Abstract
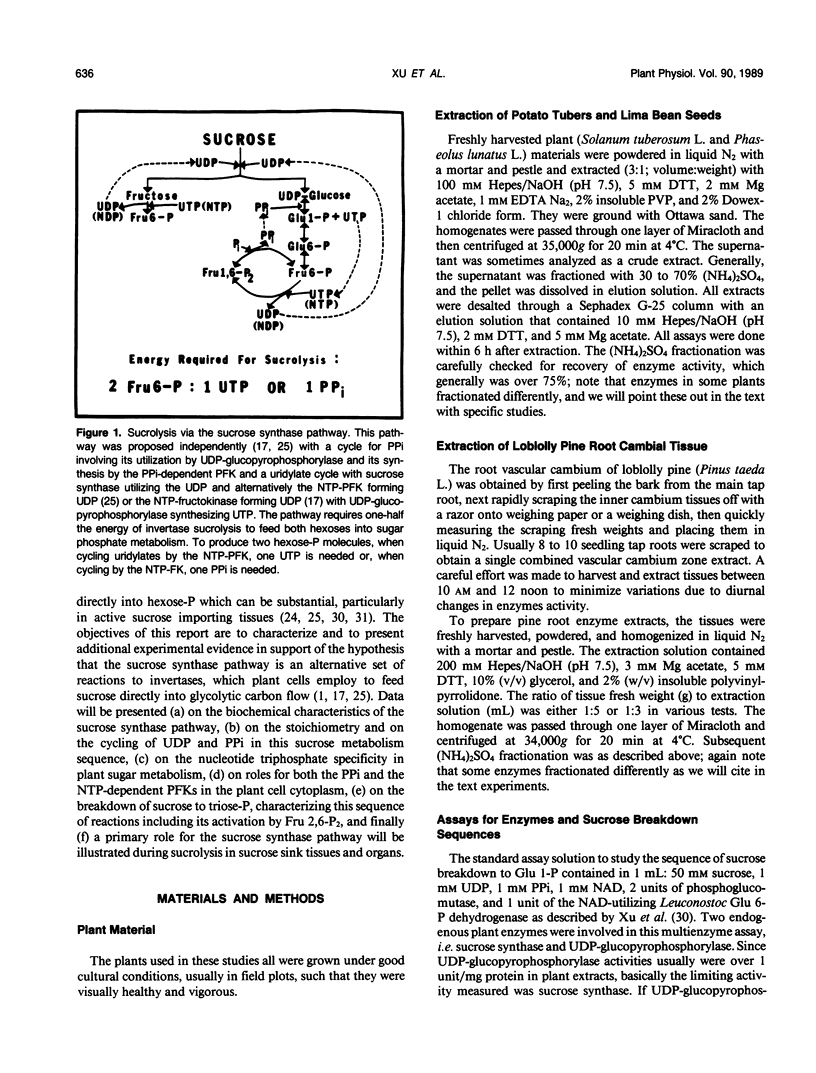
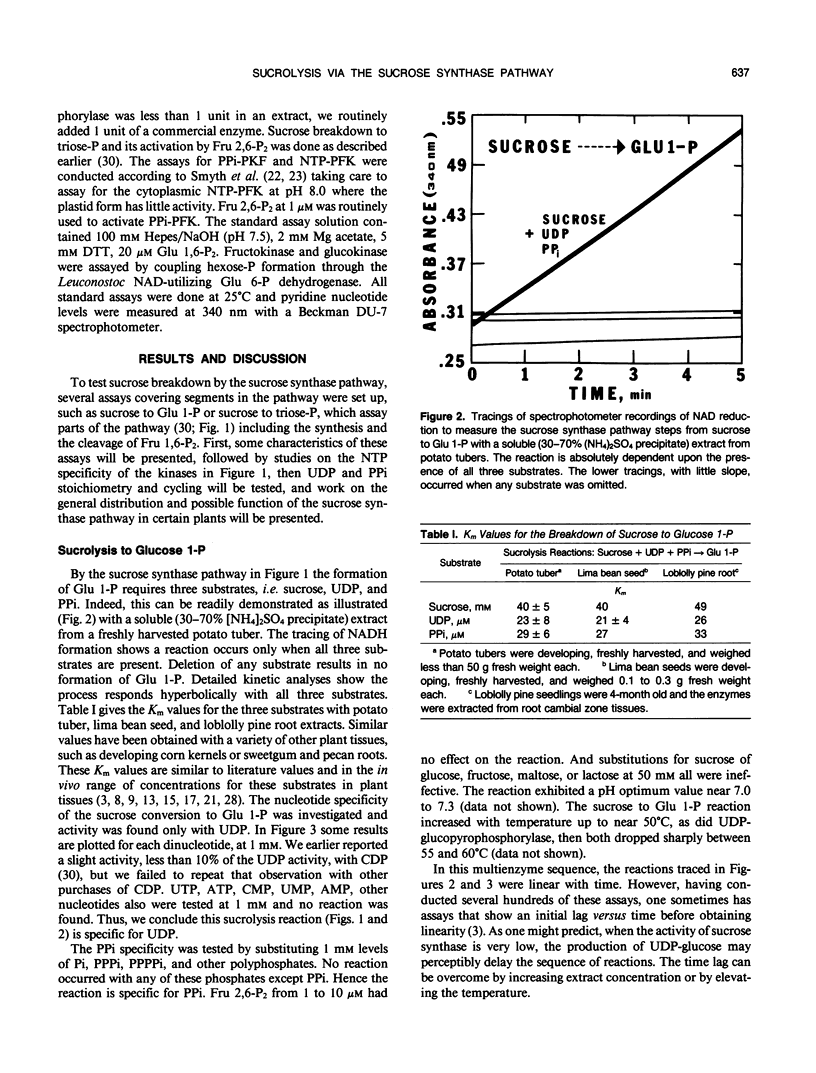
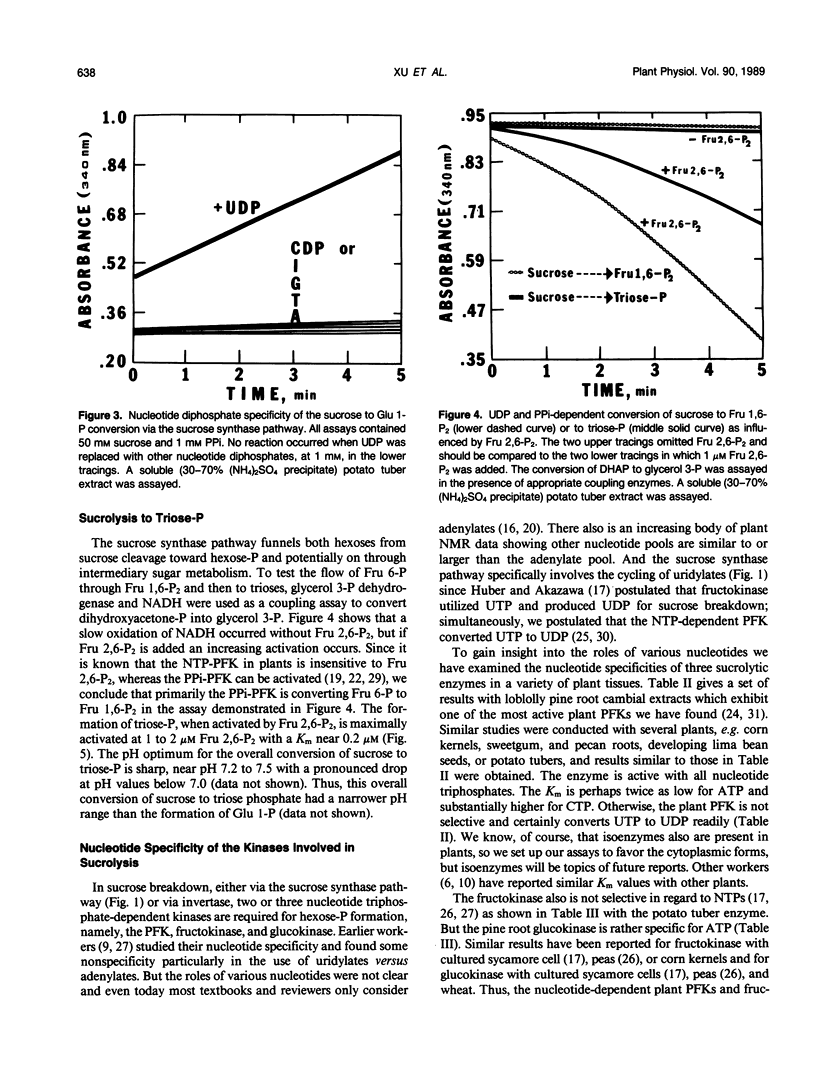
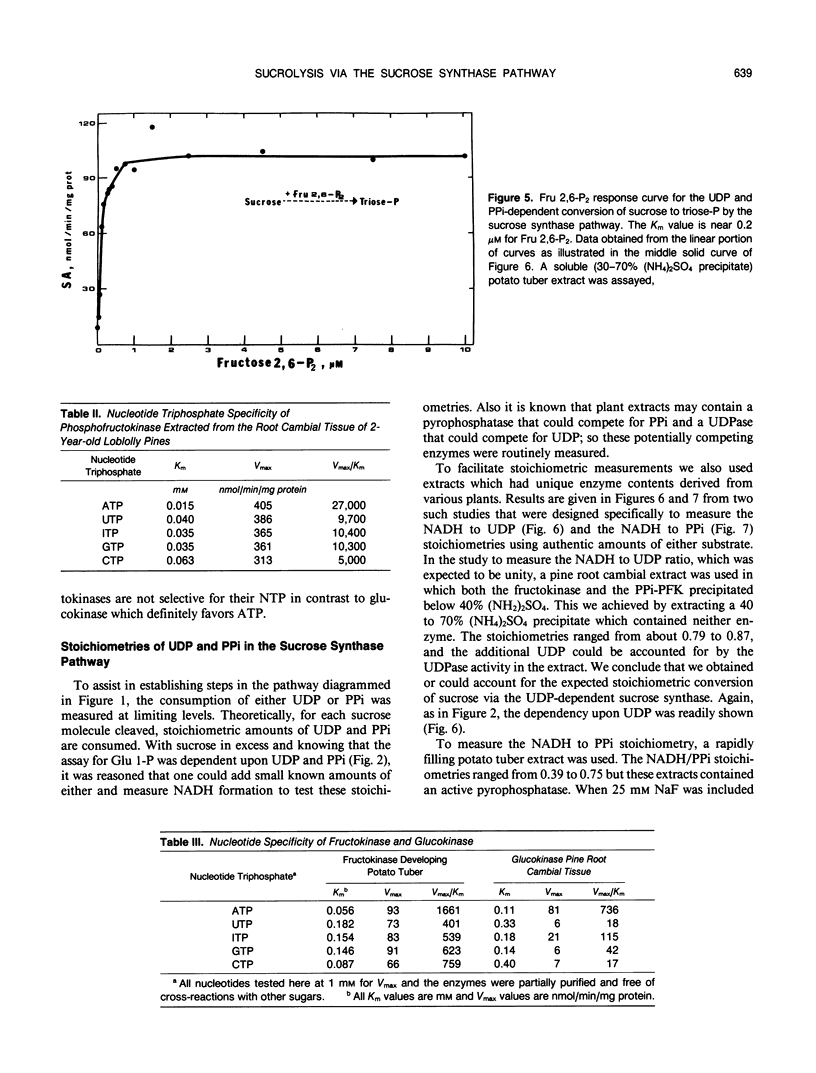
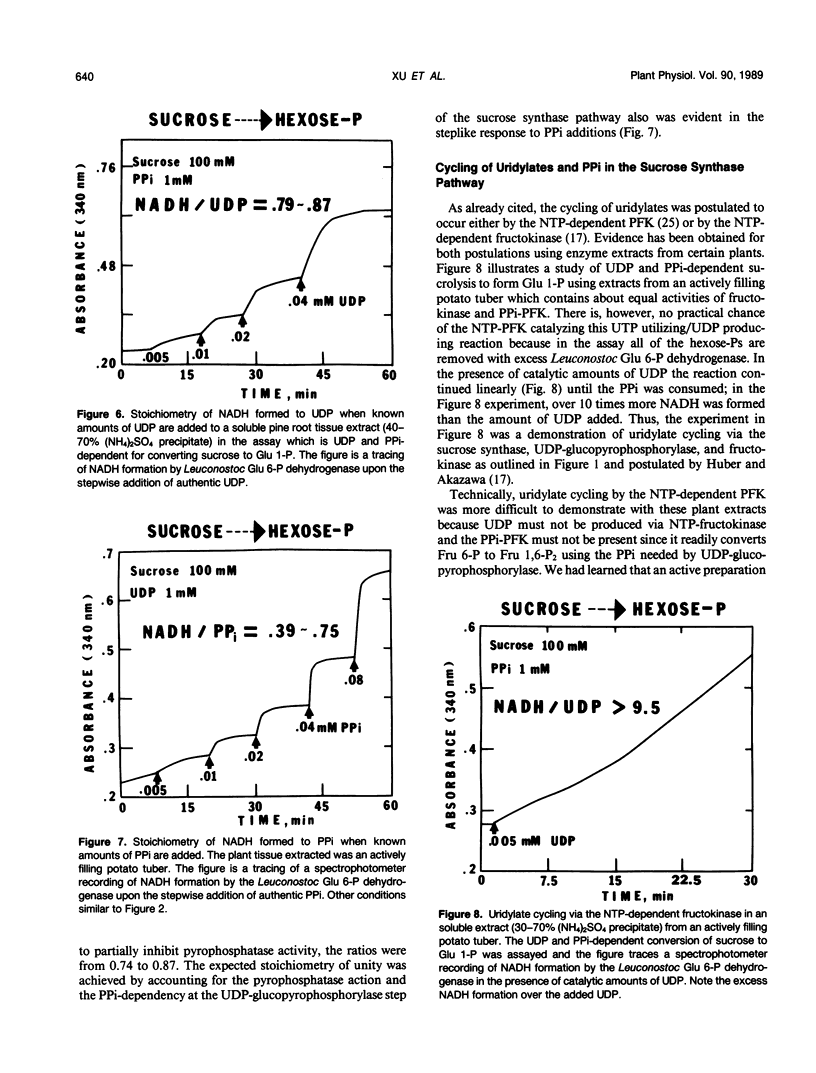
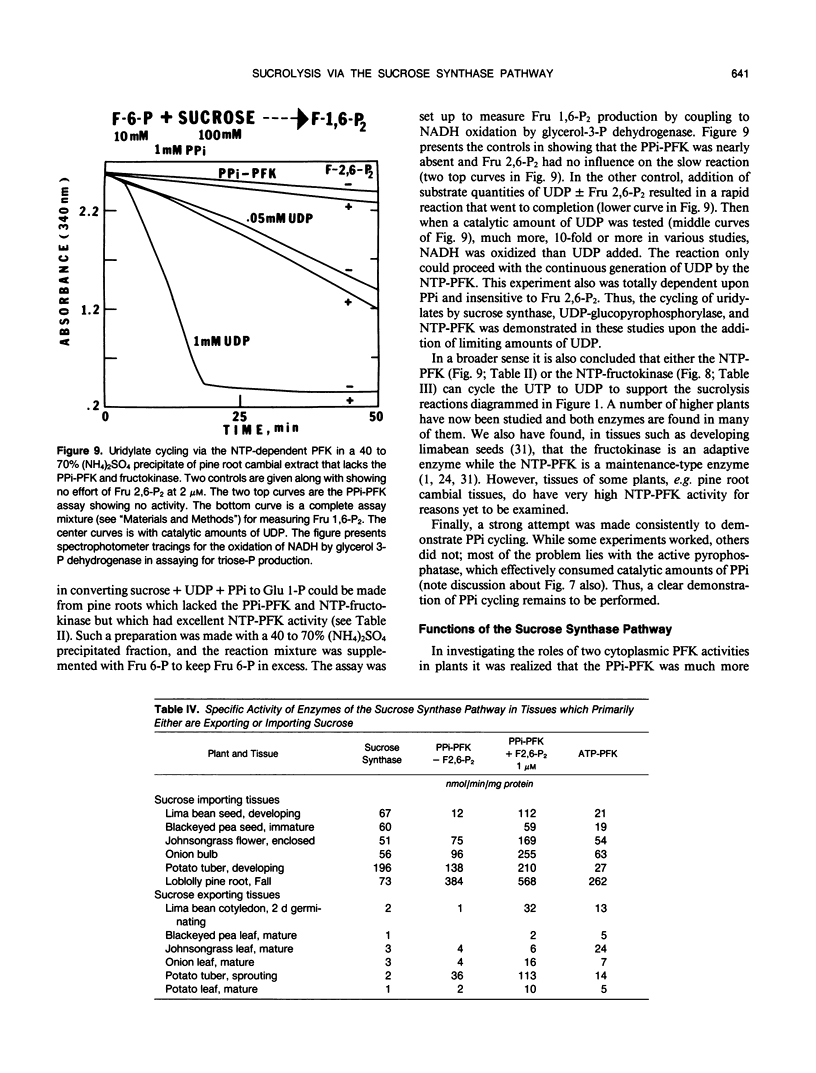
The breakdown of sucrose to feed both hexoses into glycolytic carbon flow can occur by the sucrose synthase pathway. This uridine diphosphate (UDP) and pyrophosphate (PPi)-dependent pathway was biochemically characterized using soluble extracts from several plants. The sucrolysis process required the simultaneous presence of sucrose, UDP, and PPi with their respective Km values being about 40 millimolar, 23 micromolar, and 29 micromolar. UDP was the only active nucleotide diphosphate. Slightly alkaline pH optima were observed for sucrose breakdown either to glucose 1-phosphate or to triose phosphate. Sucrolysis incrased with increasing temperature to near 50°C and then a sharp drop occurred between 55 and 60°C. The breakdown of sucrose to triose-P was activated by fructose 2,6-P2 which had a Km value near 0.2 micromolar. The cytoplasmic phosphofructokinase and fructokinase in plants were fairly nonselective for nucleotide triphosphates (NTP) but glucokinase definitely favored ATP. A predicted stoichiometric relationship of unity for UDP and PPi was measured when one also measured competing UDPase and pyrophosphatase activity. The cycling of uridylates, UDP to UTP to UDP, was demonstrated both with phosphofructokinase and with fructokinase. Enzyme activity measurements indicated that the sucrose synthase pathway has a major role in plant sucrose sink tissues. In the cytoplasmic sucrose synthase breakdown pathway, a role for the PPi-phosphofructokinase was to produce PPi while a role for the NTP-phosphofructokinase and for the fructokinase was to produce UDP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carnal N. W., Black C. C. Phosphofructokinase activities in photosynthetic organisms : the occurrence of pyrophosphate-dependent 6-phosphofructokinase in plants and algae. Plant Physiol. 1983 Jan;71(1):150–155. doi: 10.1104/pp.71.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnal N. W., Black C. C. Pyrophosphate-dependent 6-phosphofructokinase, a new glycolytic enzyme in pineapple leaves. Biochem Biophys Res Commun. 1979 Jan 15;86(1):20–26. doi: 10.1016/0006-291x(79)90376-0. [DOI] [PubMed] [Google Scholar]
- Dale E. M., Housley T. L. Sucrose synthase activity in developing wheat endosperms differing in maximum weight. Plant Physiol. 1986 Sep;82(1):7–10. doi: 10.1104/pp.82.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P. The Regulatory Properties of Purified Phaseolus aureus Sucrose Synthetase. Plant Physiol. 1972 Oct;50(4):469–472. doi: 10.1104/pp.50.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P. The Regulatory Properties of Purified Phaseolus aureus Sucrose Synthetase. Plant Physiol. 1972 Oct;50(4):469–472. doi: 10.1104/pp.50.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson G. L., Gander J. E. Uridine diphosphate glucose pyrophosphorylase from Sorghum vulgare. Purification and kinetic properties. J Biol Chem. 1972 Mar 10;247(5):1387–1397. [PubMed] [Google Scholar]
- Huber S. C., Akazawa T. A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol. 1986 Aug;81(4):1008–1013. doi: 10.1104/pp.81.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. A., Black C. C. Measurement of the pyrophosphate content of plant tissues. Plant Physiol. 1984 Jul;75(3):862–864. doi: 10.1104/pp.75.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. A., Wu M. X., Black C. C. Pyrophosphate and fructose 2,6-bisphosphate effects on glycolysis in pea seed extracts. Plant Physiol. 1984 Oct;76(2):316–320. doi: 10.1104/pp.76.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. J., Xu D. P., Black C. C. Identification of actively filling sucrose sinks. Plant Physiol. 1989 Apr;89(4):1117–1121. doi: 10.1104/pp.89.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. F., Harrison D. D., Copeland L. Fructokinase (Fraction IV) of Pea Seeds. Plant Physiol. 1977 Nov;60(5):666–669. doi: 10.1104/pp.60.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Smyth D. A., Black C. C. Regulation of pea seed pyrophosphate-dependent phosphofructokinase: Evidence for interconversion of two molecular forms as a glycolytic regulatory mechanism. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5051–5055. doi: 10.1073/pnas.81.16.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D. P., Sung S. J., Alvarez C. A., Black C. C. Pyrophosphate-dependent sucrose metabolism and its activation by fructose 2,6-bisphosphate in sucrose importing plant tissues. Biochem Biophys Res Commun. 1986 Dec 15;141(2):440–445. doi: 10.1016/s0006-291x(86)80192-9. [DOI] [PubMed] [Google Scholar]
- Xu D. P., Sung S. J., Black C. C. Sucrose metabolism in lima bean seeds. Plant Physiol. 1989 Apr;89(4):1106–1116. doi: 10.1104/pp.89.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]


