To the Editor: IFN-γ enhances cell-autonomous host defense by inducing several families of antimicrobial target genes, including immunity-related GTPases (IRGs). Animals deficient in IRGM1, the best-studied IRG, succumb to numerous bacterial and protozoal infections in a manner that nearly phenocopies that of IFN-γ–null mice (1). This infection susceptibility has been attributed to the cell-intrinsic role of IRGM1 in xenophagy and targeting of pathogen-containing vacuoles (1).
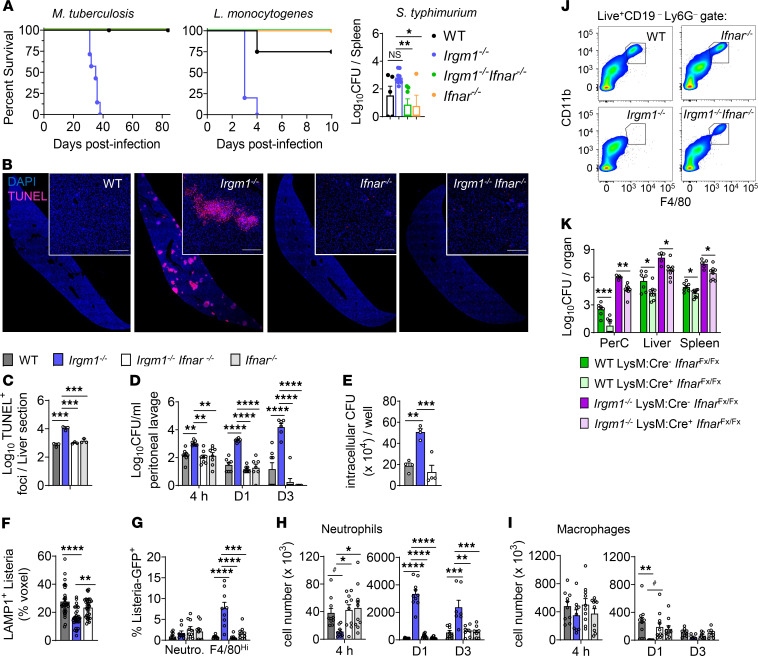
Recently, we reported that Irgm1-/- mice spontaneously produce excess type I IFN (IFN-I) (2). Although IFN-I is protective against virus, it can compromise antibacterial host defense (3). We hypothesized that IFN-I, rather than defective cell-intrinsic defenses, drives the susceptibility of Irgm1–/– mice to bacteria. Consistent with this, we found that Irgm1–/– mice succumbed to Mycobacterium tuberculosis and Listeria monocytogenes, as previously reported (1), but Irgm1–/– mice lacking the IFN-I receptor, IFNAR1 (i.e., Irgm1–/–Ifnar–/– mice), were resistant (Figure 1A). Similarly, the increased pathogen burden in Irgm1–/– mice following infection with Salmonella typhimurium was normalized in Irgm1–/–Ifnar–/– mice (Figure 1A). By contrast, during infection with Toxoplasma gondii, a pathogen for which IFN-I is host protective (4), Irgm1–/– mice had reduced survival, and this was not rescued in Irgm1–/–Ifnar–/– animals (Supplemental Figure 1A; supplemental material, including the Supplemental Methods, available online with this article; https://doi.org/10.1172/JCI171982DS1).
Figure 1. IFN-I induces susceptibility to bacterial infection in Irgm1–/– mice.
(A) Survival curves after infection with M. tuberculosis (n = 7–10) and L. monocytogenes (n = 4–5). Spleen CFU on day 21 after S. typhimurium infection (n = 4–9). (B) Liver on day 3 after L. monocytogenes infection stained for TUNEL and DAPI. Scale bar: 200 μm. (C) Quantification of TUNEL+ foci (n = 3). (D) Peritoneal lavage CFU at 4 hours, day 1, and day 3 after L. monocytogenes (n = 4–8). (E) Isolated F4/80hi peritoneal macrophages exposed to L. monocytogenes were permeabilized for CFU count after 24 hours. (F) Macrophages were stained for L. monocytogenes and lysosome (LAMP1) at 6 hours and quantified for volumetric pixels of L. monocytogenes that were LAMP1+ (n = 32 images). (G) Percentage GFP+ after 4-hour infection by GFP-tagged L. monocytogenes (n = 9–10). (H) Neutrophil and (I) F4/80hi tissue macrophage numbers in infected peritoneal lavage (n = 7–11). (J) Representative plot showing depletion of CD11b+F4/80hi macrophages at 24 hours. (K) CFU in peritoneal cavity (PerC), liver, and spleen on day 3 after L. monocytogenes (n = 5–8). Data are shown as the mean ± SEM. #P < 0.08, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 (1-way ANOVA with Tukey’s adjustment for A and C–I or Student’s t test for K).
To investigate IFN-I’s mechanism of compromising host defense in Irgm1–/– mice, we pursued the L. monocytogenes infection model. After infection, Irgm1–/– mice had elevated biomarkers of organ damage in sera (Supplemental Figure 1B) and increased inflammation and necrosis in livers and spleens (Supplemental Figure 1, C–E), phenotypes that were rescued in Irgm1–/–Ifnar–/– mice. Increased cell death in Irgm1–/– livers and spleens was dependent on IFN-I signaling (Figure 1, B and C, and Supplemental Figure 1, F and G). Compared with that in WT and Irgm1–/–Ifnar–/– organs, there was increased L. monocytogenes growth in Irgm1–/– organs (Supplemental Figure 1, H–J). Increased growth was seen by 4 hours after infection in the peritoneum (Figure 1D), the site of L. monocytogenes inoculation in our model, indicating that IFN-I suppresses clearance of L. monocytogenes upon initial encounter. Indeed, Irgm1–/– F4/80hi peritoneal macrophages internalized L. monocytogenes normally in vitro (Supplemental Figure 2A) but had reduced killing capacity (Figure 1E). This was associated with decreased lysosomal delivery of L. monocytogenes (Figure 1F and Supplemental Figure 2B), despite normal lysosomal mass (Supplemental Figure 2C) and pH (data not shown) in Irgm1–/– macrophages. L. monocytogenes–challenged Irgm1–/– F4/80hi peritoneal macrophages also had higher expression levels of STAT1, STAT2, (Y701-)PO4-STAT1, and (Y689-)PO4-STAT2 than their WT and Irgm1–/–Ifnar–/– counterparts (data not shown). In vivo, 4 hours after infection by GFP-expressing L. monocytogenes, only Irgm1–/– F4/80hi macrophages showed increased bacterial load (Figure 1G and Supplemental Figure 2D).
Given that IFN-I may induce cell death (3), we examined peritoneal myeloid cells for viability (Supplemental Figure 2E). Lytic death was increased in the Irgm1–/– peritoneum on days 1 and 3 after infection and was IFN-I–dependent (Supplemental Figure 3A). Fewer neutrophils were recruited by 4 hours after L. monocytogenes infection to Irgm1–/– peritonea, but neutrophil accumulation increased dramatically after 24 hours in an IFN-I–dependent manner (Figure 1H), and increased citrullinated histones, a marker of lytic neutrophil death by NETosis, were detected on day 3 (Supplemental Figure 3B). Increased IFN-I–dependent lytic death was also observed among F4/80hi macrophages (Supplemental Figure 3A), perhaps explaining their depletion 24 hours after infection (Figure 1, I and J). Notably, increased staining of phosphorylated mixed lineage kinase domain–like pseudokinase, a necroptosis effector, was observed only in Irgm1–/– macrophages (Supplemental Figure 3, C and D). Thus, IFN-I promotes multiple modes of proinflammatory lytic cell death in Irgm1–/– mice. Accordingly, Irgm1–/– peritoneal fluid exhibited an IFN-I–dependent increase in lactate dehydrogenase activity and proinflammatory cytokines (Supplemental Figure 3, E and F).
During peritonitis, death of resident macrophages leads to recruitment and reprogramming of Ly6ChiF4/80– monocytes into Ly6C– F4/80hi macrophages, often through an MHCII+F4/80lo/int intermediate (5). We observed emergence of a small F4/80+ population on day 3 after L. monocytogenes infection (Supplemental Figure 2E). Unlike their WT, Ifnar–/–, and Irgm1–/–Ifnar–/– counterparts, all CD11b+F4/80hi macrophages in the Irgm1–/– peritoneum at day 3 after L. monocytogenes infection retained high Ly6C and did not express TIM4 (Supplemental Figure 4, A and B), a maturity marker of peritoneal macrophages (5). The CD11b+F4/80lo population in Irgm1–/– animals remained Ly6Chi at day 3 and lacked a MHCII+ subpopulation (Supplemental Figure 4C). The receptor for colony-stimulating factor-1 (CD115), which is critical for survival and differentiation of monocytes, was repressed in Irgm1–/– Ly6Chi cells in an IFN-I–dependent manner (Supplemental Figure 4C). Ly6Chi monocytes were also elevated in Irgm1–/– blood and showed reduced CD115 and MHCII (Supplemental Figure 4E). These results suggest that excess IFN-I in Irgm1–/– mice impairs maturation of inflammatory Ly6Chi monocytes into macrophages, possibly by repressing CD115.
To specifically examine myeloid IFN-I signaling, we infected Irgm1–/– mice lacking IFNAR1 solely in myeloid cells (Irgm1–/–LysM:Cre+IfnarFx/Fx mice). These mice showed decreased necrotic death of peritoneal myeloid cells, partial rescue of CD115 in CD11b+F4/80loLy6C- cells (Supplemental Figure 4, F and G), and reduced bacterial burden (Figure 1K) compared with controls, indicating that myeloid IFN-I signaling compromises myeloid cell fate and host defense in Irgm1–/– mice.
Our findings challenge the long-prevailing paradigm that IRGM1 serves as an IFN-γ–induced cell-autonomous host defense effector (1) and suggest instead that IRGM1 supports host defense by preventing excess autocrine and/or paracrine IFN-I from compromising myeloid cell fate and function. Future studies will be required to distinguish autocrine versus paracrine mechanisms.
Supplementary Material
Version 1. 09/12/2023
In-Press Preview
Version 2. 11/01/2023
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2023, Rai et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2023;133(21):e171982. https://doi.org/10.1172/JCI171982.
Contributor Information
Prashant Rai, Email: prashant.rai@nih.gov.
Martin Sharpe, Email: martin.sharpe@nih.gov.
Charan K. Ganta, Email: charan.ganta@nih.gov.
Paul J. Baker, Email: pjb.11@outlook.com.
Katrin D. Mayer-Barber, Email: mayerk@niaid.nih.gov.
Brian E. Fee, Email: Brian.Fee@duke.edu.
Gregory A. Taylor, Email: taylo018@mc.duke.edu.
Michael B. Fessler, Email: fesslerm@niehs.nih.gov.
References
- 1.Dockterman J, Coers J. How did we get here? Insights into mechanisms of immunity-related GTPase targeting to intracellular pathogens. Curr Opin Microbiol. 2022;69:102189. doi: 10.1016/j.mib.2022.102189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rai P, et al. IRGM1 links mitochondrial quality control to autoimmunity. Nat Immunol. 2021;22(3):312–321. doi: 10.1038/s41590-020-00859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNab F, et al. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15(2):87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matta SK, et al. Toxoplasma gondii effector TgIST blocks type I interferon signaling to promote infection. Proc Natl Acad Sci U S A. 2019;116(35):17480–17491. doi: 10.1073/pnas.1904637116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ardavin C, et al. Mouse tissue-resident peritoneal macrophages in homeostasis, repair, infection, and tumor metastasis. Adv Sci (Weinh) 2023;10(11):e2206617. doi: 10.1002/advs.202206617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.