at 37°C and 1,250 rpm, the inhibition of bacterial growth was determined visually as the lowest concentration of honey completely inhibiting bacterial growth, resulting in an optically clear well (lack of visual turbidity) and expressed as a MIC value. All tests were performed in triplicate and repeated three times.
Bactericidal activity, expressed as minimal bactericidal concentration (MBC) of honey solution, was evaluated in selected honey samples No. 1, 3, 6, 7, 8, 11, 15 and 27, according to Bucekova et al (2019) [26] with some modifications Briefly, the viability of bacteria in wells with no visible bacterial growth (wells around MIC) was determined by spotted aliquots (10 μL) from each well onto MHB agar plates and incubated overnight at 37°C. The highest honey dilution at which all bacterial cells were killed was recorded as the MBC.
In addition, the viability of bacteria in honey solutions at different MBCs was determined in first 3 hours after bacterial inoculation. Aliquots (10 μL) from each timepoints (0, 1, 2 and 3 h) were subsequently spotted on MHB agar and incubated overnight at 37°C. Colony-forming units were counted and expressed as CFU/μL. As control AH, AH with 300 μM H2O2 and MHB alone were used.
Enzymatic treatment of honey samples with catalase
The selected honey samples No. 1, 3, 6, 7, 8, 11 and 15, at a concentration of 50% (w/w), were treated with catalase (2,000–5,000 U/mg protein; Sigma-Aldrich, UK) at a final concentration ranging from 1,000–2,500 U/ml at ambient temperature for 2 h. Catalase-treated honey samples were then used in the antibacterial assay to determine the MIC values against S. aureus.
Statistical analysis
The physicochemical parameters of the honey samples were expressed as mean with standard uncertainty (k = 2). The Shapiro-Wilk test of normality was used to determine the data distribution. Analysis of variance (ANOVA) with Tukey’s multiple comparison test was used to determine differences among bactericidal activities. Values with P below 0.05 were considered statistically significant. A principal components analysis (PCA) was conducted to examine the relationship among selected honey physicochemical parameters, botanical origin, activity of GOX and antibacterial activity. All statistical analyses were performed using GraphPad Prism version 9.2.0 (GraphPad Software Inc., La Jolla, CA, USA).
Results
Melissopalynological analysis
Overall, 17 botanical families were identified in honey samples; 21.2% of the samples contained pollen from one botanical family, 33.3% contained pollen from two families, and 45.5% of the samples contained mixed pollen from more than three families (Table 1 and S1 Table). The most dominant family was Anacardiaceae, present in 51.5% of the samples, followed by Myrtaceae together with Cunoniacea, found in 48.5% and 45.5% of the samples, respectively. Qualitative pollen analysis highlighted seven samples (No. 1, 3, 29, 27, 20, 10 and 18) containing only one type of pollen, with the following frequencies: Myrtaceae 89% and 72%, Cunoniacea 80%, 70%, 59%, Fagaceae 81% and Mimosaceae 85%, respectively. Moreover, Myrtaceae pollen was predominant also in samples No. 6, 8 and 32. The family Anacardiaceae was predominant in samples No. 25, 26, 12 and 4 with 70%, 61%, 46% and 45%, respectively. Cunoniacea was present in samples No. 29, 27, 20 and 13, with a pollen coverage of 80%, 70%, 59% and 58%, respectively. The lowest amount of detected pollen was observed for Zygophyllaceae, which was found in two samples (No. 31 and 32), accounting for 11% and 12%, and for Rosaceae, which was only found in one sample (No. 30), with a pollen coverage of 14%.
Table 1. Melissopalynological analysis of collected New Caledonian honey samples (n = 33).
| Family | Max. (%) | ≥46% | 45–16% | 15–4% | Present |
|---|---|---|---|---|---|
| Anacardiaceae | 70 | 3 | 10 | 4 | 17 |
| Apiaceae | 35 | - | 3 | - | 3 |
| Apocynaceae | 46 | 1 | 2 | - | 3 |
| Brassicaceae | 16 | - | 1 | - | 1 |
| Casuarinaceae | 30 | - | 1 | - | 1 |
| Cunoniaceae | 80 | 4 | 8 | 3 | 15 |
| Euphorbiaceae | 31 | - | 2 | - | 2 |
| Fagaceae | 81 | 1 | 1 | - | 2 |
| Mimosaceae | 85 | 3 | 5 | 2 | 10 |
| Myrtaceae | 89 | 5 | 9 | 2 | 16 |
| Poaceae | 22 | - | 1 | - | 1 |
| Rhizophoraceae | 40 | - | 1 | - | 1 |
| Rosaceae | 14 | - | - | 1 | 1 |
| Salicaceae | 28 | - | 1 | - | 1 |
| Zygophyllaceae | 12 | - | - | 1 | 1 |
Max.: maximum value reached by the pollen type in samples, present: number of samples where the pollen type was identified. t.: pollen type common for different plant genera.
Physicochemical parameters of honey samples
The basic physicochemical parameters of New Caledonian honey samples are listed in Table 2. Two samples, No. 18 and 30, did not meet the Codex standard criterium for the HMF level (< 80 mg/100 g for tropical honeys) and moisture (< 20%), respectively.
Table 2. Physicochemical and organoleptic characteristics of New Caledonian honey samples (n = 33).
| Sample No. | Humidity (%) | pH | EC (mS/cm) | HMF (mg/100 g) ± SD | F/G | Pfund |
|---|---|---|---|---|---|---|
| 1 | 16.44 | 4.7 | 1.267 | 6.3±2.4 | 1.39 | 5.50 |
| 2 | 18.32 | 4.2 | 1.235 | 30.1±5.1 | 1.42 | 72.72 |
| 3 | 16.55 | 4.3 | 1.306 | 12.4±2.4 | 1.30 | 11.44 |
| 4 | 16.39 | 4.5 | 0.643 | 26.7±4.5 | 1.73 | 98.71 |
| 5 | 17.23 | 4.5 | 0.604 | 20.9±3.6 | 1.42 | 76.06 |
| 6 | 16.13 | 4.8 | 1.464 | 1.5±2.4 | 1.44 | -3.05 |
| 7 | 17.33 | 4.3 | 0.796 | 11.2±2.4 | 1.16 | 119.14 |
| 8 | 16.73 | 4.5 | 1.234 | 16.5±2.8 | 1.40 | 10.69 |
| 9 | 18.6 | 4.2 | 0.970 | 32.1±5.5 | 1.34 | 70.86 |
| 10 | 19.26 | 4.4 | 0.611 | 14.8±2.5 | 1.63 | 66.03 |
| 11 | 18.43 | 3.9 | 0.558 | 19.0±3.2 | 1.13 | 28.89 |
| 12 | 14.81 | 5.0 | 0.772 | 3.6±2.4 | 1.80 | 64.92 |
| 13 | 15.61 | 4.9 | 0.810 | 5.0±2.4 | 1.67 | 54.15 |
| 14 | 16.24 | 4.8 | 0.912 | 5.8±2.4 | 1.79 | 56.00 |
| 15 | 17.14 | 4.3 | 0.752 | 1.8±2.4 | 1.24 | 21.84 |
| 16 | 16.46 | 4.2 | 0.686 | 7.8±2.4 | 1.23 | 79.03 |
| 17 | 18.24 | 4.0 | 0.578 | 52.7±9.0 | 1.31 | 94.63 |
| 18 | 17.34 | 3.9 | 0.745 | 86.9±14.8 | 1.31 | 87.20 |
| 19 | 18.74 | 4.1 | 0.794 | 17.2±2.9 | 1.28 | 44.86 |
| 20 | 17.22 | 4.1 | 0.546 | 7.8±2.4 | 1.38 | 10.69 |
| 21 | 16.16 | 4.8 | 0.713 | 6.2±2.4 | 1.61 | 63.80 |
| 22 | 17.48 | 4.4 | 0.653 | 34.4±5.8 | 1.47 | 84.23 |
| 23 | 18.28 | 4.2 | 0.570 | 7.0±2.4 | 1.23 | 79.77 |
| 24 | 18.39 | 4.1 | 0.636 | 13.2±2.4 | 1.18 | 70.49 |
| 25 | 16.06 | 4.9 | 0.784 | 2.4±2.4 | 1.45 | 79.77 |
| 26 | 15.13 | 4.8 | 0.720 | 8.9±2.4 | 1.56 | 79.77 |
| 27 | 15.43 | 5.1 | 0.918 | 3.4±2.4 | 1.83 | 43.01 |
| 28 | 15.91 | 4.5 | 0.726 | 21.1±3.6 | 1.57 | 109.86 |
| 29 | 16.44 | 4.4 | 0.628 | 43.5±7.4 | 1.59 | 91.29 |
| 30 | 20.91 | 4.1 | 0.815 | 5.1±2.4 | 1.32 | 125.08 |
| 31 | 17.79 | 4.1 | 0.660 | 21.5±3.6 | 1.23 | 89.06 |
| 32 | 17.79 | 4.1 | 0.660 | 21.5±3.6 | 1.23 | 57.49 |
| 33 | 18.16 | 4.1 | 0.404 | 8.3±2.4 | 1.25 | 95.74 |
In total, 8 out of 33 honey samples had an electric conductivity value above 0.8 mS/cm. In the collected honey samples, the pH values ranged from 3.9 to 5.1.
Protein content, GOX enzymatic activity and H2O2 accumulation in diluted honey samples
The TPC and an SDS-PAGE profile of each New Caledonian honey sample were determined (Table 3 and S1 Fig). As shown in Table 3, the mean TPC values substantially varied among samples and ranged from 448 to 1,282 μg/g of honey. The SDS-PAGE profiles of honey sample solutions were comparable with that of major royal jelly protein 1 (MRJP1) as a dominant honey protein. There was only a slight shift of MRJP1 protein in samples No. 4, 5 and 10 (S1 Fig).
Table 3. Selected biochemical characteristics of New Caledonian honey samples (n = 33).
| Sample No. | TPC (μg/g honey) ± SD | GOX (mU/mL) ± SD | H2O2 (μM) ± SD |
|---|---|---|---|
| 1 | 572.08±30.18 | 5.70±1.35 | 285.14±30.43 |
| 2 | 720.00±59.16 | 1.90±0.66 | 911.50±84.88 |
| 3 | 510.28±70.29 | 7.21±0.85 | 315.43±30.42 |
| 4 | 676.56±63.01 | 0.34±0.27 | 332.57±49.97 |
| 5 | 941.49±76.25 | 0.69±0.35 | 1252.00±28.05 |
| 6 | 785.79±133.73 | 11.67±2.42 | 306.29±32.72 |
| 7 | 1122.61±53.82 | 9.20±0.82 | 2607.43±41.11 |
| 8 | 839.01±49.73 | 6.11±0.27 | 366.29±60.80 |
| 9 | 804.64±31.34 | 5.42±0.59 | 1178.00±85.83 |
| 10 | 955.87±74.48 | 0.19±0.15 | 2059.00±108.71 |
| 11 | 887.13±43.05 | 8.08±1.48 | 2052.00±66.63 |
| 12 | 926.70±53.81 | 2.76±0.96 | 1136.50±34.46 |
| 13 | 933.58±75.85 | 2.05±0.29 | 1877.00±60.85 |
| 14 | 955.02±70.28 | 3.78±1.28 | 1060.50±70.40 |
| 15 | 1282.43±78.84 | 13.34±2.80 | 4056.73±452.45 |
| 16 | 723.92±48.80 | 9.71±1.48 | 3394.67±80.24 |
| 17 | 682.81±56.53 | 4.95±0.52 | 256.00±25.66 |
| 18 | 616.62±48.55 | 4.22±0.72 | 761.07±108.26 |
| 19 | 643.12±18.54 | 2.60±0.85 | 352.67±58.60 |
| 20 | 448.62±51.25 | 7.19±1.03 | 174.40±18.96 |
| 21 | 722.46±49.61 | 0.10±0.18 | 1256.00±28.80 |
| 22 | 1018.25±46.10 | 0.90±0.65 | 1767.00±67.30 |
| 23 | 647.47±51.42 | 6.39±0.94 | 2782.67±106.63 |
| 24 | 665.86±14.94 | 5.50±1.03 | 1184.57±130.13 |
| 25 | 607.80±18.64 | 2.26±0.24 | 1816.53±52.73 |
| 26 | 876.28±22.61 | 0.77±0.27 | 1422.29±46.80 |
| 27 | 670.03±65.17 | 3.72±1.36 | 1978.67±67.50 |
| 28 | 1227.25±41.63 | 2.78±1.02 | 710.50±45.32 |
| 29 | 1012.05±42.96 | 2.24±0.86 | 754.00±46.83 |
| 30 | 681.23±60.84 | 7.12±0.40 | 434.86±31.16 |
| 31 | 632.74±36.65 | 2.72±1.11 | 2284.00±86.73 |
| 32 | 556.26±89.86 | 1.95±1.05 | 1944.00±80.91 |
| 33 | 662.01±20.98 | 1.64±0.59 | 1553.00±76.50 |
TPC, total protein content; GOX, glucose oxidase
The enzymatic activity of GOX was evaluated in all honey samples (Table 3), resulting in great differences in enzymatic activity among the tested samples. The lowest enzymatic activity was determined in sample No. 21 (calciferous forest), with a value of 0.10 ± 0.18 mU/mL, and the highest activity was documented in sample No. 15 (anthropized environment), with value of 13.34 ± 2.80 mU/mL. Statistical analysis showed no correlation (rs = -0.072, P = 0.689) between the enzymatic activity of GOX and TPC in the analysed samples.
Similar to the enzymatic activity of GOX, there was a great variance in the concentration of H2O2, determined in 40% honey solutions after incubation for 24 h, in the analysed honey samples (Table 3), ranging from 285 to 4,057 μM. Due to large variations within the measured parameters, statistical analysis did not reveal any correlation (rs = -0.048, P = 0.790) between the level of H2O2 and the enzymatic activity of GOX in the analysed samples.
Antibacterial effects of honey samples against S. aureus and P. aeruginosa
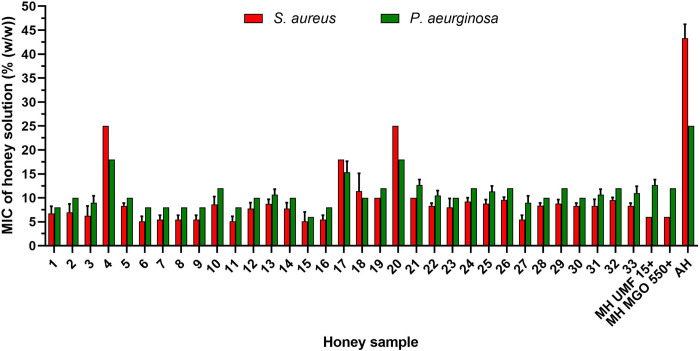
The mean MIC values of the tested honeys are shown in Fig 1 and ranged from 5.1% to 25% and from 6% to 18% for S. aureus and P. aeruginosa, respectively. Among the tested honeys, samples No. 4, 17 and 20 showed the lowest antibacterial efficacy against both bacterial species. However, none of the honey samples exhibited an antibacterial activity identical with that of artificial honey. Both manuka honey samples exhibited an equal antibacterial activity, with MIC values of 6%and 12% against S. aureus and P. aeruginosa, respectively. The antibacterial effects of eight (24% of honey samples) out of 33 New Caledonian honey samples were slightly superior to those of both manuka honeys. The most potent sample out of all tested honey samples was sample No. 15, with MIC values of 5.1% and 6.0% against S. aureus and P. aeruginosa, respectively.
Fig 1. Antibacterial activity of New Caledonian (n = 33) and manuka (UMF 15+ and MGO 550+) honey samples.
The antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa was determined by a minimum inhibitory concentration (MIC) assay. The data are expressed as mean MIC values. MH, manuka honey; AH, artificial honey (sugars only).
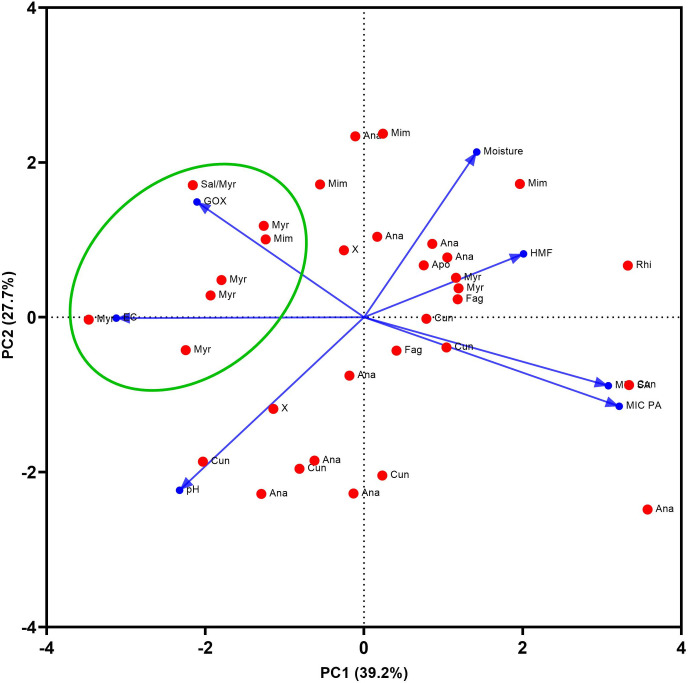
Multifactorial analysis of selected physicochemical parameters, enzymatic activity of GOX, botanical origin and antibacterial activity of honey
According to PCA, the first two components (PC1 and PC2) explained 66.9% of the total variation in the selected physicochemical parameters (moisture, pH, HMF and electric conductivity), the enzymatic activity of GOX, the honey botanical origin and the antibacterial activity of honey against S. aureus and P. aeruginosa (Fig 2). A strong negative correlation was found between the enzymatic activity of GOX and the antibacterial activity of honey samples, expressed as MIC value, against S. aureus (rs = -0.572, P < 0.001) and P. aeruginosa (rs = -0.653, P <0.001).
Fig 2. Bioplot of the principal components analysis of honey from different botanical origins.
Ana, Anacardiaceae; Cun, Cunoniacea; Myr, Myrtacaea; Apo, Apocynaceae; Fag, Fagaceae, Sal, Salicaceae, Mim, Mimosaceae; X, multifloral honey type with no dominant pollen type. The green ellipse represents the area of the most honey samples belonging to the Myrtaceae family. Blue vectors represent the following variables: EC, electric conductivity; pH; Moisture; HMF, hydroxymethylfurfural; GOX, enzymatic activity of GOX; MIC SA, antibacterial activity against Staphylococcus aureus; MIC PA, antibacterial activity against Pseudomonas aeruginosa.
Role of H2O2 in the bactericidal effect of honey samples
Based on the ability of honey samples to generate H2O2 in 40% honey solution after 24 h of incubation, eight, the most antibacterially active, honey samples with a low (samples No. 1, 3, 6 and 8) and high capacity (7, 11, 15 and 27) to produce H2O2 were selected for further detailed analysis.
The bactericidal effects of eight honey samples were determined at their MIC value of 6% against S. aureus by counting the CFUs at different time points (Fig 3). As a control, H2O2 in 6% artificial honey solution was used at an MIC of 300 μM. The statistically significant reduction in CFU counts after 1 h of incubation was observed for six out of eight honey samples and the control sample containing H2O2. A significant decrease in CFU counts was found in all honey samples after 2 h of incubation compared to the positive control (6% artificial honey in cultivation media). In addition, the complete eradication of S. aureus was observed in samples No. 6 and 15 after 2 h of incubation and in all samples after 3 h of incubation. A similar significant bactericidal effect was also documented in the control experiment with a single dose of H2O2, with the complete eradication of S. aureus after 3 h of incubation.
Fig 3. Bactericidal effects of selected New Caledonian honey samples (n = 8) at their minimal inhibitory concentrations (MICs) at different time points against Staphylococcus aureus.
Bactericidal activity was determined by counting of colony-forming units (CFUs) and expressed as number of CFU/μL. PC, positive control (culture medium only); AH, artificial honey (sugars only). * P < 0.05, ** P < 0.01, ***P < 0.001.
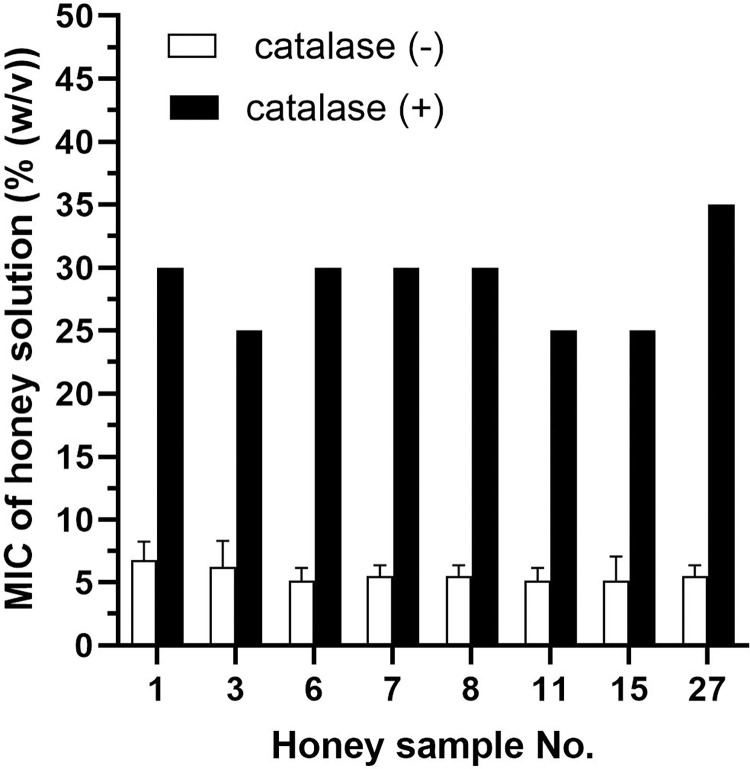
To elucidate the role of H2O2 in inhibiting bacterial growth, 50% solutions of selected honey samples were treated with catalase for 2 h (Fig 4). The removal of H2O2 in catalase-treated samples resulted in a significant increase in MIC values, which ranged from 25% to 35% in all honey samples. However, the overall antibacterial effect after catalase treatment did not reduce to the level of artificial honey, with a mean MIC value of 42.5%.
Fig 4. Antibacterial activity of selected New Caledonian honey samples (n = 8) following catalase treatment against Staphylococcus aureus.
The antibacterial activity was determined with a minimum inhibitory concentration (MIC) assay. The data are expressed as mean MIC values.
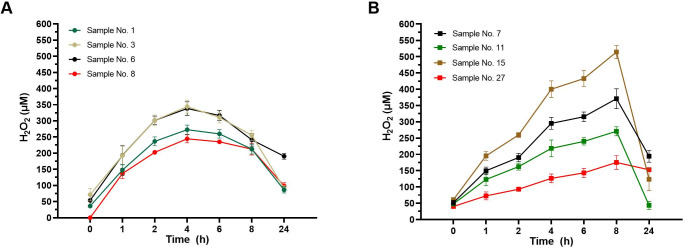
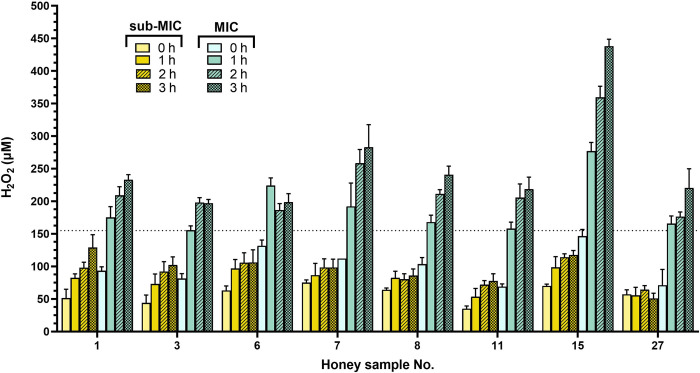
Subsequently, we analysed the kinetics of H2O2 accumulation in eight honey samples at their MIC value of 6% (Fig 5). The maximum production of H2O2 in honey samples with a low capacity to produce H2O2 after 24 h was reached after 4 h of incubation at 37°C (Fig 5A). The maximum levels of H2O2 were in a range of 244 to 344 μM. In contrast honey samples with a high capacity to produce H2O2 in 40% solution after 24 h accumulated the maximum level of H2O2 after 8 h of incubation or thereafter (Fig 5B). Although all eight honey solutions had the same concentration (6%), an obvious shift in the enzymatic kinetic profile of H2O2 production among the samples was observed.
Fig 5. Twenty-four-hour pattern of the generation of hydrogen peroxide (H2O2) in diluted honey samples.
The concentration of H2O2 was determined in selected honey samples at their MIC value of 6%. A. Honey samples No. 1, 3, 6 and 8, characterised by a low production of H2O2 at 40% dilution after 24 h. B. Honey samples No. 7, 11, 15 and 27, characterised by a high production of H2O2 at 40% dilution after 24 h. The data are expressed as mean concentration of H2O2.
The initial concentration of H2O2 in selected eight honey solutions at an MIC of 6% was below 100 μM (Fig 5), significantly lower than the used concentration of artificial added H2O2 (300 μM). We further determined the concentration of H2O2 in eight honey samples at their MICs (6%) and sub-MICs (3%) after 1, 2 and 3 h of incubation at 37°C (Fig 6). A marked increase in H2O2 production (above 150 μM) was observed in all honey samples at MIC after 1 h of incubation compared to samples at their sub-MIC values. Furthermore, none of the samples at sub-MIC was able to generate H2O2 at concentrations above 150 μM over the entire incubation period. Honey samples at MIC were able to significantly inhibit bacterial growth after 1 h of incubation (Fig 3), and a H2O2 concentration of 150 μM is the critical concentration for inhibiting the growth of S. aureus under study conditions.
Fig 6. Generation of hydrogen peroxide (H2O2) in diluted honey samples during 3 hours of incubation.
The concentration of H2O2 was determined in honey samples No. 1, 3, 6, 7, 8, 11, 15 and 27 at their MIC and sub-MIC values of 6% and 3%, respectively. The data are expressed as mean concentration of H2O2.
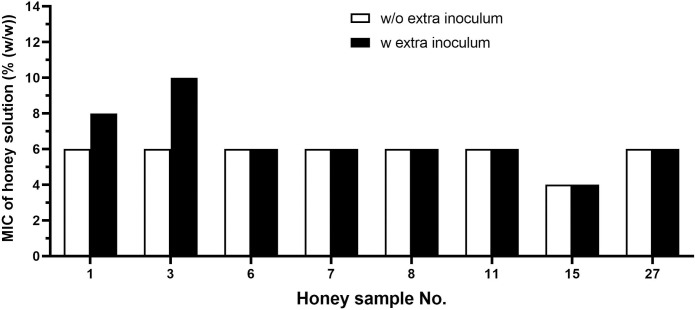
Effect of bacterial re-inoculation on honey antibacterial activity in an experimental setting
The subsequent experiments aimed to determine the antibacterial activity of selected honey samples and H2O2 after additional inoculation of S. aureus. After 4 h of incubation, each well containing either honey solution or H2O2 as antibacterial agent was re-inoculated with 10 μL of bacterial suspension at a concentration of 106 CFU/mL. At these conditions, the MIC value of H2O2 was increased from 300 to 600 μM, and additional bacterial inoculum caused an increase in the MIC values of two honey samples, No. 1 and 3, by 25% and 40%, respectively (Fig 7). However, the MIC values of six out of eight honey samples remained unchanged.
Fig 7. Antibacterial activity of selected New Caledonian honey samples (n = 8) against Staphylococcus aureus after bacterial re-inoculation at 4 h.
The antibacterial activity was determined with a minimum inhibitory concentration (MIC) assay. The data are expressed as mean MIC values.
Discussion
The characterisation of the physicochemical and biological properties of honey samples from areas with a high floral diversity and endemicity is highly attractive for beekeepers, consumers as well as the pharmaceutical industry. Honey consumer behaviour, partially due to the Covid-19 pandemic, is increasingly associated with honey health benefits and the presence of organoleptic compounds [38]. The most common biological properties that consumers are aware of in honey are its natural antibacterial properties. It is widely accepted that manuka honey possesses therapeutic advantages over other types of honey because of its high antibacterial activity. However, we showed here and elsewhere [25] that certain types of honey, including honeydew honey, had similar or even superior antibacterial activity compared to manuka honey with a high content of MGO. Although the mechanism of action of all non-manuka honey types is H2O2-dependent, the honey polyphenol content may significantly contribute to honey antibacterial activity and is a key factor responsible for the augmented antibacterial effects of certain types of honey. The levels of H2O2 in honey samples rich in polyphenols is substantially higher compared to that of monofloral honey types [25, 39]. Yet, the exact mode of polyphenol action and their effect on honey antibacterial activity has not been described in detail.
In this study, we clearly show that H2O2 is a key antibacterial compound in diluted New Caledonian honey samples. According to the PCA results, most of the honey samples containing pollen of the Myrtaceae family as the dominant pollen type were the most potent honey samples inhibiting bacterial growth. This honey type also had a high electrical conductivity Furthermore, these honey samples were also characterised by the highest values of GOX enzymatic activity when compared to the other honey botanical types. The level of accumulated H2O2 in 40% honey solutions after 24 h did not correlate with the enzymatic activity of GOX and the antibacterial activity of honey samples. In a previous study, the maximum accumulation of H2O2 occurred at 30%–50% honey dilution [40]. However, numerous studies characterising the production of H2O2 used honey diluted to 20%–30% strength [23, 28, 41–44]. Based on the results of this and of previous studies, the kinetic profile of H2O2 generation is not uniform in honey samples, and the maximum point of H2O2 production depends on the honey dilution rate and on various factors such as the concentrations of GOX enzyme, pollen-derived catalase and specific phytochemicals which enhance the enzymatic reaction [27]. The final concentrations of H2O2 can therefore vary in different honeys based on floral sources.
As mentioned above, the most potent honey samples inhibiting bacterial growth mostly contained pollen from the family Myrtaceae, which contains at least 133 genera and more than 3,800 species [45]. The family Myrtaceae is divided into two subfamilies, the capsular Leptospermoideae and the fleshy-fruited Myrtoideae. One of the most pronounced species of this family is Leptospermum scoparium, which is the botanical source of the well-known manuka honey, with different mechanisms of antibacterial action depending on the MGO level [46, 47]. Based on the enzymatic activity of GOX and the production of H2O2, supported by the results of the catalase honey treatment, we can conclude that the antibacterial effect of none of the analysed honey samples was mediated through the action of MGO.
Antibacterial effect of honey can also be attributed to action of other parameters such as bee-derived defensin-1 [48, 49], MRJP1 [50] and gluconic acid [51]. Defensin-1, an antibacterial peptide and MRJP1, the most abundant protein in honey, are expressed in hypopharyngeal bee glands and secreted during the processing of nectar into honey. A very recent proteomic study showed no difference in the amount of MRJP1 between blossom and honeydew honeys [52]. Furthermore, honeys of different botanical and geographical origin might contain the comparable amounts of MRJP1 as well as other bee-secreted proteins/peptides and therefore differences in antibacterial efficacy of various honey samples are attributed to other antibacterial parameter(s) such as H2O2 [52]. Despite of the great difference in TPC values among New Caledonian honey samples, the SDS-PAGE electrophoretic profile of honey samples was comparable, indicating MRJP1 as a dominant protein.
To the best of our knowledge, no study has, so far, determined the critical concentration limit of H2O2 in honey solutions that is required for a sufficient inhibitory activity. Under laboratory conditions, most of the selected honey samples at MICs were able to reduce CFU counts after 1 h of incubation. The critical concentration of H2O2 in honey solutions after 1 h of incubation was above 150 μM, significantly inhibiting bacterial growth. However, a single dose of H2O2 in artificial honey at a concentration of 150 μM did not inhibit the growth of S. aureus, suggesting that phytochemicals play an important role in generating more toxic reactive oxidative species (ROS). The co-occurrence of H2O2 and phytochemicals found in honey stimulate the generation of long-lived and more toxic ROS compared to H2O2 alone [53]. Similarly, Masoura et al. (2020) suggested that the H2O2 antibacterial activity of natural honeys is a determinant of the ROS-inducing effect [51]. The authors speculated that darker honeys stimulate higher ROS generation compared to light ones due to the abundance of polyphenols in dark honeys [51].
From a clinical point of view, honey is most often used undiluted in wound care, and its further dilution with wound exudates depends on the wound type and the exudate level. Moderately and heavily exudating wounds can produce up to 5 mL of exudate per 10 cm2 per 24 h [54], and it is important to avoid honey dilution to a concentration below that which gives the maximal rate of H2O2 production. Under in vitro conditions, the antibacterial efficacy of honey is determined by a standard procedure with a starting inoculum, which obviously does not correspond with wound conditions, where multiple bacterial species at different loads can be found. In this study, we investigated the capability of honey solutions at MIC values to counteract additional bacterial loads (extra inoculum) added after 4 h of incubation. The accumulated H2O2 in most of the honey solutions at their MIC values could inhibit the growth of additionally inoculated S. aureus, and the MIC values of the honey solutions remained unchanged. In contrast, the MIC value of H2O2 was doubled, from 300 to 600 μM, after the inoculation of additional bacteria, suggesting the importance of the continual slow release of H2O2 in diluted honey. A slow release of H2O2, at low concentrations, may prevent bacterial infections in wounds, and H2O2 at low concentrations is more stable. In another study, a continuous and slow release of H2O2 in diluted honey allowed the development of a hydrogel to achieve a continuous, controlled release of H2O2 for up to 72 h [55]. Also, H2O2 micro-encapsulated hydrogels demonstrated broad-spectrum antimicrobial activity with as little as 10 min of contact time [55].
According to a previous study, H2O2, at micromolar concentrations, exhibited wound healing properties by inducing vascular endothelial growth factor expression in human keratinocytes [56] and facilitating wound angiogenesis [57]. Low levels of H2O2 facilitated the regulation of wound inflammation [58]. Thus, H2O2, at low concentrations, acts as an antibacterial agent with wound healing properties, supporting the use of honey throughout the entire wound healing process.
Conclusions
Analysis of New Caledonian honey samples revealed their strong antibacterial properties, primarily based on the inhibitory action of the generated H2O2. The ability of H2O2 production in diluted honeys was not uniform among the samples. The critical concentration of H2O2 in diluted honeys responsible for inhibiting the growth of S. aureus was 150 μM. On the other hand, H2O2 itself at a concentration of 150 μM in artificial honey solution did not show any inhibitory activity, implying the important role of honey phytochemicals in the antibacterial effect of natural honey. Further research is needed to identify the honey phytochemicals which participate in strong antibacterial effects of New Caledonian honeys. Based on our findings, New Caledonian honeys represents a suitable source for medical-grade honey.
Supporting information
(TIF)
(PDF)
(PDF)
Acknowledgments
We thank New Caledonian beekeepers for providing honey samples.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic and the Slovak Academy of Sciences VEGA 2/0022/22 and the Slovak Research and Development Agency under Contract No. APVV-21-0262. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zay Ya K, Win PTN, Bielicki J, Lambiris M, Fink G. Association between antimicrobial stewardship programs and antibiotic use globally: a systematic review and meta-analysis. JAMA Netw Open. 2023;6: e2253806. doi: 10.1001/jamanetworkopen.2022.53806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magnano San Lio R, Favara G, Maugeri A, Barchitta M, Agodi A. How antimicrobial resistance is linked to climate change: an overview of two intertwined global challenges. Int J Environ Res Public Health. 2023;20: 1681. doi: 10.3390/ijerph20031681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heal CF, Banks JL, Lepper PD, Kontopantelis E, van Driel ML. Topical antibiotics for preventing surgical site infection in wounds healing by primary intention. Cochrane Database Syst Rev. 2016;11: CD011426. doi: 10.1002/14651858.CD011426.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider YK. Bacterial natural product drug discovery for new antibiotics: strategies for tackling the problem of antibiotic resistance by efficient bioprospecting. Antibiotics. 2021;10: 842. doi: 10.3390/antibiotics10070842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdelmohsen UR, Balasubramanian S, Oelschlaeger TA, Grkovic T, Pham NB, Quinn RJ, et al. Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect Dis. 2017;17: e30–e41. doi: 10.1016/S1473-3099(16)30323-1 [DOI] [PubMed] [Google Scholar]
- 6.Saadoun JH, Sogari G, Bernini V, Camorali C, Rossi F, Neviani E, et al. A critical review of intrinsic and extrinsic antimicrobial properties of insects. Trends Food Sci Technol. 2022;122: 40–48. [Google Scholar]
- 7.Abreu AC, McBain AJ, Simoes M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat Prod Rep. 2012;29: 1007–1021. doi: 10.1039/c2np20035j [DOI] [PubMed] [Google Scholar]
- 8.Sadgrove NJ, Jones GL. From Petri dish to patient: bioavailability estimation and mechanism of action for antimicrobial and immunomodulatory natural products. Front Microbiol. 2019;10: 2470. doi: 10.3389/fmicb.2019.02470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JC, Fort CL, Matl CM, Harvey BD, Demke JC, Thomas JR, et al. Effects of essential oils on scars and wound healing: a systematic review. Facial Plast Surg. 2023;39: 173–179. doi: 10.1055/a-1938-0343 [DOI] [PubMed] [Google Scholar]
- 10.Zhang F, Chen Z, Su F, Zhang T. Comparison of topical honey and povidone iodine-based dressings for wound healing: a systematic review and meta-analysis. J Wound Care. 2021;30: S28–S36. doi: 10.12968/jowc.2021.30.Sup4.S28 [DOI] [PubMed] [Google Scholar]
- 11.Hossain ML, Lim LY, Hammer K, Hettiarachchi D, Locher C. Honey-based medicinal formulations: a critical review. Appl Sci. 2021;11: 5159. [Google Scholar]
- 12.Low WL, Kenward K, Britland ST, Amin MC, C M. Essential oils and metal ions as alternative antimicrobial agents: a focus on tea tree oil and silver. Int Wound J. 2017;14: 369–384. doi: 10.1111/iwj.12611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Yan Y, Li Y, Shen C, Zhang Y. Topical effect of benzalkonium bromide on wound healing and potential cellular and molecular mechanisms. Int Wound J. 2021;18: 566–576. doi: 10.1111/iwj.13555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston M, McBride M, Dahiya D, Owusu-Apenten R, Nigam PS. Antibacterial activity of Manuka honey and its components: an overview. AIMS Microbiol. 2018;4: 655–664. doi: 10.3934/microbiol.2018.4.655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albaridi N. Antibacterial potency of honey. Int J Microbiol. 2019;2019: 2464507. doi: 10.1155/2019/2464507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Combarros-Fuertes P, Fresno J, Estevinho M, Sousa-Pimenta M, Tornadijo ME, Estevinho LM. Honey: another alternative in the fight against antibiotic-resistant bacteria? Antibiotics. 2020;9: 774. doi: 10.3390/antibiotics9110774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hulea A, Obiștioiu D, Cocan I, Alexa E, Negrea M, Neacșu AG, et al. Diversity of monofloral honey based on the antimicrobial and antioxidant potential. Antibiotics. 2022;11: 595. doi: 10.3390/antibiotics11050595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mokaya HO, Bargul JL, Irungu JW, Lattorff HMG. Bioactive constituents, in vitro radical scavenging and antibacterial activities of selected Apis mellifera honey from Kenya. Int J Food Sci Technol. 2020;55: 1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin T, Huang L, Cheng N, Wang Y, Ning Z, Huang S, et al. The in vitro and in vivo antibacterial activities of uniflorous honey from a medicinal plant, Scrophularia ningpoensis Hemsl., and characterization of its chemical profile with UPLC-MS/MS. J Ethnopharmacol. 2022;296: 115499. doi: 10.1016/j.jep.2022.115499 [DOI] [PubMed] [Google Scholar]
- 20.Tsavea E, Vardaka FP, Savvidaki E, Kellil A, Kanelis D, Bucekova M, et al. Physicochemical characterization and biological properties of pine honey produced across Greece. Foods. 2022;11: 943. doi: 10.3390/foods11070943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gkoutzouvelidou M, Panos G, Xanthou MN, Papachristoforou A, Giaouris E. Comparing the antimicrobial actions of Greek honeys from the island of Lemnos and manuka honey from New Zealand against clinically important bacteria. Foods. 2021;10: 1402. doi: 10.3390/foods10061402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YZ, Si JJ, Li SS, Zhang GZ, Wang S, Zheng HQ, et al. Chemical analyses and antimicrobial activity of nine kinds of unifloral Chinese honeys compared to manuka honey (12+ and 20+) Molecules. 2021;26: 2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sindi A, Chawn M, Hernandez M, Green K, Islam M, Locher C, et al. Anti-biofilm effects and characterisation of the hydrogen peroxide activity of a range of Western Australian honeys compared to manuka and multifloral honeys. Sci Rep. 2019;9: 17666. doi: 10.1038/s41598-019-54217-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matzen RD, Zinck Leth-Espensen J, Jansson T, Nielsen DS, Lund MN, Matzen S. The antibacterial effect In vitro of honey derived from various Danish flora. Dermatol Res Pract. 2018;2018: 7021713. doi: 10.1155/2018/7021713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bucekova M, Buriova M, Pekarik L, Majtan V, Majtan J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci Rep. 2018;8: 9061. doi: 10.1038/s41598-018-27449-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bucekova M, Jardekova L, Juricova V, Bugarova V, Di Marco G, Gismondi A, et al. Antibacterial activity of different blossom honeys: new findings. Molecules. 2019;24: E1573. doi: 10.3390/molecules24081573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brudzynski K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020;332: 127229. doi: 10.1016/j.foodchem.2020.127229 [DOI] [PubMed] [Google Scholar]
- 28.Roshan N, Rippers T, Locher C, Hammer KA. Antibacterial activity and chemical characteristics of several Western Australian honeys compared to manuka honey and pasture honey. Arch Microbiol. 2017;199: 347–355. doi: 10.1007/s00203-016-1308-3 [DOI] [PubMed] [Google Scholar]
- 29.Kato M, Kawakita A. Plant-pollinator interactions in New Caledonia influenced by introduced honey bees. Am J Bot. 2004;91: 1814–1827. doi: 10.3732/ajb.91.11.1814 [DOI] [PubMed] [Google Scholar]
- 30.Morat P, Jaffre T, Tronchet F, Munzinger J, Pillon Y, Veillon JM, et al. The taxonomic reference base Florical and characteristics of the native vascular flora of New Caledonia. Adansonia. 2012;34: 179–221. [Google Scholar]
- 31.Munzinger J, Morat P, Jaffré T, Gâteblé G, Pillon Y, Rouhan G, et al. FLORICAL: Checklist of the vascular indigenous flora of New Caledonia 2023. [cited 2023]. Available from: http://publish.plantnet-project.org/project/florical. [Google Scholar]
- 32.Erdtman G. Handbook of palynology: morphology-taxonomy-ecology: an introduction to the study of pollen grains and spores. New York: Hafner; 1969. 486 p. [Google Scholar]
- 33.Majtan J, Majtan V. Is manuka honey the best type of honey for wound care? J Hosp Infect. 2010;73: 305–306. doi: 10.1016/j.jhin.2009.08.010 [DOI] [PubMed] [Google Scholar]
- 34.EU Directive. European Commission Council Directive 2001/110/EC of 20 December 2001 relating to honey. 2002: 10–47. [Google Scholar]
- 35.>Codex Alimentarius. Codex standards for honey. Codex Stan 12–1981. 2001; Rev. 1 (1987), Rev. 2 (2001).
- 36.Ferreira ICFR, Aires E, Barreira JCM, Estevinho LM. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009;114: 1438–1443. [Google Scholar]
- 37.Clinical and Laboratory Standards Institute. Methods for dilution of antimicrobial susceptibility tests for bacteria that growth aerobically, 11th edn. CLSI document M07-A11. CLSI/NCCLS M7-A11 Wayne (PA): The Institute. 2018. [Google Scholar]
- 38.Sparacino A, Merlino VM, Blanc S, Borra D, Massaglia S. A choice experiment model for honey attributes: Italian consumer preferences and socio-demographic profiles. Nutrients. 2022;14: 4797. doi: 10.3390/nu14224797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farkasovska J, Bugarova V, Godocikova J, Majtan V, Majtan J. The role of hydrogen peroxide in the antibacterial activity of different floral honeys. Eur Food Res Technol. 2019;245: 2739–2744. [Google Scholar]
- 40.Bang LM, Bantting C, Molan PC. The effects of dilution rate on hydrogen peroxide production in honey and its implications for wound healing. J Altern Complement Med. 2003;9: 267–273. [DOI] [PubMed] [Google Scholar]
- 41.Pasias IN, Kiriakou IK, Kaitatzis A, Koutelidakis AE, Proestos C. Effect of late harvest and floral origin on honey antibacterial properties and quality parameters. Food Chem. 2018;242: 513–518. doi: 10.1016/j.foodchem.2017.09.083 [DOI] [PubMed] [Google Scholar]
- 42.Lehmann DM, Krishnakumar K, Batres MA, Hakola-Parry A, Cokcetin N, Harry E, et al. A cost-effective colourimetric assay for quantifying hydrogen peroxide in honey. Access Microbiol. 2019;1: e000065. doi: 10.1099/acmi.0.000065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sowa P, Grabek-Lejko D, Wesołowska M, Swacha S, Dżugan M. Hydrogen peroxide-dependent antibacterial action of Melilotus albus honey. Lett Appl Microbiol. 2017;65: 82–89. doi: 10.1111/lam.12749 [DOI] [PubMed] [Google Scholar]
- 44.Alygizou A, Grigorakis S, Gotsiou P, Loupassaki S, Calokerinos AC. Quantification of hydrogen peroxide in Cretan honey and correlation with physicochemical parameters. J Anal Methods Chem. 2021;2021: 5554305. doi: 10.1155/2021/5554305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson PG, O’Brien MM, Gadek PA, Quinn CJ. Myrtaceae revisited: a reassessment of infrafamilial groups. Am J Bot. 2001;88: 2013–2025. [PubMed] [Google Scholar]
- 46.Mavric E, Wittmann S, Barth G, Henle T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol Nutr Food Res. 2008;52: 483–489. [DOI] [PubMed] [Google Scholar]
- 47.Adams CJ, Manley-Harris M, Molan PC. The origin of methylglyoxal in New Zealand manuka (Leptospermum scoparium) honey. Carbohydr Res. 2009;344: 1050–1053. [DOI] [PubMed] [Google Scholar]
- 48.Kwakman PH, te Velde AA, De Boer L, Speijer D, Vandenbroucke-Grauls CM, Zaat SA. How honey kills bacteria. FASEB J. 2010;24: 2576–2582. doi: 10.1096/fj.09-150789 [DOI] [PubMed] [Google Scholar]
- 49.Valachova I, Bucekova M, Majtan J. Quantification of bee-derived defensin-1 in honey by competitive enzyme-linked immunosorbent assay, a new approach in honey quality control. Czech J Food Sci. 2016;34: 233–243. [Google Scholar]
- 50.Brudzynski K, Sjaarda C, Lannigan R. MRJP1-containing glycoproteins isolated from honey, a novel antibacterial drug candidate with broad spectrum activity against multi-drug resistant clinical isolates. Front Microbiol. 2015;16: 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masoura M, Passaretti P, Overton TW, Lund PA, Gkatzionis K. Use of a model to understand the synergies underlying the antibacterial mechanism of H2O2-producing honeys. Sci Rep. 2020;10: 17692. doi: 10.1038/s41598-020-74937-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bucekova M, Godocikova J, Kohutova L, Danchenko M, Barath P, Majtan J. Antibacterial activity and bee-derived protein content of honey as important and suitable complementary tools for the assessment of honey quality. J Food Compost Anal. 2023;123: 105610. [Google Scholar]
- 53.Brudzynski K, Abubaker K, Wang T. Powerful bacterial killing by buckwheat honeys is concentration-dependent, involves complete DNA degradation and requires hydrogen peroxide. Front Microbiol. 2012;3: 242. doi: 10.3389/fmicb.2012.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stephen-Haynes J. Considering the best ways to manage wound exudate. Nursing and Residential Care. 2015;17: 668–671. [Google Scholar]
- 55.Dogan‐Guner EM, Mohamed H, Orbey N, Goodyear N. Stabilization and controlled release of micro‐encapsulated hydrogen peroxide for wound treatment applications. J Appl Microbiol. 2019;126: 965–972. doi: 10.1111/jam.14177 [DOI] [PubMed] [Google Scholar]
- 56.Sen CK, Khanna S, Babior BM, Hunt TK, Ellison EC, Roy S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J Biol Chem. 2002;277: 33284–33290. doi: 10.1074/jbc.M203391200 [DOI] [PubMed] [Google Scholar]
- 57.Roy S, Khanna S, Nallu K, Hunt TK, Sen CK. Dermal wound healing is subject to redox control. Mol Ther. 2006;13: 211–220. doi: 10.1016/j.ymthe.2005.07.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu G, Wang Q, Lu S, Niu Y. Hydrogen peroxide: a potential wound therapeutic target. Med Princ Pract. 2017;26: 301–308. doi: 10.1159/000475501 [DOI] [PMC free article] [PubMed] [Google Scholar]