Abstract
Background
Buprenorphine maintenance treatment has been evaluated in randomised controlled trials against placebo medication, and separately as an alternative to methadone for management of opioid dependence.
Objectives
To evaluate buprenorphine maintenance compared to placebo and to methadone maintenance in the management of opioid dependence, including its ability to retain people in treatment, suppress illicit drug use, reduce criminal activity, and mortality.
Search methods
We searched the following databases to January 2013: Cochrane Drugs and Alcohol Review Group Specialised Register, Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, Current Contents, PsycLIT, CORK, Alcohol and Drug Council of Australia, Australian Drug Foundation, Centre for Education and Information on Drugs and Alcohol, Library of Congress, reference lists of identified studies and reviews. We sought published/unpublished randomised controlled trials (RCTs) from authors.
Selection criteria
Randomised controlled trials of buprenorphine maintenance treatment versus placebo or methadone in management of opioid‐dependent persons.
Data collection and analysis
We used Cochrane Collaboration methodology.
Main results
We include 31 trials (5430 participants), the quality of evidence varied from high to moderate quality.
There is high quality of evidence that buprenorphine was superior to placebo medication in retention of participants in treatment at all doses examined. Specifically, buprenorphine retained participants better than placebo: at low doses (2 ‐ 6 mg), 5 studies, 1131 participants, risk ratio (RR) 1.50; 95% confidence interval (CI) 1.19 to 1.88; at medium doses (7 ‐ 15 mg), 4 studies, 887 participants, RR 1.74; 95% CI 1.06 to 2.87; and at high doses (≥ 16 mg), 5 studies, 1001 participants, RR 1.82; 95% CI 1.15 to 2.90. However, there is moderate quality of evidence that only high‐dose buprenorphine (≥ 16 mg) was more effective than placebo in suppressing illicit opioid use measured by urinanalysis in the trials, 3 studies, 729 participants, standardised mean difference (SMD) ‐1.17; 95% CI ‐1.85 to ‐0.49, Notably, low‐dose, (2 studies, 487 participants, SMD 0.10; 95% CI ‐0.80 to 1.01), and medium‐dose, (2 studies, 463 participants, SMD ‐0.08; 95% CI ‐0.78 to 0.62) buprenorphine did not suppress illicit opioid use measured by urinanalysis better than placebo.
There is high quality of evidence that buprenorphine in flexible doses adjusted to participant need,was less effective than methadone in retaining participants, 5 studies, 788 participants, RR 0.83; 95% CI 0.72 to 0.95. For those retained in treatment, no difference was observed in suppression of opioid use as measured by urinalysis, 8 studies, 1027 participants, SMD ‐0.11; 95% CI ‐0.23 to 0.02 or self report, 4 studies, 501 participants, SMD ‐0.11; 95% CI ‐0.28 to 0.07, with moderate quality of evidence.
Consistent with the results in the flexible‐dose studies, in low fixed‐dose studies, methadone (≤ 40 mg) was more likely to retain participants than low‐dose buprenorphine (2 ‐ 6 mg), (3 studies, 253 participants, RR 0.67; 95% CI: 0.52 to 0.87). However, we found contrary results at medium dose and high dose: there was no difference between medium‐dose buprenorphine (7 ‐ 15 mg) and medium‐dose methadone (40 ‐ 85 mg) in retention, (7 studies, 780 participants, RR 0.87; 95% CI 0.69 to 1.10) or in suppression of illicit opioid use as measured by urines, (4 studies, 476 participants, SMD 0.25; 95% CI ‐0.08 to 0.58) or self report of illicit opioid use, (2 studies, 174 participants, SMD ‐0.82; 95% CI ‐1.83 to 0.19). Similarly, there was no difference between high‐dose buprenorphine (≥ 16 mg) and high‐dose methadone (≥ 85 mg) in retention (RR 0.79; 95% CI 0.20 to 3.16) or suppression of self‐reported heroin use (SMD ‐0.73; 95% CI ‐1.08 to ‐0.37) (1 study, 134 participants).
Few studies reported adverse events ; two studies compared adverse events statistically, finding no difference between methadone and buprenorphine, except for a single result indicating more sedation among those using methadone.
Authors' conclusions
Buprenorphine is an effective medication in the maintenance treatment of heroin dependence, retaining people in treatment at any dose above 2 mg, and suppressing illicit opioid use (at doses 16 mg or greater) based on placebo‐controlled trials.
However, compared to methadone, buprenorphine retains fewer people when doses are flexibly delivered and at low fixed doses. If fixed medium or high doses are used, buprenorphine and methadone appear no different in effectiveness (retention in treatment and suppression of illicit opioid use); however, fixed doses are rarely used in clinical practice so the flexible dose results are more relevant to patient care. Methadone is superior to buprenorphine in retaining people in treatment, and methadone equally suppresses illicit opioid use.
Plain language summary
Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence
Background
Methadone is widely used as a replacement for illicit opioid use such as heroin in medically‐supported opioid substitution maintenance programmes. Two other drugs have been used to help reduce illicit opioid use, specifically buprenorphine and LAAM (levo‐alpha‐acetylmethadol). LAAM is not used in current clinical practice. Buprenorphine is currently used and can reduce illicit opioid use compared with placebo, although it is less effective than methadone. Buprenorphine is an opioid drug that is not as potent as heroin and methadone, although the effects of buprenorphine may last longer. Buprenorphine can be taken once every two days. The trials include different formulations of buprenorphine: sublingual solution, sublingual tablets, combined buprenorphine/naloxone sublingual tablet and an implant.
Key results
The review of trials found that buprenorphine at high doses (16 mg) can reduce illicit opioid use effectively compared with placebo, and buprenorphine at any dose studied retains people in treatment better than placebo.
Buprenorphine appears to be less effective than methadone in retaining people in treatment, if prescribed in a flexible dose regimen or at a fixed and low dose (2 ‐ 6 mg per day). Buprenorphine prescribed at fixed doses (above 7 mg per day) was not different from methadone prescribed at fixed doses (40 mg or more per day) in retaining people in treatment or in suppression of illicit opioid use.
Summary of findings
Summary of findings for the main comparison. Summary of findings: comparison between flexible‐dose methadone and buprenorphine.
| Buprenorphine maintenance compared with methadone maintenance for opioid dependence | ||||
|
Patient or population: People with opioid dependence. Settings: Inpatient and outpatient Intervention: Buprenorphine maintenance at flexible doses Comparison: Methadone maintenance at flexible doses | ||||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Retention in treatment | RR 0.83 (0.73 to 0.95)* |
11 (1391); 5 double‐blind (788) 6 open‐label (603) |
⊕⊕⊕⊕ high | Greater retention in the methadone group. * the heterogeneity is significant. When the double‐blind studies are analysed separately the heterogeneity is not significant and the RR is 0.83 (0.72 to 0.95) |
| Morphine‐positive urines | SMD ‐0.11 (‐0.23 to 0.02) |
8 (1027) | ⊕⊕⊕⊝ moderate | No difference. |
| Self‐reported heroin use | SMD ‐0.11 (‐0.28 to 0.07) |
4 (501) | ⊕⊕⊕⊝ moderate | No difference. |
| Cocaine‐positive urines | SMD 0.10 (‐0.05 to 0.25) |
6 (919) | ⊕⊕⊕⊝ moderate | No difference. |
| Benzodiazepine‐positive urines | SMD 0.05 (‐0.09 to 0.18] |
6 (859) | ⊕⊕⊕⊝ moderate | No difference. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
Summary of findings 2. Summary of findings for the comparison between high‐dose buprenorphine and placebo.
| Buprenorphine maintenance compared with methadone maintenance for opioid dependence | ||||
|
Patient or population: People with opioid dependence. Settings: Inpatient and outpatient Intervention: Buprenorphine maintenance at high doses (16 mg) Comparison: Placebo | ||||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Retention in treatment | RR 1.82 (1.15 to 2.90) |
1001 (5) | ⊕⊕⊕⊕ high | Greater retention in buprenorphine group. |
| Morphine‐positive urines | SMD ‐1.17 (‐1.85 to ‐0.49) |
729 (3) | ⊕⊕⊕⊝ moderate | Fewer morphine‐positive urines in buprenorphine group. |
| Benzodiazepine‐positive urines | SMD ‐1.65 (‐4.94 to 1.65) |
336 (2) | ⊕⊕⊕⊝ moderate | No difference. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
Background
Description of the condition
Opioids are a class of compounds that elicit analgesic effect by binding to opioid receptors within the central and peripheral nervous system. Opioids include natural opiates such as opium (from the poppy) and morphine, and natural derivatives or synthetic compounds such as heroin (diacetylmorphine), oxycodone, buprenorphine and methadone.
Opioids produce euphoria and have been associated with recreational use. They are generally consumed by injection or inhalation of the fumes produced by heating. Regular use of opioids can lead to opioid dependence. Opioid dependence is a medical diagnosis that involves the inability to cease using opioids. The DSM‐IV criteria for dependence (cited here as the studies predominantly use DSM‐IV, whereas DSM‐V was released in 2013) require three or more of the following features:
A strong desire or sense of compulsion to take the drug;
Difficulties in controlling drug‐taking behaviour in terms of its onset, termination, or levels of use;
A physiological withdrawal state when drug use is stopped or reduced, as evidenced by: the characteristic withdrawal syndrome for the substance; or use of the same (or a closely related) substance with the intention of relieving or avoiding withdrawal symptoms;
Evidence of tolerance, such that increased doses of the drug are required in order to achieve effects originally produced by lower doses;
Progressive neglect of alternative pleasures or interests because of drug use, increased amount of time necessary to obtain or take the drug or to recover from its effects;
Persisting with drug use despite clear evidence of overtly harmful consequences, such as harm to the liver, depressive mood states or impairment of cognitive functioning.
The illegality of opioids such as heroin precludes the accurate assessment of how many people use these drugs, but recent estimates suggest there are between 15 and 39 million problem opioid users worldwide (Degenhardt 2012), and although the prevalence of opioid dependence is low (0.6 ‐ 0.8% of the global population; UNODC 2012), the burden to the individual and the community is significant. Illicit opioid dependence is a significant public health problem with heroin use associated with the spread of infectious disease (e.g., HIV, hepatitis B and C) and overdose deaths (Degenhardt 2011; Mathers 2008; Nelson 2011).
Description of the intervention
Dole and Nyswander defined opioid dependence as a "physiological disease characterised by a permanent metabolic deficiency" which was best managed by administering the opioid‐dependent person "a sufficient amount of drug to stabilise the metabolic deficiency" (Dole 1965). In the early 1960s they introduced orally‐administered maintenance doses of the synthetic opioid drug methadone as a treatment for opioid dependence. Maintenance treatment is designed to be an ongoing treatment. The substitution of legal opioids in known doses and purity provides an opportunity to stabilise the person by eliminating withdrawal, craving, participation in obtaining illegal opioids and use of needles.
Methadone maintenance treatment (MMT) has been one of the main forms of treatment for opioid dependence. As described elsewhere (Faggiano 2003; Mattick 1998; Mattick 2009), maintenance treatment with oral methadone appears to be an effective and accepted intervention for illicit opioid (heroin) dependence, and it is widely used in some countries. Yet MMT has a number of negative characteristics which potentially influence its effectiveness and which have led to an interest in alternative pharmacotherapies and methods of treatment delivery (Mattick 1998). The negative aspects of methadone are set out below.
One negative aspect of methadone is its potential to produce and/or maintain dependence on opioids, such that people experience withdrawal if a daily dose is missed, and detoxification can be a lengthy and difficult process which can discourage people from attempting withdrawal (of course, withdrawal from heroin and other opioids have similar problems). Additionally, because methadone is a full opioid agonist, there is no ceiling to the level of respiratory depression or sedation which methadone can induce, and methadone overdose can therefore be fatal (Drummer 1992). Although it is a long‐acting opioid, in some countries and settings, the inconvenience of daily dosing and clinic visits may be unattractive to clients, and restrictions imposed by the daily dosing schedule on clients' general lifestyle and on opportunities to sustain employment may also limit its acceptance to heroin users. The provision of takeaway doses of methadone results in problems of diversion of the drug for illicit use by those not in treatment, although the extent of this problem varies across countries. Finally, heroin users have developed their own 'lore' regarding methadone's negative effects, although their views may not always be accurate or favourable. Thus, despite its many advantages, methadone maintenance appears to have limited suitability for some people. In some countries there may be restrictions on the use of methadone as far as doses and duration are concerned, and this may impair adequate clinical practice. These factors may restrict the ability of methadone to attract certain users into treatment, and the examination of alternative medications to broaden the range of pharmacotherapies has been the focus of research over recent years (e.g., Ling 2003; Mitchell 2004).
How the intervention might work
There are a number of alternatives to methadone as a maintenance agent in the management of opioid dependence, as all opioids show cross‐tolerance. The most promising of these involve pharmacotherapies which treat people with a pharmaceutical‐grade opioid which has a long duration of action. These include the opiate partial agonist buprenorphine, the full agonist levo‐alpha‐acetylmethadol (LAAM) (Clark 2002; Johnson 2000) and sustained released morphine sulphate (Mitchell 2004; White 2007). LAAM is no longer used in clinical practice. In addition, heroin‐assisted treatment has been used in the management of opioid dependence, most notably in Switzerland (Perneger 1998).
This review focuses on the role of buprenorphine as a maintenance therapy in the management of opioid dependence. Buprenorphine is a potent synthetic opioid analgesic initially used for the management of acute pain. Pharmacologically, buprenorphine causes morphine‐like subjective effects and produces cross‐tolerance to other opioids.
Unlike methadone and heroin (which are full agonists), buprenorphine is a partial agonist and exerts weaker opioid effects at opioid receptor sites. This partial agonist action appears to make buprenorphine safer in overdose. Other benefits of buprenorphine may include an easier withdrawal phase and, because of the longer duration of action, the option of alternate‐day dosing.
It was during the initial development of buprenorphine as an analgesic in the 1970s that its potential utility as a substitution agent in the treatment of opioid dependence was recognised. Early work (Jasinski 1978) using buprenorphine administered by the subcutaneous route, characterised it as an opioid with low physical dependence liability with a minimal withdrawal syndrome. Subsequently, others (Fudala 1990) provided evidence that buprenorphine does produce a mild to moderate mu‐agonist withdrawal syndrome. It was thought that at doses somewhat greater than those used for analgesia it could be used in the treatment of opioid dependence (Jasinski 1978). Since that time a substantial international research effort has addressed the efficacy of buprenorphine maintenance therapy in randomised controlled trials (RCTs).
Why it is important to do this review
Clinical trials conducted in the USA. showed buprenorphine to be superior to placebo medication, but when buprenorphine and methadone maintenance were compared in a series of impressive studies using fixed doses of the drugs, the results were mixed. Some of the fixed‐dose studies showed no difference in efficacy, whereas others showed superiority for methadone, and yet others showed the reverse pattern. The investigators in these fixed‐dose studies frequently concluded that the doses of buprenorphine or methadone chosen were too low, or that poor induction regimens led to poor retention and affected trial results. A series of variable‐ (or flexible‐) dose studies have been conducted and shown essentially equivalent results for the two drugs. In these flexible‐dose studies, dose is adjusted to individual need rather than participants being randomly assigned to a set and unchanging dose, as in the fixed‐dose studies.
Given the mixed results of the early studies, it is important to attempt a systematic integration of the literature. Of particular importance is separately assessing the fixed‐ and flexible‐dose studies and considering the results in the light of the differing doses and other individual trial features. Additionally, this review separately summarises the available placebo‐controlled trial results.
This is an update of a Cochrane review, incorporating additional studies, which was first published in 2002 and updated in 2008.
Objectives
To evaluate buprenorphine maintenance compared to placebo and to methadone maintenance in the management of opioid dependence, including its ability to retain people in treatment, suppress illicit drug use, reduce criminal activity, and mortality.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials of buprenorphine maintenance versus methadone maintenance or versus placebo medication in the management of opioid dependence.
Types of participants
Individuals dependent on heroin or other opioids. We made no distinction between those using heroin and those in methadone treatment prior to entering the research trial treatment. We excluded trials of pregnant women, as a separate review of opioid maintenance therapy for pregnant women has been completed (Minozzi 2013).
Types of interventions
Experimental interventions: Buprenorphine maintenance therapy (BMT) using doses above 1 mg ("which was adopted to serve essentially as a placebo" dose in the context of heroin/opioid dependence (Ling 1998, p.477)), using sublingual tablets, an ethanol‐based solution and more recently implants, containing buprenorphine.
Control Interventions: Methadone maintenance therapy (MMT) with doses of 20 mg methadone per day or higher, or placebo, or 1 mg of buprenorphine per day (as adopted by Ling to "serve essentially as a placebo" dose in one study (Ling 1998, p.477)).
We excluded studies using methadone or buprenorphine for detoxification without a maintenance phase.
Types of outcome measures
Primary outcomes
Retention in treatment as measured by intention‐to‐treat (i.e., the number of participants still in treatment at the end of the study);
Use of opioids as measured by: a) urinalysis results positive for heroin metabolite (i.e., morphine); b) self‐reported heroin use;
Use of other substances of abuse as measured by: a) urinalysis results positive for cocaine; b) urinalysis results positive for benzodiazepines;
Criminal activity as measured by self report;
Mortality.
Secondary outcomes
Physical health;
Psychological health;
Adverse effects of medication.
Search methods for identification of studies
Electronic searches
In consultation with a drug and alcohol research information specialist, we developed a specific search strategy for each database searched with no language restrictions. We searched the Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Library, 2013, Issue 1, PubMed (January 2003 to January 2013), and EMBASE (January 2003 to January 2013); seeAppendix 1; Appendix 2; and Appendix 3.
As several drug and alcohol journals are not indexed on the main electronic databases, we also searched the following databases:
Current Contents;
PsycLIT;
CORK [www.projectcork.org/database_search/search_form.html];
Alcohol and Drug Council of Australia (ADCA) [www.adca.org.au];
Australian Drug Foundation (ADF ‐VIC) [www.adf.org.au/];
Centre for Education and Information on Drugs and Alcohol (CEIDA) [www.ceida.net.au/];
Australian Bibliographic Network (ABN).
Searching other resources
We also searched the following:
Some of the main electronic sources of ongoing trials (National Research Register, meta‐Register of Controlled Trials; Clinical Trials.gov; Agenzia Italiana del Farmaco);
Conference proceedings likely to contain trials relevant to the review (US College on Problems of Drug Dependence ‐ CPDD);
Library of Congress databases, for studies and book chapters with the key terms: buprenorphine, methadone, clinical trial, and randomised control trial;
National focal points for drug research (e.g., National Institute of Drug Abuse (NIDA), National Drug & Alcohol Research Centre (NDARC));
Reference lists of all relevant papers to identify further studies.
We consulted authors of identified RCTs for any other published or unpublished RCTs comparing the efficacy of buprenorphine and methadone maintenance as therapies for opioid dependence.
Data collection and analysis
Selection of studies
We obtained each potentially relevant study located in the search, and two of four review authors independently assessed it for inclusion. Studies were eligible irrespective of publication status or language of publication.
Data extraction and management
The two review authors who selected a study for inclusion then independently extracted the data for that study. Each review author assessed the same number of studies. We used a standardised checklist or data extraction, with a third review author acting as arbiter in cases of disagreement, and unresolved disagreements on inclusion, study quality or extraction being referred to the editor. Where required, we sought missing or clarifying information by contacting study authors. We handled multi‐arm studies (e.g., Fudala 2003) by combining relevant groups and avoiding double‐counting of participants. Measurement scales were compatible across studies for the main outcomes, with dichotomous data or means and standard deviations being used in all cases.
Assessment of risk of bias in included studies
We assessed the new studies included in this updated version and reassessed the studies already included in the existing review, using the criteria and the method indicated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The recommended approach for assessing risk of bias in studies included in Cochrane reviews is a two‐part tool, addressing six specific domains (namely randomisation sequence generation, randomisation allocation concealment, blinding of participants and assessors, incomplete outcome data, selective reporting and other potential biases). The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement, in terms of 'low', 'high' or 'unclear', relating to the risk of bias for that entry. To make these judgements we used the criteria indicated by Chapter 8 of theCochrane Handbook and their applicability to the addiction field.
For this updated review, we considered the following domains to be relevant: sequence generation, allocation concealment (avoidance of selection bias), blinding of participants, personnel and outcome assessor (performance and detection bias) and incomplete outcome data (attrition bias). See Appendix 4 for a detailed description of the criteria used. As sequence generation and allocation concealment were adequately described in only a minority of trials (leaving it unclear whether the process was or was not adequate), it was not possible to meaningfully analyse trials by stratifying on these randomisation variables. To address the better‐reported blinded/unblinded status, we report where possible the open‐label (unblinded) studies separately from the blinded comparisons. Where incomplete data were reported (especially in the case of urine analysis of ongoing drug use), we wrote to the authors of the study seeking those data and included them where available.
Measures of treatment effect
We calculated a standardised effect size for each study, based on the outcome measure reported. As the retention‐in‐treatment data are a dichotomous outcome, we calculated the risk ratio (RR) and its 95% confidence interval (CI). We estimated a standardised mean difference for continuous outcomes (urine results, self‐reported heroin use, and criminal activity). Urine data were provided in reports (or by authors in response to our request) in the form of the average number of positive urines and a standard deviation, by treatment group.
Unit of analysis issues
The urine data are presented as a continuous outcome measure but are based on data requested directly from authors. This was necessary as urine results in the literature are routinely reported as the percentage of urine samples collected per treatment group that were positive or negative for a given drug (e.g., heroin) across the study period. These 'count data' are not compatible with the analysable data fields in Review Manager 5 (RevMan) (i.e., continuous, dichotomous, individual patient data). Based on advice provided by Cochrane statisticians, we asked the study authors to calculate the number of positive urines for each participant in each treatment group and derive a mean number of positive urines with a standard deviation, allowing for analysis of urine results as continuous data. These additional data were not available for four studies at the time of writing this review, and urine results are therefore not presented for these studies (Kosten 1993; Neri 2005; Oliveto 1999; Pani 2000,).
Assessment of heterogeneity
We assessed statistically significant heterogeneity among primary outcome studies with the Chi² test and I² heterogeneity test (Higgins 2003). A significant Chi² ( P < 0.05) and I² of at least 50% was considered as statistical heterogeneity.
Data synthesis
We derived the pooled effect size estimate for each domain of measurement (retention in treatment, urine analysis results for heroin/morphine, urine analysis results for cocaine, and urine analysis results for benzodiazepines). Given the diverse treatment settings and countries of the studies involved, we used a random‐effects model.
We integrated the results from the meta‐analytic review into a discussion, taking into consideration other publications such as studies of the pharmacology of methadone and buprenorphine. We took convergence of evidence from the meta‐analysis and from the narrative review to indicate a robust conclusion.
Results
Description of studies
See the Characteristics of included studies table.
Evidence on the efficacy of buprenorphine has come from placebo‐controlled trials (Ahmadi 2002a; Ahmadi 2003b; Ahmadi 2004; Fudala 2003; Johnson 1995a; Kakko 2003; Kakko 2007; Krook 2002; Ling 1998; Ling 2010; Schottenfeld 2008), from fixed‐dosing studies of buprenorphine versus methadone maintenance treatment (Ahmadi 2003a; Ahmadi 2003b; Bickel 1988; Fischer 1999; Johnson 1992; Kosten 1993; Kristensen 2005; Ling 1996; Oliveto 1999; Pani 2000; Schottenfeld 2005; Uehlinger 1998) and from variable‐ or flexible‐dosing studies of buprenorphine versus methadone maintenance treatment (Fischer 1999; Johnson 2000; Kristensen 2005; Lintzeris 2004; Magura 2009; Mattick 2003; Neri 2005; Petitjean 2001; Soyka 2008a; Strain 1994a; Strain 1994b). Many of the earlier studies used the sublingual solution formulation (Johnson 1992; Johnson 1995a; Ling 1996; Ling 1998; Oliveto 1999; Schottenfeld 1997; Schottenfeld 2005; Strain 1994a; Strain 1994b) and a few more recent studies have used the combined buprenorphine‐naloxone tablet (Fudala 2003; Kakko 2007; Kamien 2008; Magura 2009). One study used buprenorphine implant formulation (Ling 2010). The remaining sixteen studies used the sublingual tablet formulation.
Results of the search
We identified 6495 studies through the electronic and other searches. Of these, we discarded 1794 studies as they were identified as duplicates. We eliminated a further 1733 studies after reviewing titles. We examined the full text of 61 studies, and eliminated a further 36, leaving 31 studies included in the analysis. See Figure 1.
1.
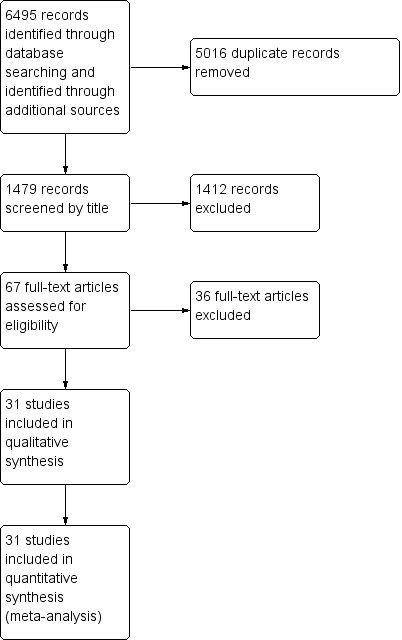
Study flow diagram.
Included studies
We include 31 studies (5430 participants) in this review.
Country of origin of the included studies Fifteen studies were from North America (Fudala 2003; Johnson 1992; Johnson 1995a; Johnson 2000; Kamien 2008; Kosten 1993; Ling 1996; Ling 1998; Ling 2010; Magura 2009; Oliveto 1999; Schottenfeld 1997; Schottenfeld 2005; Strain 1994a; Strain 1994b), nine were from Europe (Fischer 1999; Kakko 2003; Kakko 2007; Kristensen 2005; Krook 2002; Neri 2005; Pani 2000; Petitjean 2001; Soyka 2008a), four from the Middle East (Ahmadi 2002a; Ahmadi 2003a; Ahmadi 2003b; Ahmadi 2004), two from Australia (Lintzeris 2004;Mattick 2003) and one from Asia (Schottenfeld 2008).
Characteristics of the participants The majority of participants in these studies were male, consistent with the profile of heroin‐dependant users generally. They tended to be approximately 30 years of age, with different previous treatment histories and prevalence of use of other drugs, again consistent with what is known about heroin users presenting for treatment. The number of participants in these studies varied between 40 in one study (Kakko 2003) up to 736 in the study by Ling 1998. The largest comparative trial of methadone versus buprenorphine included was reported by Mattick 2003, with 405 participants. Many of the studies had quite small numbers of participants in each individual treatment group. The characteristics of the participants and the inclusion and exclusion criteria were well described in all of the studies.
Duration of intervention The interventions ranged in duration from 2 weeks through to 52 weeks. By and large, the interventions used clinically relevant doses of medication, although as noted earlier a number of the studies used predetermined fixed doses of medication (i.e., not tailored to individual treatment preference or need) and this created some limitations in terms of generalisability to day‐to‐day clinical practice, where flexible dosing is used.
Characteristics of the intervention and types of comparisons
Twenty studies involved comparisons of methadone and buprenorphine. The remaining eleven studies compared buprenorphine with placebo, where placebo was defined as either true placebo (Fudala 2003; Johnson 1995a; Kakko 2003; Kakko 2007; Krook 2002; Ling 2010; Schottenfeld 2008) or a 1 mg dose of buprenorphine (Ahmadi 2002a; Ahmadi 2003a; Ahmadi 2004; Ling 1998). Because of the use of 1 mg buprenorphine as a placebo dose, it is possible that we have underestimated the effect of buprenorphine at active doses. However, the approach is conservative and unlikely to bias results in favour of buprenorphine.
The studies selected for this review had two distinct dosing approaches. Eleven studies used flexible dosing (Fischer 1999; Johnson 2000; Kristensen 2005; Lintzeris 2004; Magura 2009; Mattick 2003; Neri 2005; Petitjean 2001; Soyka 2008a;Strain 1994a; Strain 1994b) where dose is titrated according to participant preference within a broad upper and lower dose limit. The remaining studies used fixed‐dosing schedules where participants were randomised to receive a fixed dose or a dose with a narrow dose range, without dose adjustment after stabilisation.
As most of the studies with fixed‐dosing schedules had more than one dose comparison, we have broadly classified the treatment groups as 'low dose', 'medium dose' and 'high dose' for the respective pharmacotherapy. These categories are arbitrary, driven by the doses used in the studies, and they do not reflect dose equivalence between methadone and buprenorphine. In the case of methadone, dose ranges up to 40 mg were classified as low dose, between 40 mg and 85 mg as medium dose, and more than 85 mg as high dose. In the case of buprenorphine studies where methadone was the comparator, dose ranges for buprenorphine between 2 mg and 6 mg were classified as low dose and between 7 mg and 15 mg as medium dose, and 16 mg as high dose. In the case of the buprenorphine studies where placebo (i.e. 0 mg or 1 mg) is the comparator (Johnson 1995a; Ling 1998) we included three buprenorphine dose levels; 0 ‐ 1 mg versus 2 ‐ 6 mg (Ahmadi 2002a; Ahmadi 2003a; Ahmadi 2004; Johnson 1995a; Ling 1998), 0 ‐ 1 mg versus 7 ‐ 15 mg (Ahmadi 2003a; Ahmadi 2004; Johnson 1995a; Ling 1998), and 0 ‐ 1 mg versus 16 mg (Fudala 2003; Kakko 2003; Krook 2002; Ling 1998).
Because of the design of the studies included in the review, we were also able to conduct analyses of:
low‐dose buprenorphine versus low‐dose methadone;
low‐dose buprenorphine versus medium‐dose methadone;
medium‐dose buprenorphine versus medium‐dose methadone;
high‐dose buprenorphine versus high‐dose methadone;
buprenorphine (low‐dose, medium‐dose and high‐dose) versus placebo medication.
Again, we used 1 mg of buprenorphine as a placebo dose, as it has been defined in this way by others (Ling 1998) who refer to this dose as being "adopted to serve essentially as a placebo".
The study by Johnson 2000 is classified as a flexible‐dose study and we did not include the 20 mg methadone fixed‐dose group from that study in the analyses as we did not choose to compare a low‐dose fixed methadone maintenance treatment (MMT) dose with a flexible buprenorphine maintenance treatment (BMT) dose. Specific study doses are provided in the Characteristics of included studies table.
The number of positive urines in each treatment group used to derive the mean number of positive urines with a standard deviation was not available for four studies (Kosten 1993; Neri 2005; Oliveto 1999; Pani 2000), and urine results are therefore not presented for these studies.
The reader should be aware that some of the studies used an aqueous ethanol‐based buprenorphine solution which has been reported by some to have a higher bioavailability than the marketed tablet. As the literature on dose equivalence of the solution and the tablet was sparse, and the pharmacodynamics and pharmacokinetics and dosing practices within trials were not well‐articulated, we used the doses as defined by the study authors rather than trying to estimate the dose equivalence per study. It is also noted that intra‐ and inter‐individual differences in metabolism, effects of pregnancy, and concurrent illicit drug use will all affect blood levels of medications. This approach seemed reasonable as no blood levels of the medication were given in the studies.
Four studies (Fudala 2003; Kakko 2007; Kamien 2008; Magura 2009) used the buprenorphine‐naloxone formulation.
Fudala 2003: fixed high‐dose buprenorphine‐naloxone versus placebo;
Kakko 2007: fixed medium‐dose buprenorphine‐naloxone versus placebo;
Kamien 2008: fixed medium‐dose buprenorphine‐naloxone versus medium‐dose methadone and high‐dose buprenorphine‐naloxone versus high‐dose methadone;
Magura 2009: flexible‐dose buprenorphine‐naloxone versus methadone.
We considered empirically testing these four studies separately but they involve different dosing regimens. To be consistent with the analysis in this review where studies were grouped by dosage (both the dose size and whether they are flexible or fixed), we did not combine the buprenorphine‐naloxone studies. The results from all four studies that use the combination formula showed results favouring the buprenorphine‐naloxone combination.
Excluded studies
We excluded 36 studies. Four studies (Bickel 1988; Meader 2010; Resnick 1992; Woody 2008) were essentially trials of detoxification or withdrawal. The study of Bouchez 1998 was a non‐randomised comparison of methadone, buprenorphine and morphine sulphate. Giacomuzzi 2003 was an open‐label, non‐randomised comparison of methadone and buprenorphine. Two studies involved treating people with chronic pain and iatrogenic opioid dependence (Neumann 2013; Weiss 2011).
Three studies were trials of dosing schedules (Johnson 1995b; Marsch 2005; Montoya 2004). Three studies were feasibility or efficacy trials that did not report doses or outcome variables of interest (Bond 2004; Gerra 2004; Sigmon 2004). One study was of the transfer to opioid replacement therapy (Jones 2005a), and papers by O'Connor 1998 and Lucas 2010 were trials of treatment setting, not of medication. Four studies involved pregnant women (Fischer 2006; Jones 2005b; Jones 2010b). The Pinto 2008 study was a feasibility trial and no participants were randomised, and the studies by Sacerdote 2008, Gryczynski 2013 and McKeganey 2013 were not randomised trials.
The remaining studies were a number of interim reports or secondary analysis of trials already included in this review, and data from only one article for each trial were included in the review (Eder 1998; Harris 2005; Kosten 2004; Lott 2006; Oliveto 1994; Schottenfeld 1998; Soyka 2008b; Stine 1994; Strain 1996; Uehlinger 1998; Warden 2012).
Risk of bias in included studies
See 'Risk of bias' tables in the Characteristics of included studies Table, Figure 2 and Figure 3.
2.
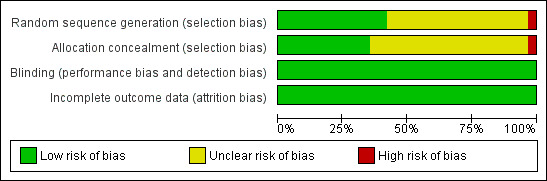
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
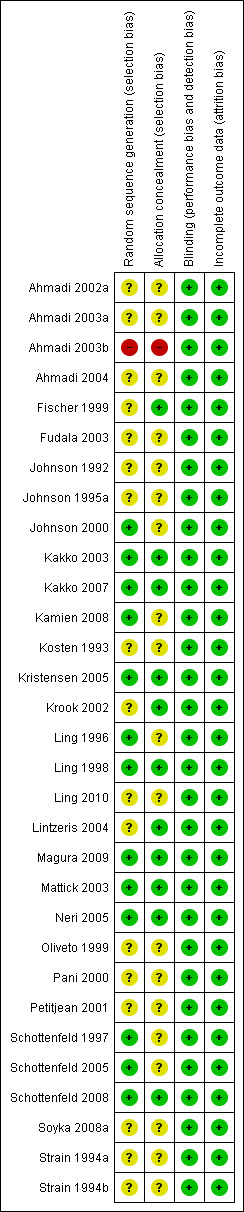
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Thirteen studies provided an adequate sequence generation for the randomisation process, including a random number table from a textbook or computer‐generated or the use of sealed envelopes. One study randomised by consecutive numerical order which we considered inadequate (Ahmadi 2003b). The remainder of the trials (n = 17) did not describe the randomisation process in sufficient detail to be clear whether the method was adequate, and are rated as being at unclear risk of bias.
Eleven studies provided an adequate method of concealment of allocation, including the use of an independent external agency, often the pharmacy. Nineteen studies did not describe the concealment of allocation process in sufficient detail to be clear that the allocation concealment method was adequate, and are rated as being at unclear risk of bias. One study reported a concealment method defined as inadequate, i.e., at high risk of bias.
Blinding
Of the 31 studies included in this review, 22 were reportedly conducted under double‐blind conditions. Ten studies (Ahmadi 2002a; Ahmadi 2003b; Ahmadi 2004;Fischer 1999; Kakko 2007; Kristensen 2005; Lintzeris 2004; Magura 2009; Neri 2005;Soyka 2008a) were open comparative trials. All the studies, including the open‐label, have been judged as being at low risk of bias for performance and detection bias because the objective outcomes are considered not to be influenced by lack of blinding. In order to maintain the double‐blind where methadone was compared with buprenorphine, participants were given both an oral solution of either active or placebo methadone syrup and a sublingual preparation of active or placebo buprenorphine (a double‐blind, double‐dummy design). In a few trials the method of maintaining the blinding was not given. Additionally, the success of the blinding was not reported in the trials.
Incomplete outcome data
All the studies have been judged to be at low risk of bias because all used the intention‐to‐treat (ITT) principle.
Effects of interventions
(01) Retention in treatment:
Comparison 01: Flexible‐dose buprenorphine versus flexible‐dose methadone:
As noted earlier, the flexible‐dose studies probably provide the best estimate of the likely impact of methadone and buprenorphine in day‐to‐day clinical practice, as they mirror clinical practice in terms of dose adjustments and in terms of the doses employed in the studies. The 11 studies (Fischer 1999; Johnson 2000; Kristensen 2005; Lintzeris 2004; Magura 2009; Mattick 2003; Neri 2005; Petitjean 2001; Soyka 2008b; Strain 1994a; Strain 1994b); 1391 participants included in the analysis, showed results in favour of methadone: risk ratio (RR) 0.83; 95% confidence interval (CI) 0.73 to 0.95. The Chi² test for heterogeneity was significant (I² = 56%, P = 0.01). Because of differences in methodology potentially creating heterogeneity among the 11 flexible‐dose studies, we conducted separate meta‐analyses of the double‐blind studies and open‐label studies. The results of these separate analyses indicated that in the double‐blind studies (five studies, 788 participants) there was a lower rate of retention for participants treated with buprenorphine: RR 0.83; 95% CI 0.72 to 0.95, with very little heterogeneity (I² = 19.0%, P = 0.29). However, very high heterogeneity was observed among open‐label studies (six studies, 603 participants; I² = 73%, P < 0.005). The results are presented in Analysis 1.1 and Figure 4
1.1. Analysis.
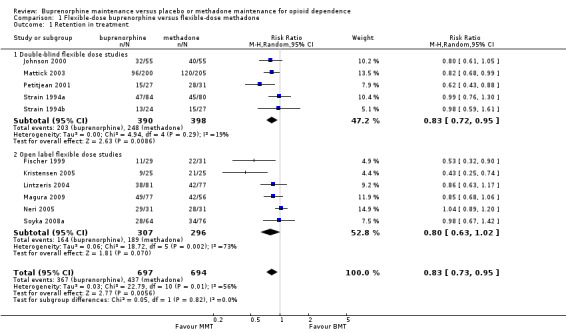
Comparison 1 Flexible‐dose buprenorphine versus flexible‐dose methadone, Outcome 1 Retention in treatment.
4.
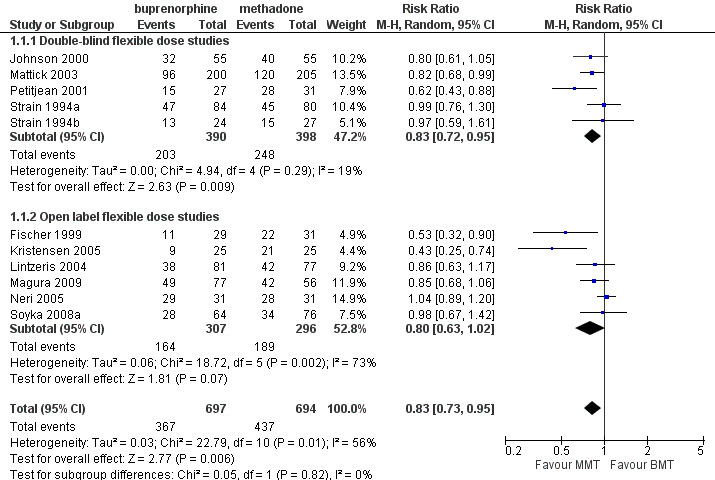
Forest plot of comparison: 1 Flexible dose buprenorphine versus flexible dose methadone, outcome: 1.1 Retention in treatment.
Comparison 02: Low‐dose buprenorphine versus low‐dose methadone:
The comparison indicated that low‐dose methadone was more likely to retain participants than low‐dose buprenorphine; RR 0.67; 95% CI 0.52 to 0.87; three studies, 253 participants (Ahmadi 2003a; Kosten 1993; Schottenfeld 1997). See Analysis 2.1.
2.1. Analysis.
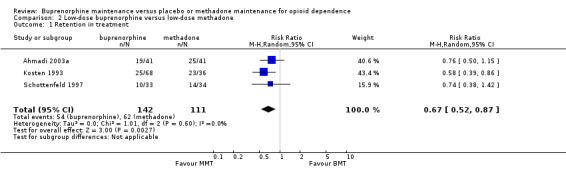
Comparison 2 Low‐dose buprenorphine versus low‐dose methadone, Outcome 1 Retention in treatment.
Comparison 03: Medium‐dose buprenorphine versus medium‐dose methadone:
There was no difference between medium‐dose buprenorphine and medium‐dose methadone in the ability to retain participants in treatment, RR 0.87; 95% CI 0.69 to 1.10; seven studies, 780 participants (Johnson 1992; Kamien 2008; Ling 1996; Oliveto 1999; Pani 2000; Schottenfeld 1997; Schottenfeld 2005). See Analysis 3.1.
3.1. Analysis.
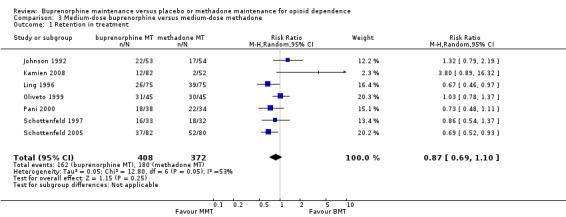
Comparison 3 Medium‐dose buprenorphine versus medium‐dose methadone, Outcome 1 Retention in treatment.
Comparison 04: High‐dose buprenorphine versus high‐dose methadone:
There were no differences between high‐dose buprenorphine and high‐dose methadone in retention: RR 0.79; 95% CI 0.20 to 3.16; one study, 134 participants (Kamien 2008). See Analysis 4.1
4.1. Analysis.
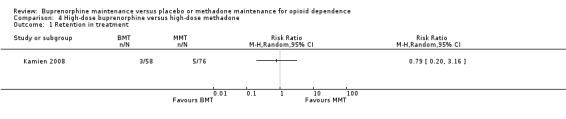
Comparison 4 High‐dose buprenorphine versus high‐dose methadone, Outcome 1 Retention in treatment.
Comparison 05: Low‐dose buprenorphine maintenance versus placebo:
The results showed a benefit for low‐dose buprenorphine over placebo in terms of retaining participants in treatment: RR 1.50; 95% CI 1.19 to 1.88); five studies, 1131 participants (Ahmadi 2002a; Ahmadi 2003a; Ahmadi 2004; Johnson 1995a; Ling 1998). SeeAnalysis 5.1.
5.1. Analysis.
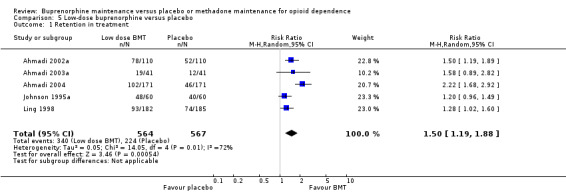
Comparison 5 Low‐dose buprenorphine versus placebo, Outcome 1 Retention in treatment.
Comparison 06: Medium‐dose buprenorphine maintenance versus placebo:
The results showed a benefit for medium‐dose buprenorphine over placebo in terms of retaining participants in treatment: RR 1.74; 95% CI 1.06 to 2.87; four studies, 887 participants (Ahmadi 2003a; Ahmadi 2004; Johnson 1995a; Ling 1998). SeeAnalysis 6.1.
6.1. Analysis.
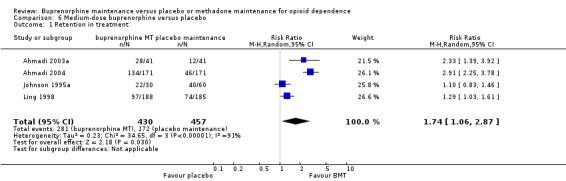
Comparison 6 Medium‐dose buprenorphine versus placebo, Outcome 1 Retention in treatment.
Comparison 07: High dose buprenorphine maintenance versus placebo:
The results showed a benefit for high‐dose buprenorphine over placebo in terms of retaining participants in treatment: RR 1.82; 95% CI 1.15 to 2.90, five studies, 1001 participants (Fudala 2003; Kakko 2003; Krook 2002; Ling 1998; Ling 2010). SeeAnalysis 7.1.
7.1. Analysis.
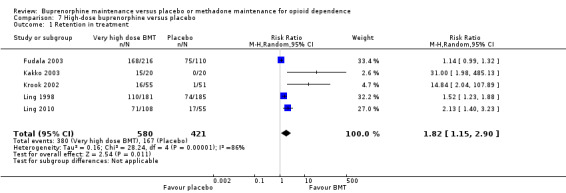
Comparison 7 High‐dose buprenorphine versus placebo, Outcome 1 Retention in treatment.
(02) Use of opioids (urinalysis):
Comparison 01: Flexible‐dose buprenorphine versus flexible‐dose methadone:
There was no difference between the two interventions in terms of heroin use, based on results of morphine urinalysis: SMD ‐0.11; 95% CI ‐0.23 to 0.02; eight studies, 1027 participants (Fischer 1999; Johnson 2000; Kristensen 2005; Mattick 2003; Petitjean 2001; Soyka 2008a; Strain 1994a; Strain 1994b; ). See Analysis 1.2.
1.2. Analysis.
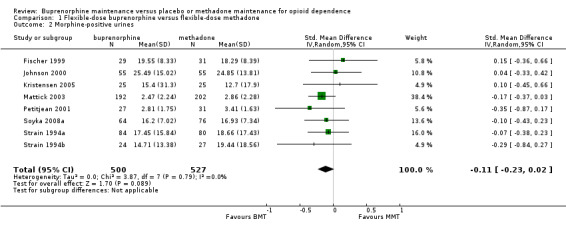
Comparison 1 Flexible‐dose buprenorphine versus flexible‐dose methadone, Outcome 2 Morphine‐positive urines.
Comparison 02: Low‐dose buprenorphine versus low‐dose methadone:
We found no differences in morphine‐positive urine; SMD ‐0.35; 95% CI ‐0.87 to 0.16; one study, 59 participants (Schottenfeld 1997) . See Analysis 2.2.
2.2. Analysis.
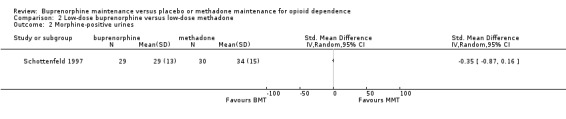
Comparison 2 Low‐dose buprenorphine versus low‐dose methadone, Outcome 2 Morphine‐positive urines.
Comparison 03: Medium‐dose buprenorphine versus medium‐dose methadone:
We found no difference between medium‐dose buprenorphine and medium‐dose methadone in terms of heroin use, based on results of morphine urinalysis: SMD 0.25; 95% CI ‐0.08 to 0.58; four studies, 476 participants (Johnson 1992; Ling 1996; Schottenfeld 1997; Schottenfeld 2005). SeeAnalysis 3.2.
3.2. Analysis.
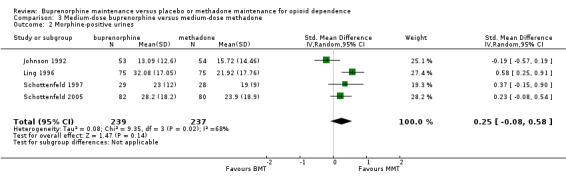
Comparison 3 Medium‐dose buprenorphine versus medium‐dose methadone, Outcome 2 Morphine‐positive urines.
Comparison 04: High‐dose buprenorphine versus high‐dose methadone:
No studies reporting urine data.
Comparison 05: Low‐dose buprenorphine maintenance versus placebo:
We found no difference between low‐dose buprenorphine and placebo as indexed by morphine‐positive urines: SMD 0.10; 95% CI ‐0.80 to 1.01; two studies, 487 participants (Johnson 1995a; Ling 1998). See Analysis 5.2.
5.2. Analysis.
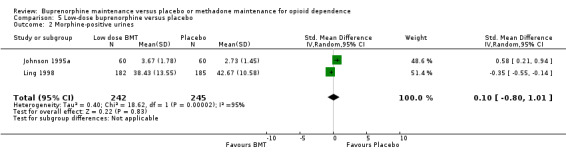
Comparison 5 Low‐dose buprenorphine versus placebo, Outcome 2 Morphine‐positive urines.
Comparison 06: Medium‐dose buprenorphine maintenance versus placebo:
We found no difference between medium‐dose buprenorphine and placebo in terms of heroin use as indexed by morphine‐positive urines: SMD ‐0.08; 95% CI ‐0.78 to 0.62; two studies, 463 participants (Johnson 1995a; Ling 1998) . See Analysis 6.2.
6.2. Analysis.
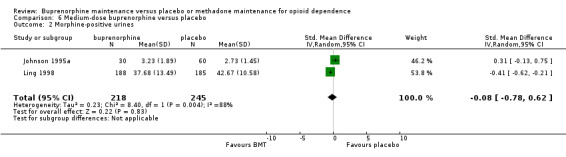
Comparison 6 Medium‐dose buprenorphine versus placebo, Outcome 2 Morphine‐positive urines.
Comparison 07: High‐dose buprenorphine maintenance versus placebo:
Participants on high‐dose buprenorphine treatment had less heroin use as indexed by morphine‐positive urines than those on placebo: SMD ‐1.17; 95% CI ‐1.85 to ‐0.49; three studies, 729 participants (Fudala 2003; Kakko 2003; Ling 1998). See Analysis 7.2.
7.2. Analysis.
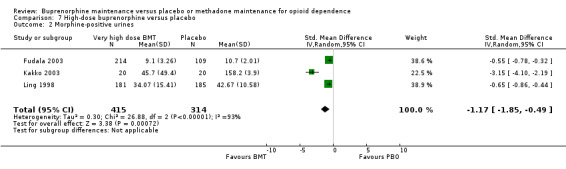
Comparison 7 High‐dose buprenorphine versus placebo, Outcome 2 Morphine‐positive urines.
(03) Use of opioids (self‐reported):
Comparison 01: Flexible‐dose buprenorphine versus flexible‐dose methadone:
We found no difference between the two interventions in terms of self‐reported heroin use: SMD ‐0.11; 95% CI ‐0.28 to 0.07; four studies, 501 participants (Johnson 2000; Lintzeris 2004; Magura 2009; Mattick 2003). See Analysis 1.3.
1.3. Analysis.
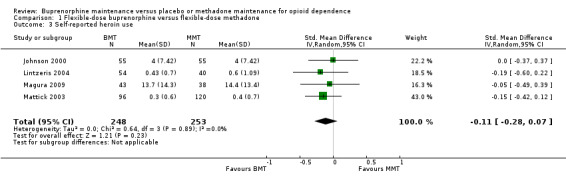
Comparison 1 Flexible‐dose buprenorphine versus flexible‐dose methadone, Outcome 3 Self‐reported heroin use.
Comparison 02: Low‐dose buprenorphine versus low‐dose methadone:
There was no difference between low‐dose buprenorphine and low‐dose methadone in self‐reported heroin use: SMD ‐0.29; 95% CI ‐0.38 to 0.96; one study, 37 participants (Kosten 1993). See Analysis 2.3.
2.3. Analysis.
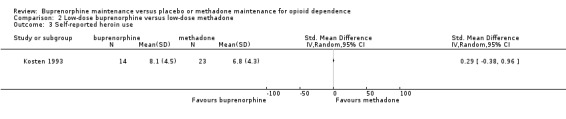
Comparison 2 Low‐dose buprenorphine versus low‐dose methadone, Outcome 3 Self‐reported heroin use.
Comparison 03: Medium‐dose buprenorphine versus medium‐dose methadone:
There was no difference in self‐reported heroin use between medium doses of buprenorphine and methadone, but again the Chi² test for heterogeneity was significant (P = 0.006): SMD ‐0.82; 95% CI ‐1.83 to 0.19; two studies, 174 participants (Kamien 2008; Pani 2000). See Analysis 3.3.
3.3. Analysis.
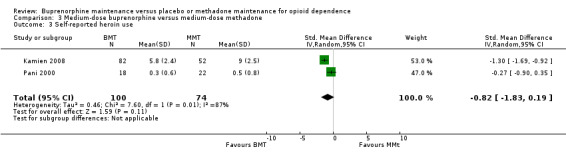
Comparison 3 Medium‐dose buprenorphine versus medium‐dose methadone, Outcome 3 Self‐reported heroin use.
Comparison 04: High‐dose buprenorphine versus high‐dose methadone:
We found no differences between high‐dose buprenorphine and high‐dose methadone in self‐reported heroin use: SMD ‐0.73; 95% CI ‐1.08 to ‐0.37; one study, 134 participants (Kamien 2008). See Analysis 4.2.
4.2. Analysis.
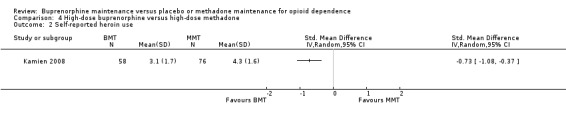
Comparison 4 High‐dose buprenorphine versus high‐dose methadone, Outcome 2 Self‐reported heroin use.
Theree were no data on self‐reported heroin use for the remaining comparisons.
(04) Use of cocaine:
Comparison 01: Flexible‐dose buprenorphine versus flexible‐dose methadone:
We found no difference between six studies of flexible dosing with buprenorphine or methadone for cocaine‐positive urines; SMD 0.10; 95% CI ‐0.05 to 0.25; 929 participants (Fischer 1999; Johnson 2000; Mattick 2003Soyka 2008a; Strain 1994al Strain 1994b). SeeAnalysis 1.4.
1.4. Analysis.
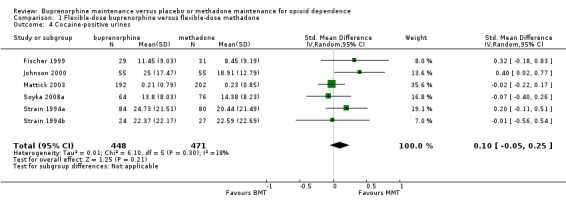
Comparison 1 Flexible‐dose buprenorphine versus flexible‐dose methadone, Outcome 4 Cocaine‐positive urines.
Comparison 02: Low‐dose buprenorphine versus low‐dose methadone:
There were no differences in cocaine‐positive urines between low‐dose usage of buprenorphine and methadone; SMD 0.08; 95% CI ‐0.43 to 0.59; one study, 59 participants (Schottenfeld 1997). See Analysis 2.4.
2.4. Analysis.
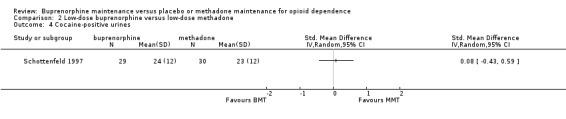
Comparison 2 Low‐dose buprenorphine versus low‐dose methadone, Outcome 4 Cocaine‐positive urines.
Comparison 03: Medium‐dose buprenorphine versus medium‐dose methadone:
We found no difference between medium dosage of buprenorphine and methadone for cocaine‐positive urines: SMD 0.21; 95% CI ‐0.06 to 0.47; two studies, 57 participants (Schottenfeld 1997; Schottenfeld 2005). See Analysis 3.4.
3.4. Analysis.
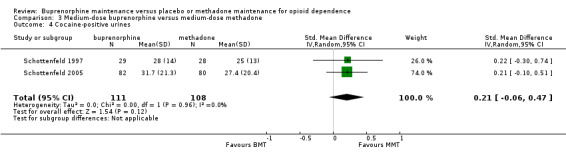
Comparison 3 Medium‐dose buprenorphine versus medium‐dose methadone, Outcome 4 Cocaine‐positive urines.
Comparison 4: High‐dose buprenorphine versus high‐dose methadone:
There were no data for this comparison on cocaine‐positive urines.
Comparison 05: Low‐dose buprenorphine maintenance versus placebo:
We found no difference between low‐dose buprenorphine and placebo for cocaine‐positive urines: SMD 0.26; 95% CI ‐0.10 to 0.62; one study, 120 participants (Johnson 1995a). See Analysis 5.3.
5.3. Analysis.
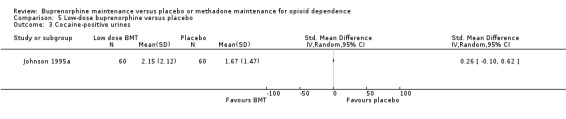
Comparison 5 Low‐dose buprenorphine versus placebo, Outcome 3 Cocaine‐positive urines.
Comparison 06: Medium‐dose buprenorphine maintenance versus placebo:
There was an advantage for placebo over medium‐dose buprenorphine for cocaine‐positive urines: SMD 0.50; 95% CI 0.05 to 0.94; one study, 90 participants (Johnson 1995a). See Analysis 6.3.
6.3. Analysis.
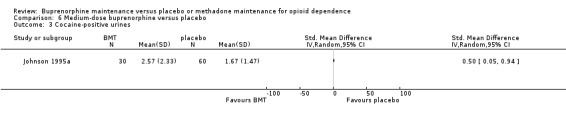
Comparison 6 Medium‐dose buprenorphine versus placebo, Outcome 3 Cocaine‐positive urines.
Comparison 07: High‐dose buprenorphine maintenance versus placebo:
We found no difference between high‐dose buprenorphine and placebo for cocaine‐positive urines: SMD 0.08; 95% CI ‐0.16 to 0.32; one study, 296 participants (Fudala 2003). See Analysis 7.3.
7.3. Analysis.
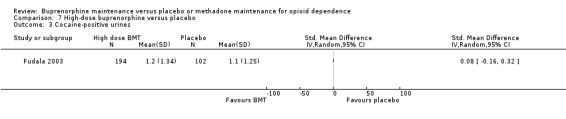
Comparison 7 High‐dose buprenorphine versus placebo, Outcome 3 Cocaine‐positive urines.
(05) Use of benzodiazepines:
Comparison 01: Flexible‐dose buprenorphine versus flexible‐dose methadone:
We found no difference across six studies comparing flexible dosing of buprenorphine and methadone for benzodiazepine‐positive urines: SMD 0.05; 95% CI ‐0.12 to 0.22; 859 participants (Fischer 1999; Kristensen 2005; Mattick 2003; Soyka 2008a; Strain 1994a; Strain 1994b).SeeAnalysis 1.5.
1.5. Analysis.
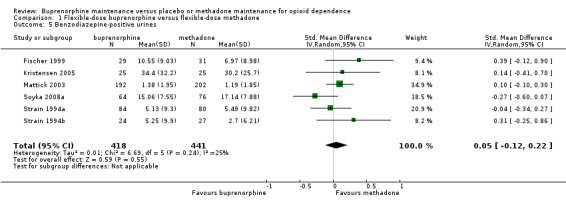
Comparison 1 Flexible‐dose buprenorphine versus flexible‐dose methadone, Outcome 5 Benzodiazepine‐positive urines.
Comaprison 02: Low‐dose buprenorphine versus low‐dose methadone:
No data available for benzodiazepine‐positive urines.
Comparison 03: medium‐dose buprenorphine versus medium‐dose methadone:
No data available for benzodiazepine‐positive urines.
Comparison 04: high‐dose buprenorphine versus high‐dose methadone:
No data available for benzodiazepine‐positive urines.
Comparison 05: Low‐dose buprenorphine maintenance versus placebo:
We found no difference between low‐dose buprenorphine and placebo for benzodiazepine‐positive urines: SMD 0.03; 95% CI ‐0.33 to 0.38; one study, 120 participants (Johnson 1995a). See Analysis 5.4.
5.4. Analysis.
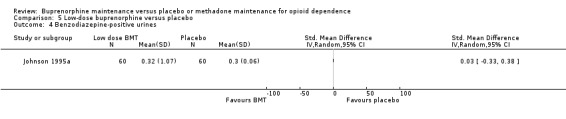
Comparison 5 Low‐dose buprenorphine versus placebo, Outcome 4 Benzodiazepine‐positive urines.
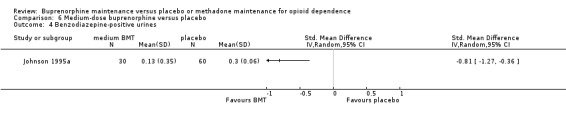
Comparison 06: Medium‐dose buprenorphine maintenance versus placebo:
Buprenorphine was superior to placebo for benzodiazepine‐positive urines; SMD ‐0.81; 95% CI ‐1.27 to ‐0.36; one study, 90 participants (Johnson 1995a). See Analysis 6.4.
6.4. Analysis.
Comparison 6 Medium‐dose buprenorphine versus placebo, Outcome 4 Benzodiazepine‐positive urines.
Comparison 07: High‐dose buprenorphine maintenance versus placebo:
We found no difference between high‐dose buprenorphine and placebo for benzodiazepine‐positive urines: SMD ‐1.65; 95% CI ‐4.94 to 1.65; two studies, 336 participants (Fudala 2003; Kakko 2003). See Analysis 7.4.
7.4. Analysis.
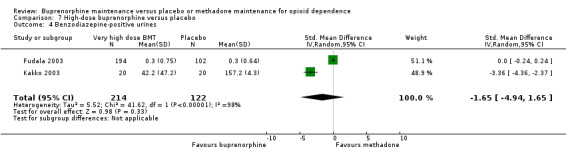
Comparison 7 High‐dose buprenorphine versus placebo, Outcome 4 Benzodiazepine‐positive urines.
(06) Criminal activity:
Comparison 01: Flexible‐dose buprenorphine versus flexible‐dose methadone:
We found no difference between the buprenorphine and methadone groups: SMD ‐0.10; 95% CI ‐0.31 to 0.12; two studies, 328 participants (Magura 2009; Mattick 2003). See Analysis 1.6.
1.6. Analysis.
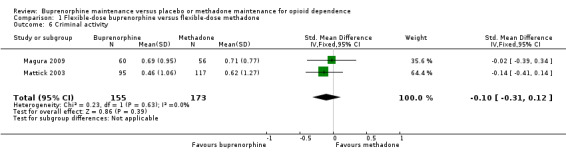
Comparison 1 Flexible‐dose buprenorphine versus flexible‐dose methadone, Outcome 6 Criminal activity.
For the other comparisons, no data on criminal activity were reported in the included studies.
(07) Mortality:
Five studies provided mortality data (Kakko 2003; Krook 2002; Ling 1996; Ling 1998; Schottenfeld 2008). No deaths were reported in the Krook 2002; Ling 1998 or Schottenfeld 2008 studies. Kakko 2003 reported a 20% mortality in control participants at one year while the Ling 1996study reported two deaths unrelated to the study medication (i.e., stab wounds and cancer).
(08) Adverse events:
Ten studies report collecting data on adverse events or side effects (Fudala 2003; Johnson 2000; Kamien 2008; Krook 2002; Ling 1996; Ling 1998; Mattick 2003; Pani 2000; Petitjean 2001; Soyka 2008b).The frequency and types of adverse events varied by study. There were no attempts to compare the adverse events statistically in Mattick 2003, Fudala 2003 or Ling 1996. Ling 1996 reported there were "numerous and diverse adverse events reported for both groups" and "adverse events equally represented in all groups" (Ling 1998). Those studies that did compare adverse events statistically found no difference between groups in the frequency of adverse events (Pani 2000; Petitjean 2001). The methadone group in Petitjean 2001 reported significantly more sedation (58% versus 26%). Krook 2002 reported that the two groups were statistically similar, with the exception that more of the placebo group reported exanthema. The side effects were reportedly "similar among groups" (Johnson 2000).
Discussion
Summary of main results
Buprenorphine is an effective medication for maintenance treatment of opioid dependence, as it is superior to placebo in retaining people in treatment based on all the 14 placebo‐controlled comparisons. However, based on objective urinanalysis results, buprenorphine is only superior to placebo in suppression of heroin use at high doses (defined here as 16 mg or more) but not at low or medium doses (defined here as 15 mg or less).
Before discussing the results of the buprenorphine and methadone comparisons, we remind the reader that we have distinguished between the fixed‐dose studies and flexible‐dose studies, as the former do not relate to clinical reality, where doses are in fact titrated by the clinician in response to individual need. The flexible‐dose studies are more clinically relevant and deserve more attention in consideration of the meta‐analytic review findings. The results of the meta‐analyses here clearly indicate that methadone is better able to retain participants than buprenorphine in flexible‐dosing approaches (at least in the double‐blind efficacy trials). Turning to reduction in heroin use, within the flexible‐dose studies, there was no evidence of any difference between methadone and buprenorphine in their ability to suppress heroin use (self‐reported or urinanalysis results).
One explanation which has been advanced by authors in some of the studies included here for the poorer retention in buprenorphine treatment (Fischer 1999; Petitjean 2001) is that they inducted participants too slowly onto buprenorphine, and that this was the cause of the poorer retention in that medication group. It is possible that retention is affected by too‐slow induction, and given the apparent relative safety of buprenorphine it may be possible to induct people to higher doses at a more rapid rate and to overcome the problem of slightly poorer retention for buprenorphine compared with methadone. However, there are a number of other possible explanations for the poorer retention on buprenorphine than methadone. In particular, it may well be that buprenorphine, being a partial agonist, does not retain people because it does not have a full opioid effect and is less satisfying to those allocated to it. Another possibility is that people in the initial stages of dosing who have recently ingested heroin suffer a mild withdrawal syndrome by virtue of buprenorphine (a partial agonist) displacing heroin (a full agonist) from opioid receptors in the central nervous system, and this mild withdrawal may lead to leaving treatment. A further possibility is that buprenorphine is simply easier to withdraw from and, on that basis, those using it are more at liberty to leave treatment without the severe withdrawal syndrome that can accompany methadone withdrawal. These factors may all act together to cause buprenorphine to have a slightly poorer outcome for retention than methadone. Future research should be undertaken to address this particular issue.
Turning to other outcomes, the results from two trials suggest there is no difference between methadone and buprenorphine for reducing criminal activity. There is evidence from other literature showing criminal activity is decreased in those who are in methadone treatment (Lind 2005). The majority of the studies that reported mortality data reported no deaths, and the two studies that reported deaths were among the controls (Kakko 2003) or not related to study medication (Ling 1998). Other research suggests that mortality is decreased among those in opioid substitution treatment (Soyka 2011), although methadone dosing in the first two weeks of induction is associated with heightened risk of death from methadone overdose (Clausen 2008; Degenhardt 2009; Degenhardt 2011; Gibson 2008).
The majority of studies did not compare adverse events statistically, although those that did found only one difference between methadone and buprenorphine with one study reporting more sedation among participants on methadone (Johnson 2000).
Overall completeness and applicability of evidence
The evidence is reasonably complete with regard to the effectiveness of buprenorphine relative to placebo, and buprenorphine relative to methadone, in maintenance for the important outcomes of retention and illicit opioid use. More data on the impacts on criminal activity, mortality and adverse events would be desirable. However, it may not be possible to generate such data from randomised trials, especially for impacts on crime and mortality, as randomised controlled trials are typically too short in duration to show differential effects in these domains.
Quality of the evidence
The clinical trials represented in this review are of reasonable quality, and whilst many of them did not fully explain how randomisation was concealed, they appear to have used doses which are clinically relevant and to have treated participants for significant periods of time. Moreover, despite the tendency of randomised studies to include selected populations, characteristics of drug users enrolled in the studies included in this review appear to be heterogeneous enough to allow generalisability of the results across different clinical and cultural settings. Based on the nature of the trials, it would appear the external validity or generalisability of the results is quite good, particularly from those trials which have used large sample sizes and adequate doses.
Potential biases in the review process
To overcome bias at the study level, we have used: (a) predetermined main outcomes; (b) the main outcomes being independent of the study reporting; (c) including all the reported main outcomes from the studies; and (d) additional data from the authors (which was generally provided).
At the review level, to reduce our risks of bias, we (a) used predetermined study inclusion criteria; (b) employed wide‐ranging and thorough searches of the literature; (c) relied on independent coding of the data, with separate resolution of disagreements; and (d) subjected the review to repeated independent refereeing and comment through Cochrane Collaboration methods. For example, as mentioned above, we addressed the potential problem of selective outcome reporting by contacting the authors of the studies to obtain results for main outcome variables. We note that we have reported data for retention for all but one study (Johnson 1995a), where retention was reported by dose change and not by remaining in the treatment condition. There is no evidence of selective reporting for the retention measure. The data for urine results were included, after we asked authors to provide such data and there was good compliance with this request, so that measured outcomes were provided to us and included in the review.
We have also included in the review an indication of which studies contributed data to the outcomes, for the interested reader (see Figures and Tables, Data Analysis, and elsewhere in text).
Agreements and disagreements with other studies or reviews
Similar conclusions have been reached by other meta‐analytic reviews of these treatments (Barnett 2001; Connock 2007; West 2000), and the dose‐response of methadone is well‐established (Faggiano 2003).
Authors' conclusions
Implications for practice.
The implications of this review are clear for clinical practice. Despite the results showing that buprenorphine (especially at high doses) is an effective maintenance therapy for heroin dependence when compared with placebo, methadone maintenance treatment at flexible doses is better able to retain people in treatment. Both treatments suppress heroin use. Buprenorphine should be supported as a medication to use in substitution maintenance treatment, where higher doses of methadone cannot be administered or methadone is not tolerated, or simply to provide patient and clinician choice. Given buprenorphine's different pharmacological properties, it may have advantages in some settings and under some policies where its relative safety and alternate‐day administration are useful clinically compared to methadone.
Implications for research.
There does not appear to be any need for further randomised control trials of the relative efficacy of methadone compared with buprenorphine. There does appear to be a need to undertake studies which will clarify factors responsible for retention in the first few weeks or months of treatment in buprenorphine versus methadone. One way of addressing this issue would be to compare a standard induction as used in some of the trials reported herein with a rapid induction onto buprenorphine, with the potential to have a further comparison of induction onto methadone. Problems in the methods of induction onto buprenorphine within the trials analysed might partly explain the inferiority of buprenorphine shown in this review. It would be ideal if such a trial were to be conducted under double‐blind conditions, particularly in terms of the rapid versus standard induction onto buprenorphine. Other outcome measures could be included in future studies, such as self‐reported drug use, criminal activity, physical health, and psychological health, which were too infrequently and irregularly reported in the literature analysed in the current review.
There is enough research evidence to show that buprenorphine in low, medium or high doses is more effective than placebo in retaining people in treatment, and in reducing heroin use at high doses. Future trials involving placebo (or indeed short‐term maintenance where people are terminated from treatment after a few weeks of intervention) should consider the ethical implications of providing substandard (i.e., placebo or short‐term treatment), given the strength of the available evidence.
What's new
| Date | Event | Description |
|---|---|---|
| 5 December 2013 | New search has been performed | Risk of bias tables added for all studies. Seven clinical trials have been added to the review. Studies that utilised the combined buprenorphine/naloxone combination product were included in this update. A study using the buprenorphine implant formulation was also included (Ling 2010). Additional urine data from Schottenfeld 2005 were included. A further 2 studies were considered but excluded from the review. A flow diagram reflecting the search results was added. |
| 5 December 2013 | New citation required but conclusions have not changed | new citation |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 5 December 2013 | New search has been performed | Substantive amendment with additional studies included |
| 6 December 2010 | New search has been performed | Added studies |
Acknowledgements
We acknowledge the authors of the original papers analysed here who provided data on urine results in a form that was compatible with the Cochrane software, so that we could conduct meta‐analysis. Simona Vecchi and Alexandra Aitken assisted with the search strategy. Eva Congreve assisted with the original search strategy and literature searches.
Appendices
Appendix 1. CENTRAL search strategy
MeSH descriptor: [Opioid‐Related Disorders] explode all trees
((opioid* or opiat*) and (abus* or dependen* or disorder*)):ti,ab,kw (Word variations have been searched)
#1 or #2
"heroin":ti,ab,kw (Word variations have been searched)
(opioid* or opiat*):ti,ab,kw (Word variations have been searched)
#4 or #5
MeSH descriptor: [Buprenorphine] explode all tree
MeSH descriptor: [Methadone] explode all tree
"buprenorphine":ti,ab,kw (Word variations have been searched)
"methadone":ti,ab,kw (Word variations have been searched)
#7 or #8 or #9 or #10
#3 and #6 and #11 from 2011 to 2013, in Trials (Word variations have been searched
Appendix 2. PubMed search strategy
"Opioid‐Related Disorders"[Mesh]
((drug*[tiab] OR substance[tiab] OR opioid*[tiab] OR opiat*[tiab]) AND (abuse*[tiab] OR addict*[tiab] OR depend*[tiab] OR disorder*[tiab]))
(#1) OR #2
opioid*[tiab] OR opiat*[tiab]
heroin[tiab]
"Heroin"[Mesh]
((#4) OR #5) OR #6
(#3) AND #7
"buprenorphine"[MeSH Terms]
buprenorphine[tiab]
Methadone[MeSH]
Methadone[tiab]
(((#9) OR #10) OR #11) OR #12
randomized controlled trial [pt]
controlled clinical trial [pt]
placebo [tiab]
drug therapy [sh]
randomly [tiab]
trial [tiab]
groups [tiab]
((((((#14) OR #15) OR #16) OR #17) OR #18) OR #19) OR #20
animals [mh] NOT humans [mh]
(#21) NOT #22
((#8) AND #13) AND #23
Appendix 3. EMBASE search strategy
'addiction'/exp
'drug abuse'/exp
((drug OR substance OR opioid* OR opiat*) NEXT/3 (abuse* OR addict* OR depend* OR disorder*)):ab,ti
#1 OR #2 OR #3
opioid*:ab,ti OR opiat*:ab,ti OR heroin*:ab,ti OR narcot*:ab,ti
'diamorphine'/exp
#5 OR #6
#4 AND #7
'buprenorphine'/exp OR buprenorphine:ab,ti
'methadone'/exp OR methadone:ab,ti
#9 OR #10
'crossover procedure'/exp
'double blind procedure'/exp
'single blind procedure'/exp
'controlled clinical trial'/exp
'clinical trial'/exp
placebo:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti
random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR (cross:ab,ti AND over:ab,ti)
'randomized controlled trial'/exp
#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19
#8 AND #11 AND #20
#8 AND #11 AND #20 AND [humans]/lim AND [embase]/lim
Appendix 4. Criteria for risk of bias assessment
|
Item |
Judgment |
Description |
||
| Random sequence generation (selection bias) | low risk | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation. | ||
| high risk | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention. | |||
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk. | |||
| Allocation concealment (selection bias) | low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled, randomisation); sequentially‐numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. | ||
| high risk | Investigators enrolling participants could possibly foresee assignments because one of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |||
| Unclear risk | Insufficient information to permit judgement of low or high risk This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement. | |||
| Blinding of participants, personnel and outcome assessor (performance and detection bias) |
low risk |
Blinding of participants, providers and outcome assessor and unlikely that the blinding could have been broken; Either participants or providers were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias. No blinding, but the objective outcome measurement are not likely to be influenced by lack of blinding. |
||
| high risk | No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding; Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken; Either participants or outcome assessor were not blinded, and the non‐blinding of others likely to introduce bias. |
|||
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |||
| Incomplete outcome data (attrition bias) For all outcomes except retention in treatment or drop out |
low risk |
No missing outcome data; Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; Missing data have been imputed using appropriate methods; All randomised participants are reported/analysed in the group they were allocated to by randomisation irrespective of non‐compliance and co‐interventions (intention‐to‐treat). |
||
| high risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation . |
|||
| Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of low or high (e.g., number randomised not stated, no reasons for missing data provided; number of drop outs not reported for each group). |
Data and analyses
Comparison 1. Flexible‐dose buprenorphine versus flexible‐dose methadone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Retention in treatment | 11 | 1391 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.73, 0.95] |
| 1.1 Double‐blind flexible dose studies | 5 | 788 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.72, 0.95] |
| 1.2 Open label flexible dose studies | 6 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.02] |
| 2 Morphine‐positive urines | 8 | 1027 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.23, 0.02] |
| 3 Self‐reported heroin use | 4 | 501 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.28, 0.07] |
| 4 Cocaine‐positive urines | 6 | 919 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.05, 0.25] |
| 5 Benzodiazepine‐positive urines | 6 | 859 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.12, 0.22] |
| 6 Criminal activity | 2 | 328 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.31, 0.12] |
Comparison 2. Low‐dose buprenorphine versus low‐dose methadone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Retention in treatment | 3 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.52, 0.87] |
| 2 Morphine‐positive urines | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Self‐reported heroin use | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Cocaine‐positive urines | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Medium‐dose buprenorphine versus medium‐dose methadone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Retention in treatment | 7 | 780 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.10] |
| 2 Morphine‐positive urines | 4 | 476 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.08, 0.58] |
| 3 Self‐reported heroin use | 2 | 174 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐1.83, 0.19] |
| 4 Cocaine‐positive urines | 2 | 219 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.06, 0.47] |
Comparison 4. High‐dose buprenorphine versus high‐dose methadone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Retention in treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Self‐reported heroin use | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Low‐dose buprenorphine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Retention in treatment | 5 | 1131 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.19, 1.88] |
| 2 Morphine‐positive urines | 2 | 487 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.80, 1.01] |
| 3 Cocaine‐positive urines | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Benzodiazepine‐positive urines | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 6. Medium‐dose buprenorphine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Retention in treatment | 4 | 887 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.06, 2.87] |
| 2 Morphine‐positive urines | 2 | 463 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.78, 0.62] |
| 3 Cocaine‐positive urines | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Benzodiazepine‐positive urines | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 7. High‐dose buprenorphine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Retention in treatment | 5 | 1001 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.15, 2.90] |
| 2 Morphine‐positive urines | 3 | 729 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐1.85, ‐0.49] |
| 3 Cocaine‐positive urines | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Benzodiazepine‐positive urines | 2 | 336 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐4.94, 1.65] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmadi 2002a.
| Methods | Three‐group, single‐blind (participants unaware of dose of buprenorphine), randomised clinical trial. Concealment of randomisation not stated. | |
| Participants | Geographic region: Iran.
N = 330.
Mean age: 38 years.
97% male. Inclusion criteria: DSM‐IV opioid dependence and daily opium use for at least 12 months. Exclusion criteria: another medical condition, a diagnosis of alcohol dependence, or current use of anticonvulsants, neuroleptics or methadone; and a score 7 or higher on the Addiction Severity Index, or if in need of psychological or psychiatric treatment. |
|
| Interventions | 18 weeks of maintenance, fixed dosing with buprenorphine sublingual tablet of 1 mg, 2 mg, or 4 mg daily. | |
| Outcomes | Retention in treatment. | |
| Funding source | None reported. | |
| Declarations of interest | None reported. | |
| Notes | Participants were offered weekly 1‐hour individual counselling sessions and if 6 consecutive days were missed participants were re‐inducted. If participants had more than 3 re‐inductions or missed 7 consecutive doses they were discharged. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Assigned to the medication randomly". Method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open label. Objective outcome measurement not influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Ahmadi 2003a.
| Methods | Four‐group, double‐blind, randomised clinical trial. Participants were matched into treatment groups by level and duration of dependence. Concealment of randomisation not stated. | |
| Participants | Geographic region: Iran.
N = 164.
Mean age: 31 years (BMT) and 34 years (MMT).
100% male. Inclusion criteria: DSM‐IV opioid dependence, male gender, daily heroin use for at least 6 months. Exclusion criteria: another serious medical condition, a diagnosis of alcohol dependence, prescribed anticonvulsants, neuroleptics or methadone during the previous month; or a score of 7 or higher on the Addiction Severity Index. |
|
| Interventions | 18 weeks of maintenance, fixed dosing. Buprenorphine (sublingual tablet) 1 mg, 3 mg or 8 mg daily, or methadone tablet of 30 mg daily. | |
| Outcomes | Retention in treatment. | |
| Funding source | "Supported by internal funds" of Shiaz University of Medical Sciences. | |
| Declarations of interest | None reported. | |
| Notes | Offered weekly 1‐hour individual counselling sessions. If participants missed 6 consecutive days they were re‐inducted. If participants had more than 3 re‐inductions or missed 7 consecutive days they were discharged. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allocated randomly" ‐ method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Ahmadi 2003b.
| Methods | Three‐group, randomised open‐label trial with participants randomised to treatment groups in consecutive numerical order by an investigational team. Participants were divided into treatment groups by dose and duration of buprenorphine misuse. Concealment of randomisation not stated. | |
| Participants | Geographic region: Iran.
N = 204.
Mean age; 31 years (BMT), 31 years (MMT).
100% male. Inclusion criteria: Male gender, DSM‐IV diagnosis of opioid dependence, "daily use of injection buprenorphine for at least 6 months". Exclusion criteria: another medical condition, a diagnosis of alcohol dependence, prescribed anticonvulsants, neuroleptics or methadone during the previous month, or a score of 7 or higher on the Addiction Severity Index. |
|
| Interventions | 24 weeks of maintenance, with fixed dosing. Buprenorphine (sublingual tablet) 5 mg daily, methadone 50 mg daily, or naltrexone 50 mg daily. | |
| Outcomes | Retention in treatment. | |
| Funding source | None reported. | |
| Declarations of interest | None reported. | |
| Notes | Offered weekly half‐hourly individual counselling sessions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomised to treatment groups in consecutive numerical order". |
| Allocation concealment (selection bias) | High risk | "Randomised to treatment groups in consecutive numerical order". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label. Objective outcome measurement not influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Ahmadi 2004.
| Methods | Three‐group, double‐blind randomised trial. Concealment of randomisation not stated. | |
| Participants | Geographic region: Iran.
N = 513.
Mean age: 38 years,
96% male. Inclusion criteria: DSM‐IV opioid dependence. Exclusion criteria: 18 years or younger or 85 years and older, another serious medical condition, a diagnosis of alcohol dependence, prescribed anticonvulsants, neuroleptics or methadone in the previous month, had a score of 7 or higher on the psychiatric problem scale of the Addiction Severity Index. |
|
| Interventions | 12 months of maintenance, fixed dosing. Buprenorphine (sublingual tablet) 1 mg, 3 mg or 8 mg daily. | |
| Outcomes | Retention in treatment. | |
| Funding source | None reported. | |
| Declarations of interest | None reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Assigned randomly" ‐ method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐ blind". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Fischer 1999.
| Methods | Two‐group, open‐label, randomised clinical trial, with participants randomised "externally . . . independent of the investigators", but the method of concealment of allocation is unstated. | |
| Participants | Geographic region: Austria.
N = 60.
Age range: 18 ‐ 39 years.
68% male. Inclusion criteria: DSM‐IV diagnosis of opioid dependence, aged 18 ‐ 45 years, willing to follow the maintenance programme and avoid using illegal drugs wherever possible. Exclusion criteria: dependence on other drugs (except cannabis), pregnancy, HIV positivity or seriously illness. |
|
| Interventions | 24 weeks of maintenance, flexible dosing. Buprenorphine (sublingual tablet) mean dose 7.5 mg/day (range 2 mg to 8 mg). Methadone mean dose 63 mg/d (range 20 mg to 80 mg). | |
| Outcomes | Retention in treatment, urinalysis for opioids, cocaine, and benzodiazepines. | |
| Funding source | None reported. | |
| Declarations of interest | None reported. | |
| Notes | Prior to entering trial, all participants were screened for 1 week and maintained with slow‐release oral morphine (Kapanol CSR). Weekly group psychotherapy was provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified. |
| Allocation concealment (selection bias) | Low risk | "Randomised externally using a source independent of investigators". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label. Objective outcome measurement not influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Fudala 2003.
| Methods | Two‐group, double‐blind placebo‐controlled trial. Concealment of randomisation not stated. | |
| Participants | Geographic region: USA.
N = 326.
Mean age: 38 years (placebo) and 37 years (BMT).
65% male. Inclusion criteria: DSM‐IV opioid dependence, aged 18 ‐ 59. Exclusion criteria: Pregnant or breast‐feeding women, medical conditions that made participation hazardous, a current, primary Axis I psychiatric diagnosis other than opiate, caffeine or nicotine dependence, the use of methadone, LAAM or naltrexone within 14 days of enrolment. |
|
| Interventions | 4 weeks of maintenance, fixed dosing. Buprenorphine (sublingual tablet) 16 mg versus placebo. | |
| Outcomes | Urinalysis and self report of cravings. | |
| Funding source | NIDA, USA, via Dept of Veterans Affairs Cooperative Studies Program (Inter‐agency agreement number 3YO1/DA/30011/04). Medication and placebo tablets provided by Reckitt and Colman Pharmaceuticals/ReckittBenckiser. | |
| Declarations of interest | None reported. | |
| Notes | A third treatment group received 16mg buprenorphine in combination with naloxone 4mg. The 4 week trial was followed by a 48‐52 week open label trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" ‐ method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind, placebo‐controlled". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Johnson 1992.
| Methods | Three‐group, double‐blind, double‐dummy, randomised clinical trial stratified by age, gender, and Clinical Institute Narcotic Assessment (CINA) scores, but the method of concealment of allocation is unstated. | |
| Participants | Geographic region: USA.
N = 162.
Age range: 21 ‐ 50 years.
70% male. Inclusion criteria: age 21 ‐ 50, self‐reported addiction of at least 4 months, 2+ use of heroin per day, USD 50 or more expended on heroin per day, evidence of withdrawal based on self report, urine positive for opioids. Exclusion criteria: acute or chronic medical or psychiatric conditions, pregnancy. |
|
| Interventions | 17 weeks of maintenance, fixed dosing. Buprenorphine (solution) 8 mg/day. Methadone 20 mg/day or 60 mg/day. | |
| Outcomes | Retention in treatment, urinalysis for opioids, abstinence. | |
| Funding source | NIDA Intramural Research Budget, USA. | |
| Declarations of interest | None reported. | |
| Notes | Participants were offered but not required to attend 30 ‐ 60 minutes of individual counselling per week. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy, double‐blind. Paper stated when the blind was broken for the first participant for a dose adjustment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Johnson 1995a.
| Methods | Three‐group, double‐blind, placebo‐controlled, randomised clinical trial, but the method of concealment of allocation is unstated. | |
| Participants | Geographic region: USA.
N = 150.
Age range: 18 ‐ 50 years.
69% male. Inclusion criteria: 18 ‐ 50 years of age, positive urine for opioids (not methadone), met federal guidelines for methadone treatment, DSM‐IIIR diagnosis of opioid dependence. Exclusion criteria: major medical conditions, chronic medications, history of serious psychiatric illness, prior drug abuse with buprenorphine, treatment at clinic in past 3 months, currently pregnant. |
|
| Interventions | 2 weeks of maintenance, fixed dosing. Buprenorphine (solution) 0 mg, 2 mg or 8 mg daily. | |
| Outcomes | % on original dose, % requesting a dose change, urinalysis for opioids and cocaine, dose adequacy. | |
| Funding source | "USPHS grant R18DA06165". | |
| Declarations of interest | None reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned ‐ method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat used in meta analyses. |
Johnson 2000.
| Methods | Four‐group, triple‐dummy, double‐blind, randomised clinical trial using a random number generator, but the method of the concealment of allocation is unstated. | |
| Participants | Geographic region: USA.
N = 220.
Age range: 21 ‐ 55 years.
65% male. Inclusion criteria: 21 ‐ 55 years of age, DSM‐IV diagnosis of opioid dependence, urine positive for opioids. Exclusion criteria: serious psychiatric or medical conditions, currently pregnant. |
|
| Interventions | 17 weeks of maintenance, flexible dosing. Mean doses not specified. Buprenorphine (formulation not specified) 16 mg to 32 mg on 2 weekdays, with 50% higher dose over weekends. Methadone 60 ‐ 100mg/day. LAAM 75 ‐ 115 mg on 2 weekdays, with 40% higher dose over weekends, and a fixed dose 20 mg/day methadone control group. | |
| Outcomes | Retention in treatment, urinalysis for opioids, continuous abstinence from opioids, self‐reported drug use, participant rating of severity of drug problem, urinalysis for cocaine, continuous abstinence from cocaine, breath alcohol readings, side effects, and sex differences. | |
| Funding source | NIDA grants (P50DA05273, K02DA00332, KO5DA00050, Y01DA40032), USA. | |
| Declarations of interest | Johnson consultant to Roxane Pharmaceuticals (LAAM manufacturer), Reckitt and Colman (buprenorphine manufacturer), and Schering‐Plough (buprenorphine distributor). | |
| Notes | 45% of participants met DSM‐IV criteria for cocaine abuse or dependence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator used. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and clinic staff unaware of treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Kakko 2003.
| Methods | Two‐group, double‐blind placebo‐controlled trial. Participants allocated by a random number table from a textbook, but the concealment of randomisation not stated. | |
| Participants | Geographic region: Sweden.
N = 40.
Mean age: 32 years (placebo) and 29 years (BMT).
70% male (placebo) and 75% male (BMT). Inclusion criteria: DSM‐IV history of heroin dependence for at least a year, aged 20 years or older. Exclusion criteria: individuals who fulfilled requirements for methadone maintenance in Sweden (i.e. 4 years of multiple daily heroin use documented in hospital records and 3 or more unsuccessful treatment attempts in abstinence‐oriented treatment programmes). Individuals co‐dependent on alcohol, amphetamines, cannabinoids or benzodiazepines. |
|
| Interventions | 12 months of maintenance, fixed dosing. Buprenorphine (sublingual tablet) supervised daily for at least 6 months, possible take home doses thereafter. Fixed‐dose 16 mg buprenorphine or tapered 6‐day regimen of buprenorphine followed by placebo. | |
| Outcomes | Retention in treatment and urinalysis. | |
| Funding source | Untied educational grant from Schering‐Plough Sweden. | |
| Declarations of interest | Untied educational grant from Schering‐Plough Sweden. | |
| Notes | All participants received cognitive‐behaviour therapy, group therapy and weekly individual counselling. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned.... by the clinical trials unit... with the use of a random numbers table". |
| Allocation concealment (selection bias) | Low risk | Pharmacy controlled randomisation. Participants given pre‐assembled medication packs corresponding with randomised number and pre‐assembled by pharmacy (not by personnel involved in study or participants). "Code translation table retained in a safe" . "Only the pharmacists who participated in the randomisation process had access". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. Treatment packs "were of identical appearance". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Kakko 2007.
| Methods | Two‐group, double‐blind randomised trial using a computer‐generated random sequence. The randomisation code insulated from research staff, only the pharmacy had access. | |
| Participants | Geographic region: Sweden.
N = 96.
Mean age: 34.8 years (Bup/nlx) and 36.5 years (MMT).
90% male (Bup/nlx) and 69% male (MMT). Inclusion criteria: DSM‐IV heroin dependence, aged 20 years or older. Exclusion criteria: severe psychiatric illness, severe medical condition, treatment with anti‐seizure drugs or disulphiram, pregnancy or breast‐feeding. |
|
| Interventions | 24 days double‐blind induction phase followed by single‐blind maintenance phase for total of 6 months. Buprenorphine/naloxone (sublingual tablets) mean dose 29.6 mg/day. Methadone mean dose 110 mg/day. | |
| Outcomes | Retention in treatment and urinalysis. | |
| Funding source | Swedish National Drug Policy Co‐ordinator, Stockholm County, and Schering‐Plough Sweden. | |
| Declarations of interest | Two authors received research and travel grants from Schering‐Plough, one author consulted for Merck Pharmaceuticals, and Kakko received honoraria from Schering‐Plough Australia, Schering‐Plough Sweden, and ReckittBenckiser Australia. | |
| Notes | All participants received cognitive‐behaviour therapy, group therapy and weekly individual counselling. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | "Only research pharmacist and deputy had access to codes". "research pharmacy insulated from trial staff". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. "identical looking tablets". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Kamien 2008.
| Methods | Four‐group, double‐blind, double‐dummy randomised clinical trial, but the method of concealment of allocation is unstated. | |
| Participants | Geographic region: USA.
N = 268.
Mean age: 37.2 years (BMT 8 mg), 38.9 years (BMT 16 mg), 40.3 years (45 mg MMT), 38.1 years (90 mg MMT).
41% male (BMT 8 mg), 58% male (BMT 16 mg), 42% male (45 mg MMT), 50% male (90 mg MMT). Inclusion criteria: DSM‐IV heroin dependence, aged 18 years or older. Exclusion criteria: active psychosis, manic‐depressive illness, organic psychiatric disorders or serious medical illness. |
|
| Interventions | 17 weeks of maintenance, fixed dosing. Buprenorphine‐naloxone (sublingual tablets) 8 mg/day or 16 mg/day. Methadone 45 mg/day or 90 mg/day. | |
| Outcomes | Retention in treatment, urinalysis. | |
| Funding source | NIDA, USA. | |
| Declarations of interest | One author employed by Schering‐Plough Corporation. | |
| Notes | All participants received manualised mandated behavioural counselling. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Minimum likelihood allocation was used to randomly assign patients". |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double dummy. "Oral solution first, followed by tablets." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Kosten 1993.
| Methods | Four‐group, double‐dummy, double‐blind, randomised clinical trial, but the method of concealment of allocation is unstated. | |
| Participants | Geographic region: USA.
N = 125 (N = 140 intention‐to‐treat).
Mean age: 32 years.
73% male. Inclusion criteria: DSM‐III opioid dependence, 1‐year history of opioid dependence, urine positive for opioids, withdrawal symptoms evident. Exclusion criteria: Nil reported. |
|
| Interventions | 24 weeks of maintenance, fixed dosing. Buprenorphine (solution) 2 mg/day or 6 mg/day. Methadone 35 mg/day or 65 mg/day. | |
| Outcomes | Retention in treatment, urinalysis, self‐reported drug use, opioid withdrawal ratings. | |
| Funding source | NIDA grants (R01‐DA0566, P50‐DA04060, R18‐DA06190, K02‐DA00112). | |
| Declarations of interest | None reported. | |
| Notes | No alcohol or sedative dependence, but 65% of participants met DSM‐III‐R criteria for cocaine dependence. For the first 6 weeks participants attended twice‐weekly relapse prevention group therapy, and for the remaining time, weekly group therapy. 3 types of analysis were used (intention‐to‐treat, completer, and efficacy). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐bind, "patients received both an oral and sublingual vehicle". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat used in meta‐analyses. |
Kristensen 2005.
| Methods | Two‐group, open‐label randomised clinical trial. 25 unmarked closed envelopes with each study medication were placed in a hat and drawn by each participant following randomisation. | |
| Participants | Geographic region: Norway. N = 50. Mean age: 36 years. 76% male. Inclusion criteria: ICD10 diagnosis of opioid dependence. Exclusion criteria: younger than 25 years. |
|
| Interventions | 180 days of flexible dosing (methadone) and fixed dosing (buprenorphine). Buprenorphine (sublingual tablets) mean dose 16 mg per day. Methadone dose of 106 mg per day. | |
| Outcomes | Retention in treatment, urinalysis. | |
| Funding source | The study was funded by "Sosial‐og helsedepartementet". | |
| Declarations of interest | Two authors supported to attend international congress by Schering‐Plough. | |
| Notes | 50 long term (> 10 years) opioid dependent participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised. Unmarked, sealed envelopes participants drew from a hat. |
| Allocation concealment (selection bias) | Low risk | Unmarked, sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label. Objective outcome measurement not influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Krook 2002.
| Methods | Two‐group, placebo‐controlled, double‐blind randomised clinical trial. The randomisation code was available only to the pharmacy staff. | |
| Participants | Geographic region: Norway.
N = 106.
Mean age = 38 years.
66% male. Inclusion criteria: at least 25 years old, current DSM‐IV diagnosis of opioid dependence, more than 10 years history of opioid dependence, and a written plan for rehabilitation process. Exclusion criteria: not having completed prior drug‐free treatment, serious illness, pregnancy. |
|
| Interventions | 12 weeks of maintenance, fixed dosing with 16 mg buprenorphine sublingual tablet or 0 mg placebo tablet. | |
| Outcomes | Retention in treatment, self‐reported heroin use. | |
| Funding source | Grants from Norwegian Social and Health Department, and Schering‐Plough A/S Norway. | |
| Declarations of interest | None reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Assigned randomly" ‐ method not specified. |
| Allocation concealment (selection bias) | Low risk | "Randomisation code kept in pharmacy and was available only to pharmacy staff." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. "all patients had to start with same dose". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Ling 1996.
| Methods | Three‐group, double‐dummy, double‐blind, randomised clinical trial with a computer‐generated random numbers list, but the method of concealment of allocation is unstated. | |
| Participants | Geographic region: USA. N = 225. Age range: 18 ‐ 65 years. 60% male. Exclusion criteria: current involvement in a methadone maintenance program, acute hepatitis, DSM III‐R dependence on alcohol, sedative‐hypnotics, cocaine, amphetamines; pregnancy/breast‐feeding, current use of anticonvulsants, disulphiram or neuroleptics. | |
| Interventions | 52 weeks of maintenance (efficacy evaluation based on first 26 weeks), fixed dosing. Buprenorphine (solution) 8 mg/day. Methadone 30 mg/day or 80 mg/day. All participants were encouraged to attend weekly individual counselling sessions. Medication and counselling were free. | |
| Outcomes | Retention in treatment, urinalysis for opioids, cocaine, amphetamines, benzodiazepines, craving, and opioid withdrawal symptoms. | |
| Funding source | NIDA grants (R18‐DA082). | |
| Declarations of interest | None reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random numbers list". |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind". "Each patient received both an oral solution and a sublingual form of medication". "doses were prepared weekly by a research pharmacist who was the only individual locally who had knowledge of the drug assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Ling 1998.
| Methods | Four‐group, double‐blind, randomised multi‐site clinical trial using a random number table to pre‐label medications by pharmacy staff. | |
| Participants | Geographic region: USA (12 sites).
N = 736.
Mean age: 36 years.
68% male. Inclusion criteria: DSM‐III diagnosis of opioid dependence, state or federal criteria for methadone maintenance treatment, daily use of opiates in past six months. Exclusion criteria: MMT in last 30 days, alcohol dependence, serious medical condition including AIDS, current use of neuroleptics, anticonvulsants or disulphiram, pregnancy. |
|
| Interventions | 16 weeks of maintenance, fixed dosing. Buprenorphine (solution) 1 mg, 4 mg, 8 mg or 16 mg/day. | |
| Outcomes | Retention in treatment, urinalysis for illicit opioids, craving, and global ratings by participants and staff. | |
| Funding source | NIDA grants. | |
| Declarations of interest | None reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random numbers table". |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled randomisation. "assigning patients to the medication that had been labelled in a blinded fashion by the research pharmacy using a random numbers table". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and staff at the sites were blind to medication and dose. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Ling 2010.
| Methods | Two‐group, randomised, double‐blind placebo‐controlled trial with participants stratified by sex and site. | |
| Participants | Geographic region: USA (3 sites).
N = 163.
Mean age: 35.8 years (buprenorphine implant), 39.3 years (placebo).
72% male (buprenorphine implant), 40% male (placebo). Inclusion criteria: 18 ‐ 65 years of age, DSM‐IV diagnosis of opioid dependence. Exclusion criteria: dependence on other psychoactive drugs (other than nicotine), current use of non‐prescribed benzodiazepines, treatment in past 90 days, chronic pain requiring opioid treatment, abnormal LFTs. |
|
| Interventions | 24 weeks of 4 buprenorphine implants (80 mg per implant) or 4 placebo implants | |
| Outcomes | Retention in treatment, urinalysis. | |
| Funding source | Titan Pharmaceuticals. | |
| Declarations of interest | Authors in receipt of support from ReckittBenckiser, Titan Pharmaceuticals, and other companies (see paper for extensive declarations). | |
| Notes | Open‐label phase where participants were inducted onto sublingual buprenorphine followed by a double‐blind implant phase. After implant, participants could receive supplemental sublingual buprenorphine‐naloxone tablets. Participants could receive an additional implant if required. Treatment failure was defined as receiving a 5th implant and subsequently requiring 3 or more days per week of sublingual buprenorphine‐naloxone treatment for 2 consecutive weeks or 8 or more days of supplemental buprenorphine‐naloxone treatment over 4 consecutive weeks at any time. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised (stratified by sex and site)" ‐ method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "the physicians who placed and removed implants...did not serve as...investigators". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Lintzeris 2004.
| Methods | Two‐group, open‐label, randomised clinical trial, with participants block‐randomised and allocated to treatment group by an independent agency. Separate randomisation schedules were used for participants in methadone maintenance and heroin users seeking treatment. | |
| Participants | Geographic region: Australia (3 sites).
N = 158.
Mean age: 32 years (heroin intake), 29 years (methadone intake).
57.6% male. Inclusion criteria: diagnosis of heroin dependence, or in methadone maintenance treatment for 8 weeks or less and able to reduce to 60 mg of methadone (to allow randomisation to ongoing methadone treatment or to buprenorphine). Exclusion criteria: under 18 years of age, pregnant and/or breast‐feeding. |
|
| Interventions | 26 weeks of maintenance, flexible dosing. Buprenorphine (sublingual tablets) mean dose 14.5 mg. Methadone mean dose 51.2 mg (methadone intake), 49.4 mg (heroin intake). | |
| Outcomes | Retention in treatment and self‐reported heroin use. | |
| Funding source | None reported. | |
| Declarations of interest | None reported. | |
| Notes | Clinicians were advised to exercise caution but not necessarily exclude participants with concomitant medical or psychiatric conditions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" ‐ method not specified. |
| Allocation concealment (selection bias) | Low risk | "Subjects were (block) randomised and allocated by an independent agency". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label. Objective outcome measurement not influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Magura 2009.
| Methods | Two‐group, open‐label, randomised clinical trial, with randomisation through a random numbers generator. Participants randomised and allocated to treatment group by receipt of sealed envelope created by investigator not involved in recruitment. | |
| Participants | Geographic region: USA (NYC prison inmates).
N = 133.
100% male. Inclusion criteria: Inmates 18 ‐ 65 years eligible for treatment, with 90 days jail term or less, residing in area after release from jail. Exclusion criteria: female gender, already in methadone maintenance treatment or took non‐prescribed street methadone in past 3 days, or evidence of psychosis or HIV infection and NESB. |
|
| Interventions | 12 weeks of maintenance, flexible dosing with a ceiling dose of 32 mg for BMT and 70 mg for MMT. Buprenorphine/naloxone (sublingual tablets) median dose 12 mg (range 4 ‐ 20 mg). Methadone median dose 30 mg (10 ‐ 70 mg). | |
| Outcomes | Retention in treatment, self‐reported heroin use, crime (re‐incarceration). | |
| Funding source | NIDA grant (R21‐DA020583), and buprenorphine donated by ReckiitBenckiser. | |
| Declarations of interest | "Authors declare they have no conflict of interest". | |
| Notes | Inmates. 17 participants randomised to receive buprenorphine dropped out prior to receiving medication. For this analysis used intention‐to‐treat although in published articles the 17 were not included in analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator. |
| Allocation concealment (selection bias) | Low risk | Sealed, pre‐numbered envelopes. Random number generator used and conducted by someone not involved in recruitment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label. Objective outcome measurement not influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat used in meta‐analyses. |
Mattick 2003.
| Methods | Two‐group, double‐dummy, double‐blind, randomised multi‐site clinical trial stratified for sex, randomised in blocks of 10, randomisation list generated in USA and controlled by the dispensing pharmacist separate from the clinical staff and research staff. | |
| Participants | Geographic region: Australia (3 sites).
N = 405.
Mean age: 30 years.
67% male. Inclusion criteria: 18 years or older, opioid dependence, lived within commuting distance of the clinic, able to provide informed consent. Exclusion criteria: acute liver disease, pregnancy/breast‐feeding, dependence on alcohol or sedative/hypnotics, use of anti‐convulsants or neuroleptics on a daily basis, methadone treatment in the last month. |
|
| Interventions | 13 weeks of maintenance, flexible dosing. Buprenorphine (sublingual tablets) mean dose 10.1 mg (range 2 mg to 32 mg/day). Methadone mean dose 52.1mg (range 20 mg to 150 mg/day). | |
| Outcomes | Retention in treatment, urinalysis for opioids, self‐reported heroin use and criminal behaviour. | |
| Funding source | Australian Government, NSW and SA Government Departments of Health, and UNSW. Reckitt and Colman Pty Ltd. | |
| Declarations of interest | None reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence. |
| Allocation concealment (selection bias) | Low risk | "randomisation process relied on sealed envelopes, not seen or accessible by the research or clinical staff, kept locked in a secure area of pharmacy separate from clinical dosing area and available to dispensing pharmacist only". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Prescribing doctors, staff conducting the dosing and patients were unaware of treatment assignment". "one active medication (either methadone or buprenorphine) and one placebo medication (placebo syrup or tablet)". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat used in meta‐analyses. |
Neri 2005.
| Methods | Two group, open label randomised clinical trial, with randomisation undertaken using a random numbers table, and the investigators had no access to the random number table. | |
| Participants | Geographic region: Italy,
N = 62,
mean age = 25 years,
89% male. Inclusion criteria: DSM‐IV classified as suffering narcotic addiction/heroin abuse. Exclusion criteria: patients with severe psychiatric illness (dementia, psychosis and cognitive impairment) who were unable to answer questions, and those with dependence on alcohol, amphetamines, cannabis, and benzodiazepines. |
|
| Interventions | "12 months" of maintenance, flexible dosing. Buprenorphine (sublingual tablets) mean dose 30.4mg (+/‐2.8mg) every three days. Methadone dose described as "medium dose 100mg/day" (mean dose of methadone was not provided). | |
| Outcomes | Retention in treatment, urinalysis for opiate metabolites. | |
| Funding source | None reported. | |
| Declarations of interest | None reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number used. |
| Allocation concealment (selection bias) | Low risk | Investigators had no access to the random number table. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label. Objective outcome measurement not influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Oliveto 1999.
| Methods | Two‐group, double‐dummy, double‐blind randomised clinical trial. The randomisation list was controlled by the dispensing pharmacist, but the method concealment of randomisation is not stated. | |
| Participants | Geographic region: USA.
N = 180.
Mean age: 34 years.
69% male. Inclusion criteria: Opioid dependence (documented prior MMT or precipitated withdrawal with naloxone challenge) and reported regular cocaine use. Exclusions: history of psychosis, current alcohol or sedative dependence, current suicidal tendency, current prescribed psychoactive medication, pregnant or breast‐feeding, notable medical conditions and prior buprenorphine treatment. |
|
| Interventions | 13 weeks of maintenance, fixed dosing. Buprenorphine (solution) 12 mg/day. Methadone 65 mg/day. | |
| Outcomes | Retention in treatment, urinalysis for opioids, self‐reported heroin and cocaine use. | |
| Funding source | NIDA US Public Health grant (DA05626, K02‐DA00112, P50‐DA04060). | |
| Declarations of interest | None reported. | |
| Notes | Weekly group therapy and individual monthly therapy provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Simple randomisation procedure" ‐ method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double dummy, double‐blind". Pharmacist was non‐ blind. "All patients received a liquid to swallow and a liquid to hold under their tongue". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Pani 2000.
| Methods | Two‐group, double‐dummy, double‐blind, randomised multi‐site clinical trial, but the method of concealment of randomisation is unstated. | |
| Participants | Geographic region: Italy. N = 72. Mean age: 28 years. 86% male. Exclusions: serious medical conditions, hypnotic‐sedative or alcohol dependence. | |
| Interventions | 24 weeks of maintenance, fixed dosing. Buprenorphine (tablets) 8 mg/day. Methadone 60 mg/day. | |
| Outcomes | Retention in treatment, urinalysis for opioids, craving, self‐reported heroin use, psychosocial adjustment and psychopathology. | |
| Funding source | Italian Ministry of Health and University of Cagliari, Molteni dei fratelli Aliti Spa, Reckitt and Colman, Boehringer Mannheim Italia SpA. | |
| Declarations of interest | None reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" "randomisation was balanced" ‐ method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Each patient received one oral administration (syrup) and one sublingual (tablet)". " also received increasing dose of placebo tablets or placebo syrup". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Petitjean 2001.
| Methods | Two‐group, double‐dummy, double‐blind randomised trial, but the method of concealment of allocation is unstated. | |
| Participants | Geographic region: Switzerland.
N = 58.
Mean age: 27 years.
83% male. Inclusion criteria: DSM‐IIIR diagnosis of opioid dependence, local criteria for MMT (greater than 18 years of age, unwilling to undergo abstinence‐oriented treatment). Exclusions: previous treatment with buprenorphine, treatment with methadone in last 30 days, sedative‐hypnotic or alcohol dependence, serious medical or psychiatric illness, pregnancy. |
|
| Interventions | 6 weeks of maintenance, flexible dosing. Buprenorphine (tablets) mean dose 10.5 mg (range 8 ‐ 16 mg/day), methadone mean dose 69.8 mg (range 30 ‐120 mg/day). | |
| Outcomes | Retention in treatment, urinalysis for opioids and cocaine, heroin craving, and adverse events. | |
| Funding source | Federal Office of Public Health grant (316‐94‐8043). | |
| Declarations of interest | None reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All subjects received...an oral liquid formulation ...followed by a sublingual tablet." "Research staff blinded to treatment condition questions the subjects..". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Schottenfeld 1997.
| Methods | Four‐group, double‐dummy, double‐blind, randomised clinical trial, using a computer‐generated random number list, but the method of concealment of the allocation is unstated. | |
| Participants | Geographic region: USA.
N = 116.
Mean age: 32 years.
68% male. Inclusion criteria: DSM III‐R dependence on opioids and cocaine. Exclusion criteria: current alcohol or sedative dependence, current psychosis or suicide risk, pregnancy, inability to read/understand the ratings forms and checklists. |
|
| Interventions | 24 weeks of maintenance, fixed dosing. Buprenorphine (solution) 4 mg/day or 12 mg/day. Methadone 20 mg/day or 65 mg/day. | |
| Outcomes | Retention in treatment, urinalysis for opioids and cocaine, self‐reported opioid and cocaine use, self‐reported withdrawal symptoms. | |
| Funding source | NIDA grants (K02‐DA0112, R18‐DA06190, R01‐DA06266), USA. | |
| Declarations of interest | None reported. | |
| Notes | All participants were required to participate in weekly relapse prevention group counselling sessions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. Oral medication (or placebo) and buprenorphine solution (or placebo). "A research pharmacist prepared all the medications and was the only person with knowledge of the drug assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Schottenfeld 2005.
| Methods | Four‐group, double‐dummy, double‐blind, randomised clinical trial, using a computerised urn randomisation procedure, but the method of concealment of allocation is unstated. | |
| Participants | Geographic region: USA.
N = 162.
Mean age: 36 years.
66% male. Inclusion criteria: at least 18 years old, at least 1 year of documented opioid dependence, DSM‐IV for opioid dependence and cocaine abuse. Exclusion criteria: current alcohol or sedative dependence, significant medical condition, current psychosis, bipolar disorder, major depression or suicide risk, pregnancy, inability to read/understand English. |
|
| Interventions | 24 weeks of maintenance, fixed dosing. Buprenorphine (solution) 12 mg. Methadone 65 mg/day. | |
| Outcomes | Retention in treatment, urinalysis for opioids and cocaine. | |
| Funding source | NIDA grants (R01‐DA09413, K24‐DA‐00445, T01‐DA‐13108, R01‐DA‐09803‐04A2, R01‐DA‐012979), USA. | |
| Declarations of interest | None reported. | |
| Notes | All participants were required to participate in manual guided individual counselling (twice weekly for 12 weeks and weekly thereafter). Participants were also randomly assigned to contingency management or performance feedback. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned using a computerised urn randomisation procedure". |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind ‐ oral liquid and placebo plus a sublingual liquid and placebo. "A research pharmacist who had no direct contact with any of the patients prepared all medications in advance". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Schottenfeld 2008.
| Methods | Three‐group double‐blind double‐dummy randomised clinical trial using a computerised randomisation procedure. All study personnel except the pharmacist were blind to treatment allocation. | |
| Participants | Geographic region: Malaysia.
N = 126.
Mean age: 36 years.
66% male. Inclusion criteria: DSM‐IV of opioid dependence, positive urine test for opioids. Exclusion criteria: dependence on alcohol, benzodiazepines or sedatives; elevated LFTs; evidence of danger to self or others, psychosis or major depression, or life‐threatening medical condition. |
|
| Interventions | 6 months maintenance, fixed dosing. Buprenorphine (sublingual tablets) 8 mg for the first week, followed by 16 mg on Mon ‐ Wed and 24 mg on Fri. | |
| Outcomes | Retention in treatment, days to first heroin use, days to heroin relapse and days of abstinence (all tested by urinalysis), and adverse events. | |
| Funding source | NIDA grants (R01‐DA14178, K24‐DA000445) USA, State of Connecticut Department of Mental Health and Addiction Services, and buprenorphine donated by ReckittBenckiser. | |
| Declarations of interest | "We declare that we have no conflict of interest". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by computer programme. |
| Allocation concealment (selection bias) | Low risk | Study medication prepared by research pharmacist. Randomisation disclosed only to research pharmacist. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy, double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Soyka 2008a.
| Methods | Two‐group open‐label randomised clinical trial. The method of concealment of allocation is unstated. | |
| Participants | Geographic region: Germany.
N = 140.
Mean age: 31.2 years buprenorphine group, 27.9 years methadone group. 66% male. Inclusion criteria: at least 18 years of age, opioid dependence, history of heroin abuse. Exclusion criteria: acute psychosis, any regular opioid substitution treatment or any regular psychosocial treatment in the month prior to study entry. |
|
| Interventions | 6 months maintenance, flexible dosing. Buprenorphine (sublingual tablets) mean dose 10.7 mg/day, methadone mean dose 49.1 mg/day. | |
| Outcomes | Retention in treatment, urinalysis for opioids, cocaine, benzodiazepines, cannabis and amphetamines. Withdrawal symptoms and side effects. | |
| Funding source | German Federal Ministry of Research and Technology (BMBF 01EB0440‐0441\01EB0142). | |
| Declarations of interest | One author a consultant for Sanofi and Essex, Forrest Labatoraties, and Alkernes Incorporated. | |
| Notes | Participants also treated with standardised psychotherapeutic interventions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" ‐method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐ label. Objective outcome measurement not influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Strain 1994a.
| Methods | Two‐group, double‐dummy, double‐blind, randomised clinical trial, but the method of concealment of allocation is unstated. | |
| Participants | Geographic region: USA.
N = 164.
Mean age: 32 years.
71% male. Inclusion criteria: DSM‐IIIR diagnosis of opioid dependence, 1 year of intravenous opioid use, urine positive for opioids. Exclusion criteria: chronic medical or major mental illness, pregnancy, prior methadone episodes longer than 21 days, previous buprenorphine treatment for opioid dependence, illicit methadone‐positive urine. |
|
| Interventions | 6 months maintenance, flexible dosing. Buprenorphine (solution) mean dose 8.9 mg/day (range 2 mg to 16 mg/day). Methadone mean dose 54 mg/day (range 20 mg to 90 mg/day). | |
| Outcomes | Retention in treatment, compliance with treatment (attendance and counselling contact), urinalysis for opioids, cocaine, benzodiazepines (3 x week). | |
| Funding source | NIDA grants (DA06120, DA06165). | |
| Declarations of interest | None reported. | |
| Notes | Participants were assigned an individual counsellor and individualised treatment plan for weekly meetings as well as weekly group therapy based on a relapse prevention model. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" ‐ method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy, double‐blind. "subjects and staff were unaware of the phases and details of the dosing schedule". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
Strain 1994b.
| Methods | Two‐group, double‐dummy, double‐blind, randomised clinical trial, stratified for race and gender, but the method of concealment of allocation is unstated. | |
| Participants | Geographic region: USA.
N = 51.
Mean age: 33 years.
71% male. Inclusion criteria: self‐reported cocaine use in previous 30 days or cocaine positive urine. Exclusion: chronic medical or major mental illness, pregnancy, prior methadone treatment lasting longer than 21 days, previous treatment episode with buprenorphine for opioid dependence. |
|
| Interventions | 16 weeks maintenance, flexible dosing. Buprenorphine (solution) mean dose 11.2 mg, (range 2 mg to 16 mg/day). Methadone mean dose 66.6 mg (range 20 mg to 90 mg/day). | |
| Outcomes | Retention in treatment, compliance with treatment (attendance and counselling contacts), urinalysis for opioids, cocaine, benzodiazepines. | |
| Funding source | USPHS grants (R18‐DA06120, R18‐DA06165, K20‐DA00166, K05‐DA00050). | |
| Declarations of interest | None reported. | |
| Notes | Participants were assigned an individual counsellor and given weekly group therapy focusing on education and relapse prevention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" ‐ method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy, double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. |
BMT: buprenorphine maintenance treatment LAAM: levo‐alpha‐acetylmethadol LFT: liver function test MMT: methadone maintenance treatment NESB: Non‐English‐speaking background NIDA: National Institute on Drug Abuse
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bickel 1988 | Excluded for the type of treatment not in the inclusion criteria: detoxification rather than maintenance, as participants were stabilised on buprenorphine for 3 weeks and then doses decreased to withdrawal. |
| Bond 2004 | Excluded for the type of outcome not in the inclusion criteria: feasibility study of methadone and buprenorphine dosing in community pharmacies. No dose information is provided. |
| Bouchez 1998 | Excluded for the study design not in the inclusion criteria: non‐randomised comparison of methadone, buprenorphine, and morphine sulphate in 39 participants (with n = 9, n = 22, and n = 8, respectively). |
| Eder 1998 | Excluded for the study design not in the inclusion criteria: Interim report of the Fischer et al. (1999) study which is already included in the review. |
| Fischer 2006 | Excluded for the type of participants not in the inclusion criteria: involves pregnant women. |
| Gerra 2004 | Excluded for the study design not in the inclusion criteria: compared the efficacy of buprenorphine and methadone in a clinical non‐experimental setting. |
| Giacomuzzi 2003 | Excluded for the study design not in the inclusion criteria: open‐label, non‐randomised, 2‐site comparison of methadone and buprenorphine. |
| Gryczynski 2013 | Excluded as the participants were not randomised. |
| Harris 2005 | Excluded for the study is a duplicate publication: secondary analysis of the data previously published by Lintersi (2004).This study investigated the cost effectiveness of buprenorphine compared to methadone. |
| Johnson 1995b | Excluded for the type of treatment not in the inclusion criteria: compared daily and alternate‐day dosing of buprenorphine, where the alternate‐day dosing group receive placebo on the alternate days. |
| Jones 2005a | Excluded for the type of treatment not in the inclusion criteria: randomised controlled trial comparing the transfer onto methadone or buprenorphine from short‐acting morphine. |
| Jones 2005b | Excluded for the type of participants not in the inclusion criteria: involves pregnant women. |
| Jones 2010a | Excluded for the type of participants not in the inclusion criteria: involves pregnant women. |
| Jones 2010b | Excluded for the type of participants not in the inclusion criteria: involves pregnant women. |
| Kosten 2004 | Excluded for the study is a duplicate publication: secondary analysis of the data previously published by Oliveto (1999). |
| Lott 2006 | Excluded for the study is a duplicate publication: secondary analysis of the data previously published by Johnson (2000). |
| Lucas 2010 | Excluded for the type of treatment not in the inclusion criteria: compared buprenorphine maintenance in a clinic setting versus referral to opioid treatment (that may involve buprenorphine). |
| Marsch 2005 | Excluded for the type of treatment not in the inclusion criteria: randomised clinical trial of the relative efficacy of different dosing schedules. |
| McKeganey 2013 | Excluded as the participants were not randomised. |
| Meader 2010 | Excluded for the type of treatment not in the inclusion criteria: meta analysis of detoxification trials. |
| Montoya 2004 | Excluded for the type of treatment not in the inclusion criteria: examined 4 different dosing schedules of buprenorphine. Placebo buprenorphine was given in the withdrawal phase of the study. |
| Neumann 2013 | Excluded as participants have iatrogenic opioid dependence. |
| O'Connor 1998 | Excluded for the type of treatment not in the inclusion criteria: compared buprenorphine maintenance in a primary care setting versus buprenorphine delivered in a specialist opioid replacement (i.e., methadone) clinic setting. It is not a comparison of buprenorphine in a primary care clinic versus methadone in a methadone clinic. |
| Oliveto 1994 | Excluded for the study is a duplicate publication: secondary analysis of the data published by Kosten (1993). |
| Pinto 2008 | Excluded as the participants were not randomised. |
| Resnick 1992 | Excluded for the type of treatment not in the inclusion criteria: examines the efficacy of maintenance buprenorphine compared to dose reductions of buprenorphine, following 4 ‐12 weeks of buprenorphine maintenance. |
| Sacerdote 2008 | Excluded as the participants were not randomised. |
| Schottenfeld 1998 | Excluded for the study is a duplicate publication: data from a secondary analysis of the data previously published by Kosten (1993). |
| Sigmon 2004 | Excluded for the outcome not in the inclusion criteria: No outcome data available on variables of interest. |
| Soyka 2008b | Excluded for the study is a duplicate publication: data from a secondary analysis of the data previously published by Soyka (2008a). |
| Stine 1994 | Excluded for the study is a duplicate publication: drawing on the data previously published by Kosten (1993). |
| Strain 1996 | Excluded for the study is a duplicate publication: drawing on the data previously published by Strain (1994a). |
| Uehlinger 1998 | Excluded for the study is a duplicate publication: presents preliminary data analyses that are reported in full in Petitjean (2000). |
| Warden 2012 | Excluded for the study is a duplicate publication: presents preliminary data analyses that are reported in full in Woody (2008) and is a detoxification trial rather than a maintenance trial. |
| Weiss 2011 | Excluded for the type of treatment not in the inclusion criteria: detoxification trial, rather than a maintenance trial and involves participants with iatrogenic dependence. |
| Woody 2008 | Excluded for the type of treatment not in the inclusion criteria: detoxification trial, rather than a maintenance trial. |
Characteristics of studies awaiting assessment [ordered by study ID]
Ahmadi 2002b.
| Methods | Randomly assigned to buprenorphine dose. Participants blinded to dose. |
| Participants | 105 heroin‐dependent. |
| Interventions | 17‐week buprenorphine/naloxone (2 and 4 mg sublingual dose) versus placebo (1 mg) |
| Outcomes | Retention in treatment. |
| Notes | Excluded in previous review as reportedly used buprenorphine/naloxone combination. Contacted author for confirmation. |
Cropsey 2011.
| Methods | Randomised to buprenorphine or placebo. Double‐blind. |
| Participants | 36 opioid‐dependent women who had contact with criminal justice system. |
| Interventions | 12 weeks maintenance medication. |
| Outcomes | Retention in treatment and urinalysis. |
| Notes | Contacted author for clarification of retention data as open‐label and randomised data reported together. |
Differences between protocol and review
None.
Contributions of authors
RPM, JK and CB reviewed the papers, with JK and RPM coding data from the papers for meta‐analysis. RPM conceptualised the reviews and JK conducted the initial literature searches. For the 2014 updated versions of the review CB conducted the literature searches and RPM and CB reviewed and coded the papers and wrote the analysis sections. RPM wrote the discussion with CB. MD contributed to the writing of the final version of the review.
Sources of support
Internal sources
National Drug and Alcohol Research Centre, University of New South Wales, Sydney, Australia.
External sources
Australian Government Department of Health, Canberra, Australia.
Declarations of interest
The first reviewer, RPM, is the first author on one trial of buprenorphine versus methadone for maintenance therapy in opioid dependence (see reference in the Included Studies). RPM has received untied educational funds from ReckittBenckiser for studies of post‐marketing surveillance of buprenorphine, to conduct research required by the Australian Government for the registration and/or subsidising of this medication for use in Australia. RPM has not received any personal income from ReckittBenckiser. There are no other declarations of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ahmadi 2002a {published data only}
- Ahmadi J. A controlled trial of buprenorphine treatment for opium dependence: The first experience from Iran. Drug and Alcohol Dependence 2002;66(2):111‐4. [DOI] [PubMed] [Google Scholar]
Ahmadi 2003a {published data only}
- Ahmadi J. Methadone versus buprenorphine maintenance for the treatment of heroin‐dependent outpatients. Journal of Substance Abuse Treatment 2003;24(3):217‐20. [DOI] [PubMed] [Google Scholar]
Ahmadi 2003b {published data only}
- Ahmadi J, Ahmadi K, Ohaeri J. Controlled, randomised trial in maintenance treatment of intravenous buprenorphine dependence with naltrexone, methadone or buprenorphine: A novel study. European Journal of Clinical Investigation 2003;33(9):824‐9. [DOI] [PubMed] [Google Scholar]
Ahmadi 2004 {published data only}
- Ahmadi J, Babaee‐Beigi M, Alishahi M, Maany I, Hidari T. Twelve‐month maintenance treatment of opium‐dependent patients. Journal of Substance Abuse Treatment 2004;26(1):61‐4. [DOI] [PubMed] [Google Scholar]
Fischer 1999 {published and unpublished data}
- Fischer G, Gombas W, Eder H, Jagsch R, Peternell A, Stuhlinger G, et al. Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction 1999;94(9):1337‐47. [DOI] [PubMed] [Google Scholar]
Fudala 2003 {published and unpublished data}
- Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, et al. Office‐based treatment of opiate addiction with a sublingual‐tablet formulation of buprenorphine and naloxone. New England Journal of Medicine 2003;349(10):949‐58. [DOI] [PubMed] [Google Scholar]
Johnson 1992 {published and unpublished data}
- Johnson R, Jaffe J, Fudala P. A controlled trial of buprenorphine treatment for opioid dependence. JAMA 1992;267(20):2750‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Johnson 1995a {published and unpublished data}
- Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. A placebo controlled trial of buprenorphine as a treatment for opioid dependence. Drug and Alcohol Dependence 1995;40(1):17‐25. [DOI] [PubMed] [Google Scholar]
Johnson 2000 {published and unpublished data}
- Johnson RE, Chutuape MA, Strain, EC, Walsh SL, Stitzer ML, Begelow GE. A comparison of levomethadyl acetate, buprenorphine and methadone for opioid dependence. New England Journal of Medicine 2000;343(18):1290‐7. [DOI] [PubMed] [Google Scholar]
Kakko 2003 {published and unpublished data}
- Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1‐year retention and social function after buprenorphine‐assisted relapse prevention treatment for heroin dependence in Sweden: A randomised, placebo‐controlled trial. Lancet 2003;361(9358):662‐8. [DOI] [PubMed] [Google Scholar]
Kakko 2007 {published data only}
- Kakko J, Grönbladh L, Svanborg KD, Wachenfeldt J, Rück C, Rawlings B, et al. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomised controlled trial. American Journal of Psychiatry 2007;164(5):797‐803. [DOI] [PubMed] [Google Scholar]
Kamien 2008 {published data only}
- Kamien JB, Branstetter SA, Amass L. Buprenorphine‐naloxone versus methadone maitenance therapy: A randomised double‐blind trial with opioid dependent patients. Heroin Addiction and Related Clinical Problems 2008;10(4):5‐18. [Google Scholar]
Kosten 1993 {published data only}
- Kosten T, Schottenfeld R, Ziedonis D, Falcioni J. Buprenorphine versus methadone maintenance for opioid dependence. Journal of Nervous and Mental Disease 1993;181(6):358‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kristensen 2005 {published data only}
- Kristensen Ø, Espegren O, Asland R, Jakobsen E, Lie Ø, Seiler S. Buprenorphine and methadone to opiate addicts‐‐a randomized trial (English abstract only). Tidsskrift for den Norske Laegeforening 2005;125(2):148‐51. [PMID: 15665884] [PubMed] [Google Scholar]
Krook 2002 {published data only}
- Krook AL, Brørs O, Dahlberg J, Grouff K, Magnus P, Røysamb E, et al. A placebo‐controlled study of high dose buprenorphine in opiate dependents waiting for medication‐assisted rehabilitation in Oslo, Norway. Addiction 2002;97(5):533‐42. [DOI] [PubMed] [Google Scholar]
Ling 1996 {published and unpublished data}
- Ling W, Wesson D, Charuvastra C, Klett J. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Archives of General Psychiatry 1996;53(5):401‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ling 1998 {published and unpublished data}
- Ling W, Charuvastra C, Collins JF, Batki S, Brown LS Jr, Kintaudi P, et al. Buprenorphine maintenance treatment of opiate dependence: A multicenter, randomised clinical trial. Addiction 1998;93(4):475‐86. [DOI] [PubMed] [Google Scholar]
Ling 2010 {published data only}
- Ling W, Casadonte P, Beglow G, Kampman KM, Patkar A, Bailey GL, et al. Buprenorphine implants for treatment of opioid dependence: A randomized controlled trial. JAMA 2010;304(14):1576‐83. [DOI] [PubMed] [Google Scholar]
Lintzeris 2004 {published data only}
- Lintzeris N, Ritter A, Panjari M, Clark N, Kutin J, Bammer G. Implementing buprenorphine treatment in community settings in Australia: Experiences from the buprenorphine implementation trial. American Journal on Addictions 2004;13 Suppl 1:S29‐S41. [DOI] [PubMed] [Google Scholar]
Magura 2009 {published data only}
- Magura S, Lee JD, Hershberger J, Joseph H, Marsch L, Shropshire C, et al. Buprenorphine and methadone maintenance in jail and post‐release: A randomized clinical trial. Drug and Alcohol Dependence 2009;99(1‐3):222‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattick 2003 {published data only}
- Mattick RP, Ali R, White J, O'Brien S, Wolk S, Danz C. Buprenorphine versus methadone maintenance therapy: A randomised double‐blind with 405 opioid‐dependent patients. Addiction 2003;98(4):441‐52. [DOI] [PubMed] [Google Scholar]
Neri 2005 {published data only}
- Neri S, Bruno CM, Pulvirenti D, Malaguarnera M, Italiano C, Mauceri B, et al. Randomised clinical trial to compare the effects of methadone and buprenorphine on the immune system in drug abusers. Psychopharmacology 2005;179(3):700‐4. [DOI] [PubMed] [Google Scholar]
Oliveto 1999 {published data only}
- Oliveto AH, Feingold A, Schottenfeld R, Jatlow P, Kosten TR. Desipramine in opioid‐dependent cocaine abusers maintained on methadone or buprenorphine. Archives of General Psychiatry 1999;56(9):812‐20. [DOI] [PubMed] [Google Scholar]
Pani 2000 {published data only}
- Pani PP, Maremmani I, Pirastu R, Tagliamonte A, Gessa GL. Buprenorphine: A controlled trial in the treatment of opioid dependence. Drug and Alcohol Dependence 2000;60(1):39‐50. [DOI] [PubMed] [Google Scholar]
Petitjean 2001 {published and unpublished data}
- Petitjean S, Stohler R, Déglon JJ, Livoti S, Waldvogel D, Uehlinger C, et al. Double‐blind randomised trial of buprenorphine and methadone in opiate dependence. Drug and Alcohol Dependence 2001;62(1):97‐104. [DOI] [PubMed] [Google Scholar]
Schottenfeld 1997 {published and unpublished data}
- Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Archives of General Psychiatry 1997;54(8):713‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schottenfeld 2005 {published data only}
- Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, Kosten TR. Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. American Journal of Psychiatry 2005;162(2):340‐9. [DOI] [PubMed] [Google Scholar]
Schottenfeld 2008 {published and unpublished data}
- Schottenfeld RS, Chawarski MC, Mazlan M. Maintenance treatment with buprenorphine and naltrexone for heroin dependence in Malaysia: a randomised, double‐blind, placebo‐controlled trial. Lancet 2008;371(9631):2192‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soyka 2008a {published data only}
- Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome:results form a randomised study. International Journal of Neuropsychopharmacology 2008;11(5):641‐53. [DOI] [PubMed] [Google Scholar]
Strain 1994a {published and unpublished data}
- Strain E, Stitzer M, Liebson I, Bigelow G. Comparison of buprenorphine and methadone in the treatment of opioid dependence. American Journal of Psychiatry 1994;151(7):1025‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strain 1994b {published and unpublished data}
- Strain E, Stitzer M, Liebson I, Bigelow G. Buprenorphine versus methadone in the treatment of opioid dependent cocaine users. Psychopharmocology 1994;116(4):401‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bickel 1988 {published data only}
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. A clinical trial of buprenorphine: Comparison with methadone in the detoxification of heroin addicts. Clinical Pharmacology and Therapeutics 1988;43(1):72‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bond 2004 {published data only}
- Bond C, Matheson C, Lawrie T, Cameron I, Robinson A, McNamee P, et al. A randomised comparison of methadone against buprenorphine in the treatment of opioid dependency: a feasibility study. International Journal of Pharmacy Practice 2004;Supplement 2004:R52. [Google Scholar]
Bouchez 1998 {published data only}
- Bouchez J, Beauverie P, Touzeau D. Substitution with buprenorphine in methadone‐ and morphine sulfate‐dependent patients. Preliminary results. European Addiction Research 1998;4(Suppl 1):8‐12. [DOI] [PubMed] [Google Scholar]
Eder 1998 {published data only}
- Eder H, Fischer G, Gombas W, Jagsch R, Stuehlinger G, Kasper S. Comparison of buprenorphine and methadone maintenance in opiate addicts. European Addiction Research 1998;4(Suppl 1):3‐7. [DOI] [PubMed] [Google Scholar]
Fischer 2006 {published data only}
- Fischer G, Ortner R, Rohrmeister K, Jagsch R, Baewert A, Langer M, et al. Methadone versus buprenorphine in pregnant addicts: A double‐blind, double‐dummy comparison study. Addiction 2006;101(2):275‐81. [DOI] [PubMed] [Google Scholar]
Gerra 2004 {published data only}
- Gerra G, Borella F, Zaimovic A, Moi G, Bussandri M, Bibici C, et al. Buprenorphine versus methadone for opioid dependence: Predictor variables for treatment outcome. Drug and Alcohol Dependence 2004;75(1):37‐45. [DOI] [PubMed] [Google Scholar]
Giacomuzzi 2003 {published data only}
- Giacomuzzi SM, Riemer Y, Ertl M, Kemmler G, Rössler H, Hinterhuber H, et al. Buprenorphine versus methadone maintenance treatment in an ambulant setting: A health‐related quality of life assessment. Addiction 2003;98(5):693‐702. [DOI] [PubMed] [Google Scholar]
Gryczynski 2013 {published data only}
- Gryczynski J, Mitchell SG, Jaffe JH, Kelly SM, Myers CP, O'Grady KE, et al. Retention in methadone and buprenorphine treatment among African Americans. Journal of Substance Abuse Treatment 2013;45(3):287‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2005 {published data only}
- Harris AH, Gospodarevskaya E, Ritter A. A randomised trial of the cost effectiveness of buprenorphine as an alternative to methadone maintenance treatment for heroin dependence. Pharmacoeconomics 2005;23(1):77‐91. [DOI] [PubMed] [Google Scholar]
Johnson 1995b {published data only}
- Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. Buprenorphine treatment of opioid dependence: Clinical trial of daily versus alternate‐day dosing. Drug and Alcohol Dependence 1995;40(1):27‐35. [DOI] [PubMed] [Google Scholar]
Jones 2005a {published data only}
- Jones HE, Johnson RE, Jasinski DR, Milio L. Randomised controlled study transitioning opioid‐dependent pregnant women from short‐acting morphine to buprenorphine or methadone. Drug and Alcohol Dependence 2005;78(1):33‐8. [DOI] [PubMed] [Google Scholar]
Jones 2005b {published data only}
- Jones HE, Johnson RE, Jasinski DR, O'Grady KE, Chisholm CA, Choo RE, et al. Buprenorphine versus methadone in the treatment of pregnant opioid‐dependent patients: Effects on the neonatal abstinence syndrome. Drug and Alcohol Dependence 2005;79(1):1‐10. [DOI] [PubMed] [Google Scholar]
Jones 2010a {published data only}
- Jones HE, O'Grady KE, Johnson RE, Velez M, Jansson LM. Infant neurobehaviour following prenatal exposure to methadone and buprenorphine: results from the neonatal intensive care unit network neurobehavioural scale. Substance Use and Misuse 2010;45(13):2244‐57. [DOI] [PubMed] [Google Scholar]
Jones 2010b {published data only}
- Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, et al. Neonatal abstinence syndrome after methadone and buprenorphine exposure. New England Journal of Medicine 2010;363(24):2320‐2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kosten 2004 {published data only}
- Kosten T, Falcioni J, Oliveto A, Feingold, A. Depression predicts higher rates of heroin use on desipramine with buprenorphine than with methadone. American Journal on Addictions 2004;13(2):191‐201. [DOI] [PubMed] [Google Scholar]
Lott 2006 {published data only}
- Lott DC, Strain EC, Brooner RK, BIgelow GE, Johnson RE. HIV risk behaviors during pharmacologic treatment for opioid dependence: A comparison of levomethadyl acetate [corrected], buprenorphine, and methadone. Journal of Substance Abuse Treatment 2006;31(2):187‐94. [DOI] [PubMed] [Google Scholar]
Lucas 2010 {published data only}
- Lucas GM, Chaudry A, Hsu J, Woodson T, Lau B, Olsen Y, et al. Clinic‐based treatment of opioid‐dependent HIV‐Infected patients versus referral to an opioid treatment program. A randomized trial. Annals of Internal Medicine 2010;152(11):704‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marsch 2005 {published data only}
- Marsch LA, Bickel WK, Badger GJ, Jacobs EA. Buprenorphine treatment for opioid dependence: relative efficacy of daily, twice and thrice weekly dosing. Drug and Alcohol Dependence 2005;77(2):195‐204. [DOI] [PubMed] [Google Scholar]
McKeganey 2013 {published data only}
- McKeganey N, Russell C, Cockayne L. Medically assisted recovery from opiate dependence within the context of the UK drug strategy: methadone and Suboxone (buprenorphine‐naloxone) patients compared. Journal of Substance Abuse Treatment 2013;44(1):97‐102. [DOI] [PubMed] [Google Scholar]
Meader 2010 {published data only}
- Meader N. A comparison of methadone, buprenorphine and alpha2 adrenergic agonists for opioid detoxification: A mixed treatment comparison meta analysis. Drug and Alcohol Dependence 2010;108(1‐2):110‐4. [DOI] [PubMed] [Google Scholar]
Montoya 2004 {published data only}
- Montoya ID, Gorelick DA, Preston KL, Schroeder JR, Umbricht A, Cheskin LJ, et al. Randomized trial of buprenorphine for treatment of current opiate and cocaine dependence. Clinical Pharmacology and Therapeutics 2004;75(1):34‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neumann 2013 {published data only}
- Neumann AM, Blondell RD, Jaanimägi MS, Giambrone AK, Homish GG, Lozano JR, et al. A preliminary study comparing methadone and buprenorphine in patients with chronic pain and coexistent opioid addiction. Journal of Addictive Diseases 2013;32(1):68‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 1998 {published data only}
- O'Connor PG, Oliveto AH, Shi JM, Triffleman EG, Carroll KM, Kosten TR, et al. A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinic. American Journal of Medicine 1998;105(2):100‐5. [DOI] [PubMed] [Google Scholar]
Oliveto 1994 {published data only}
- Oliveto A, Kosten T, Schottenfeld R, Ziedonis D, Falcioni J. Cocaine use in buprenorphine vs methadone maintained patients. American Journal on Addictions 1994;3(1):43‐8. [Google Scholar]
Pinto 2008 {published data only}
- Pinto H, Rumball D, Maskrey V, Holland R. A pilot study for a randomized controlled and patient preference trial of buprenorphine versus methadone maintenance treatment in the management of opiate dependent patients. Journal of Substance Use 2008;13(2):73‐82. [Google Scholar]
Resnick 1992 {published data only}
- Resnick RB, Galanter M, Pycha C, Cohen A, Grandison P, Flood N. Buprenorphine: An alternative to methadone for heroin dependence treatment.. Psychopharmacology Bulletin 1992;28(1):109‐13. [PubMed] [Google Scholar]
Sacerdote 2008 {published data only}
- Sacerdote P, Franchi S, Gerra G, Leccese V, Panerai AE, Somaini L. Buprenorphine and methadone maintenance treatment of heroin addicts preserves immune function. Brain, Behavior, and Immunity 2008;22(4):606‐13. [DOI] [PubMed] [Google Scholar]
Schottenfeld 1998 {published data only}
- Schottenfeld R, Pakes J, Kosten T. Prognostic factors in buprenorphine‐ versus methadone‐maintained patients. Journal of Nervous and Mental Disease 1998;186(1):35‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sigmon 2004 {published data only}
- Sigmon S, Wong C, Chausmer A, Liebson I, Bigelow G. Evaluation of an injection depot formulation of buprenorphine: placebo comparison. Addiction 2004;99(11):1439‐49. [DOI] [PubMed] [Google Scholar]
Soyka 2008b {published data only}
- Soyka M, Lieb M, Kagerer S, Zingg C, Koller G, Lehnert P, et al. Cognitive functioning during methadone and buprenorphine treatment: Results of a randomized clinical trial. Journal of Clinical Psychopharmacology 2008;28(6):699‐703. [DOI] [PubMed] [Google Scholar]
Stine 1994 {published data only}
- Stine SM, Kosten TR. Reduction of opiate withdrawal‐like symptoms by cocaine abuse during methadone and buprenorphine maintenance. Am J Drug Alcohol Abuse 1994;20(4):445‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strain 1996 {published data only}
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Buprenorphine versus methadone in the treatment of opioid dependence: self reports, urinalysis, and addiction severity index. Journal of Clinical Psychopharmacology 1996;16(1):58‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Uehlinger 1998 {published and unpublished data}
- Uehlinger C, Déglon J, Livoti S, Petitjean S, Waldvogel D, Ladewig D. Comparison of buprenorphine and methadone in the treatment of opioid dependence. Eur Addict Res 1998;4 Suppl 1:13‐8. [DOI] [PubMed] [Google Scholar]
Warden 2012 {published data only}
- Warden D, Subramaniam GA, Carmody T, Woody GE, Minhajuddin A, Poole SA, et al. Predictors of attrition with buprenorphine/naloxone treatment in opioid dependent youth. Addictive Behaviors 2012;37(9):1046‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2011 {published data only}
- Weiss RD, Sharpe Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, et al. Adjunctive counseling during brief and extended buprenorphine‐naloxone treatment for prescription opioid dependence: A 2‐phase randomized controlled trial. Archives of General Psychiatry 2011;68(12):1238‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woody 2008 {published data only}
- Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, et al. Extended vs short‐term buprenorphine‐naloxone for treatment of opioid‐addicted youth: A randomized trial. JAMA 2008;300(17):2003‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ahmadi 2002b {published data only}
- Ahmadi, J. Buprenorphine maintenance treatment of heroin dependence: the first experience from Iran. Journal of Substance Abuse Treatment 2002;22:157‐9. [DOI] [PubMed] [Google Scholar]
Cropsey 2011 {published data only}
- Cropsey KL, Lane PS, Hale GJ, Jackson DO, Clark CB, Ingersoll KS, et al. Results of a pilot randomized controlled trial of buprenorphine for opioid dependent women in the criminal justice system. Drug and Alcohol Dependence 2011;119(3):172‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Barnett 2001
- Barnett PG, Rodgers JH, Bloch D. A meta‐analysis comparing buprenorphine to methadone for treatment of opiate dependence. Addiction 2001;96(5):683‐90. [DOI] [PubMed] [Google Scholar]
Clark 2002
- Clark NC, Lintzeris N, Gijsbers A, Whelan G, Dunlop A, Ritter A, Ling WW. LAAM maintenance vs methadone maintenance for heroin dependence. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD002210] [DOI] [PubMed] [Google Scholar]
Clausen 2008
- Clausen T, Anchersen K, Waal H. Mortality prior to, during and after opioid maintenance treatment (OMT): A national prospective cross‐registry study. Drug and Alcohol Dependence 2008;94(1‐3):151‐7. [DOI] [PubMed] [Google Scholar]
Connock 2007
- Connock M, Juarez‐Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al. Methadone and buprenorphine for the management of opioid dependence; a systematic review and economic evaluation. Health Technology Assessment 2007;11(9):1‐190. [DOI] [PubMed] [Google Scholar]
Degenhardt 2009
- Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state‐wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug and Alcohol Dependence 2009;105(1‐2):9‐15. [DOI] [PubMed] [Google Scholar]
Degenhardt 2011
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, et al. Mortality among regular or dependent users of heroin and other opioids: A systematic review and meta‐analysis of cohort studies. Addiction 2011;106(1):32‐51. [DOI] [PubMed] [Google Scholar]
Degenhardt 2012
- Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet 2012;379(9810):55‐70. [DOI] [PubMed] [Google Scholar]
Dole 1965
- Dole VP, Nyswander M. A medical treatment for diacetylmorphine (heroin) addiction: a clinical trial with methadone hydrochloride. JAMA 1965;193:646‐50. [DOI] [PubMed] [Google Scholar]
Drummer 1992
- Drummer OH, Opeskin K, Syrjanen M, Cordner SM. Methadone toxicity causing death in ten subjects starting on a methadone maintenance program.. American Journal of Forensic Medicine and Pathology 1992;13(4):346–50. [DOI] [PubMed] [Google Scholar]
Faggiano 2003
- Faggiano F, Vigna‐Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD002208] [DOI] [PubMed] [Google Scholar]
Fudala 1990
- Fudala PJ, Jaffe JH, Dax EM, Johnson RE. Use of buprenorphine in the treatment of opioid addiction. II. Physiologic and behavioral effects of daily and alternate‐day administration and abrupt withdrawal. Clinical Pharmacology and Therapeutics 1990;47(4):525‐34. [DOI] [PubMed] [Google Scholar]
Gibson 2008
- Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O'Brien S. Exposure to opioid maintenance treatment reduces long‐term mortality. Addiction 2008;103(3):462‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jasinski 1978
- Jasinski D, Pevnick J, Griffith J. Human pharmacology and abuse potential of the analgesic buprenorphine. Archives of General Psychiatry 1978;35(4):501‐6. [DOI] [PubMed] [Google Scholar]
Lind 2005
- Lind B, Chen S, Weatherburn D, Mattick R. The effectiveness of methadone maintenance treatment in controlling crime. An Australian aggregate‐level analysis. Journal of Criminology 2005;45(2):201‐11. [Google Scholar]
Ling 2003
- Ling W, Wesson DR. Clinical efficacy of buprenorphine: comparisons to methadone and placebo. Drug and Alcohol Dependence 2003;70(2 Suppl):S49‐57. [DOI] [PubMed] [Google Scholar]
Mathers 2008
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet 2008;372(9651):1733‐45. [DOI] [PubMed] [Google Scholar]
Mattick 1998
- Mattick RP, Oliphant D, Ward J, Hall W. The effectiveness of other opioid replacement therapies: LAAM, heroin, buprenorphine, naltrexone and injectable maintenance. Methadone maintenance and other opioid replacement therapies. Amsterdam: Harwood Academic Publishers, 1998:123‐60. [DOI: 10.1002/14651858] [DOI] [Google Scholar]
Mattick 2009
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002209.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Minozzi 2013
- Minozzi S, Amato L, Bellisario C, Ferri M, Davoli M. Maintenance agonist treatments for opiate dependent pregnant women. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD006318.pub3] [DOI] [PubMed] [Google Scholar]
Mitchell 2004
- Mitchell TB, White JM, Somogyi AA, Bochner F. Slow‐release oral morphine versus methadone: a crossover comparison of patient outcomes and acceptability as maintenance pharmacotherapies for opioid dependence. Addiction 2004;99(8):940‐5. [DOI] [PubMed] [Google Scholar]
Nelson 2011
- Nelson PK, Mathers BM, Cowie B, Hagan H, Jarlais D, Horyniak D, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011;378(9791):571‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perneger 1998
- Perneger TV, Giner F, Rio M, Mino A. Randomised trial of heroin maintenance programme for addicts who fail in conventional drug treatments. BMJ 1998;317(7150):13‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soyka 2011
- Soyka M, Träder A, Klotsche J, Backmund M, Bühringer G, Rehm J, et al. Six‐year mortality rates of patients in methadone and buprenorphine maintenance therapy: results form a nationally representative cohort study. Journal of Clinical Psychopharmacology 2011;31(5):678‐80. [DOI] [PubMed] [Google Scholar]
UNODC 2012
- UNODC. World Drug Report 2012. www.unodc.org/unodc/en/data‐and‐analysis/WDR‐2012.html (accessed 6 January 2014) 2012; Vol. Sales No E.12.XI.1.
West 2000
- West S, O'Neal K, Graham C. A meta‐analysis comparing the effectiveness of buprenorphine and methadone. Journal of Substance Abuse 2000;12(4):405‐14. [DOI] [PubMed] [Google Scholar]
White 2007
- White JM, Lopatko OV. Opioid maintenance: a comparative review of pharmacological strategies. Expert Opinion on Pharmacotherapy 2007;8(1):1‐11. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Mattick 2008
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD002207.pub3] [DOI] [PubMed] [Google Scholar]


