Abstract
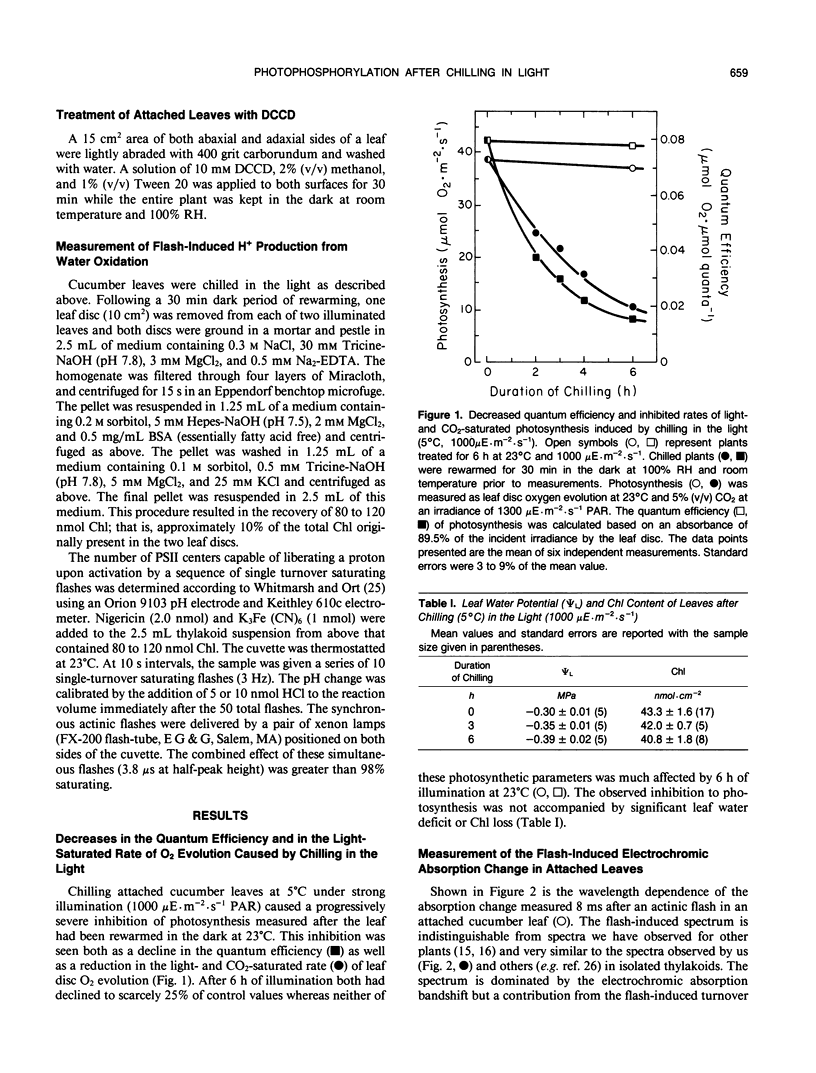
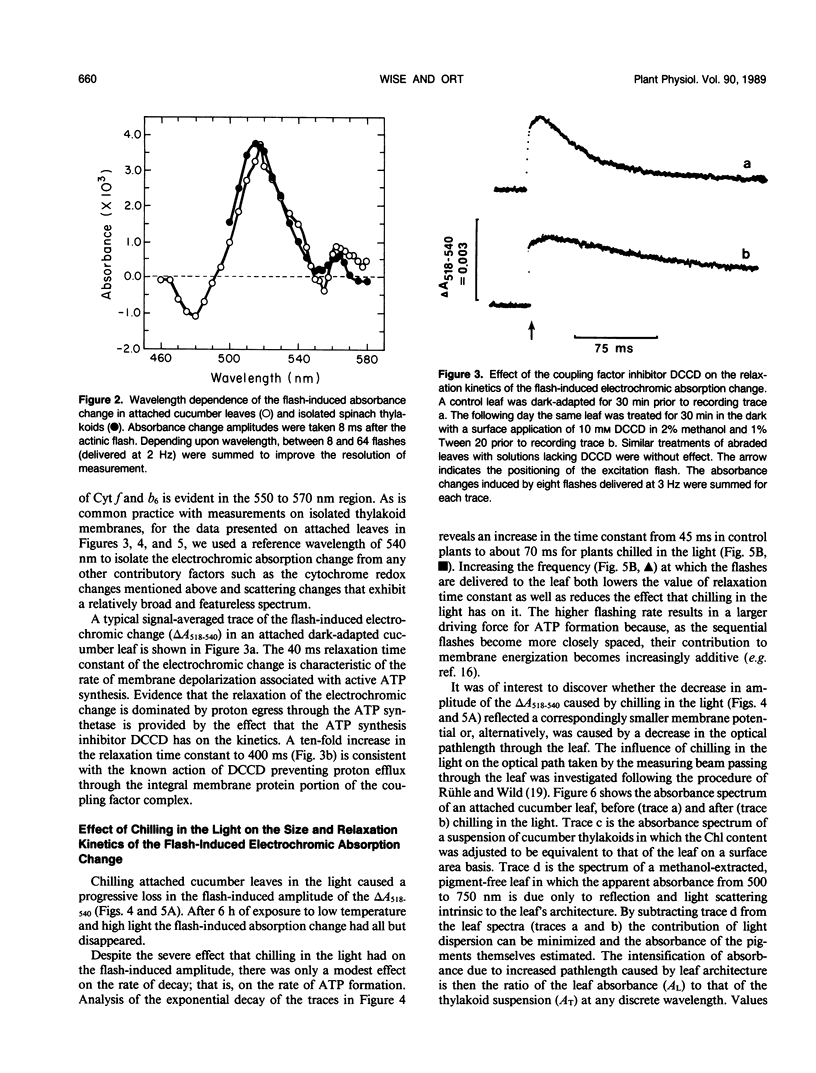
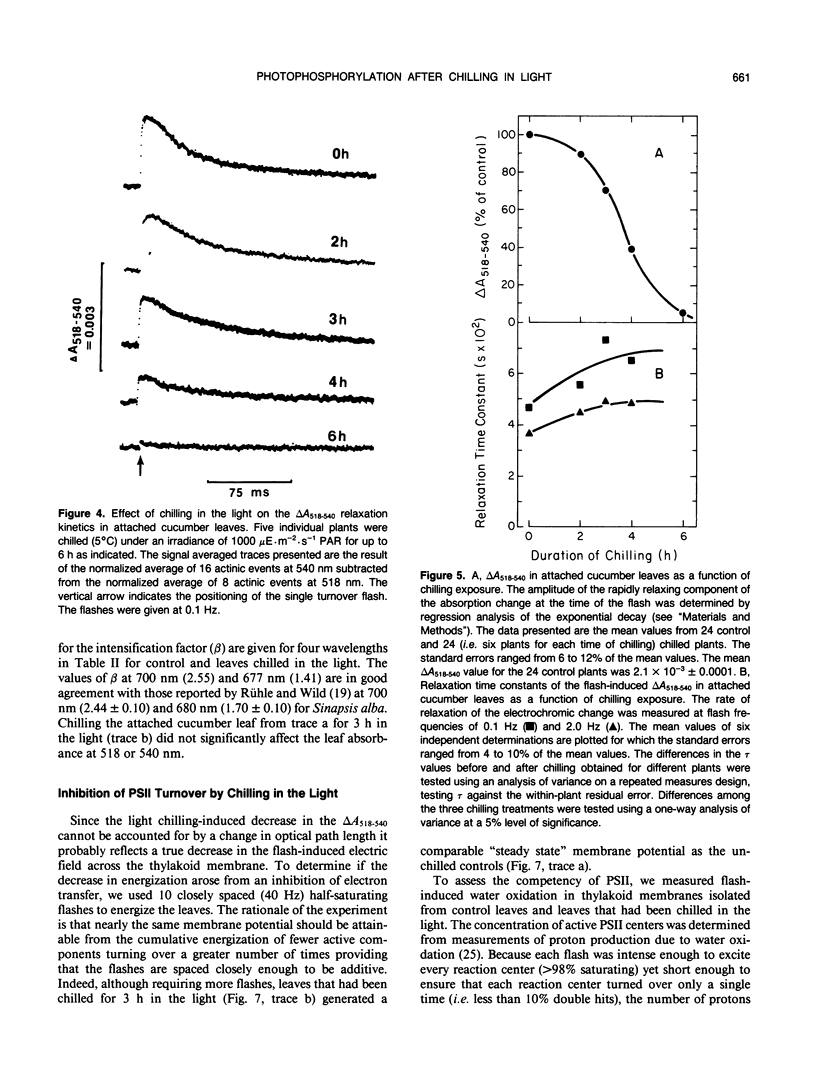
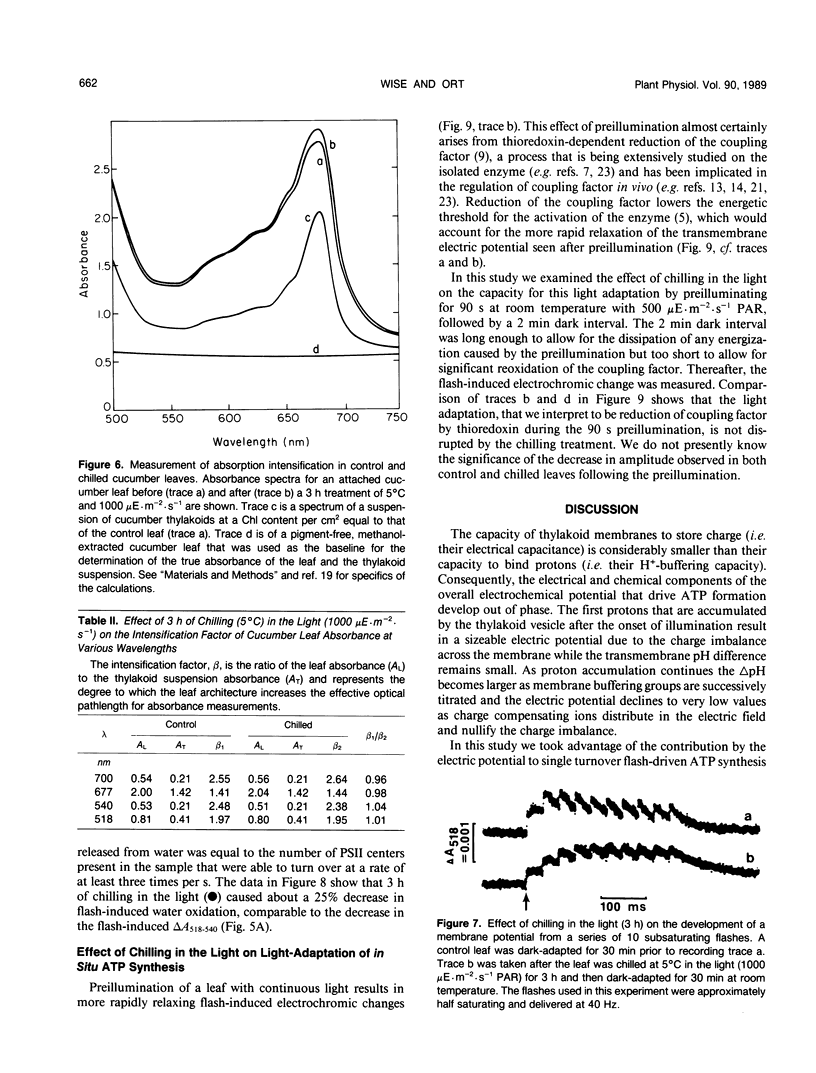
The response of in situ photophosphorylation in attached cucumber (Cucumis sativus L. cv Ashley) leaves to chilling under strong illumination was investigated. A single-beam kinetic spectrophotometer fitted with a clamp-on, whole leaf cuvette was used to measure the flash-induced electrochromic absorbance change at 518 minus 540 nanometers (ΔA518−540) in attached leaves. The relaxation kinetics of the electric field-indicating ΔA518−540 measures the rate of depolarization of the thylakoid membrane. Since this depolarization process is normally dominated by proton efflux through the coupling factor during ATP synthesis, this technique can be used, in conjuction with careful controls, as a monitor of in situ ATP formation competence. Whole, attached leaves were chilled at 5°C and 1000 microeinsteins per square meter per second for up to 6 hours then rewarmed in the dark at room temperature for 30 minutes and 100% relative humidity. Leaf water potential, chlorophyll content, and the effective optical pathlength for the absorption measurements were not affected by the treatment. Light- and CO2-saturated leaf disc oxygen evolution and the quantum efficiency of photosynthesis were inhibited by approximately 50% after 3 hours of light chilling and by approximately 75% after 6 hours. Despite the large inhibition to net photosynthesis, the measurements of ΔA518−540 relaxation kinetics showed photophosphorylation to be largely unaffected by the chilling and light exposure. The amplitude of the ΔA518-540 measures the degree of energization of the photosynthetic membranes and was reduced significantly by chilling in the light. The cause of the decreased energization was traced to impaired turnover of photosystem II. Our measurements showed that the chilling of whole leaves in the light caused neither an uncoupling of photophosphorylation from photosynthetic electron transport nor any irreversible inhibition of the chloroplast coupling factor in situ. The sizeable inhibition in net photosynthesis observed after chilling in the light cannot, therefore, be attributed to any direct effect on photophosphorylation competence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Drake B., Raschke K. Prechilling of Xanthium strumarium L. Reduces Net Photosynthesis and, Independently, Stomatal Conductance, While Sensitizing the Stomata to CO(2). Plant Physiol. 1974 Jun;53(6):808–812. doi: 10.1104/pp.53.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber M. P. Effect of Light and Chilling Temperatures on Chilling-sensitive and Chilling-resistant Plants. Pretreatment of Cucumber and Spinach Thylakoids in Vivo and in Vitro. Plant Physiol. 1977 May;59(5):981–985. doi: 10.1104/pp.59.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graan T., Ort D. R. Quantitation of the rapid electron donors to P700, the functional plastoquinone pool, and the ratio of the photosystems in spinach chloroplasts. J Biol Chem. 1984 Nov 25;259(22):14003–14010. [PubMed] [Google Scholar]
- Hangarter R. P., Grandoni P., Ort D. R. The effects of chloroplast coupling factor reduction on the energetics of activation and on the energetics and efficiency of ATP formation. J Biol Chem. 1987 Oct 5;262(28):13513–13519. [PubMed] [Google Scholar]
- Ketcham S. R., Davenport J. W., Warncke K., McCarty R. E. Role of the gamma subunit of chloroplast coupling factor 1 in the light-dependent activation of photophosphorylation and ATPase activity by dithiothreitol. J Biol Chem. 1984 Jun 10;259(11):7286–7293. [PubMed] [Google Scholar]
- Martin B., Ort D. R., Boyer J. S. Impairment of photosynthesis by chilling-temperatures in tomato. Plant Physiol. 1981 Aug;68(2):329–334. doi: 10.1104/pp.68.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeler T. C., Naylor A. W. A Comparison of the Effects of Chilling on Thylakoid Electron Transfer in Pea (Pisum sativum L.) and Cucumber (Cucumis sativus L.). Plant Physiol. 1988 Jan;86(1):147–151. doi: 10.1104/pp.86.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty K. M., Jackson J. B. Correlation between ATP synthesis and the decay of the arotenoid band shift after single flash activation of chromatophores from Rhodopseudomonas capsulata. Biochim Biophys Acta. 1979 Sep 11;547(3):463–473. doi: 10.1016/0005-2728(79)90027-6. [DOI] [PubMed] [Google Scholar]
- Shahak Y. Activation and Deactivation of H-ATPase in Intact Chloroplasts. Plant Physiol. 1982 Jul;70(1):87–91. doi: 10.1104/pp.70.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. O., Rowley J. A. Plants under Climatic Stress: I. Low Temperature, High Light Effects on Photosynthesis. Plant Physiol. 1971 May;47(5):713–718. doi: 10.1104/pp.47.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejos R. H., Arana J. L., Ravizzini R. A. Changes in activity and structure of the chloroplast proton ATPase induced by illumination of spinach leaves. J Biol Chem. 1983 Jun 25;258(12):7317–7321. [PubMed] [Google Scholar]
- Whitmarsh J., Ort D. R. Stoichiometries of electron transport complexes in spinach chloroplasts. Arch Biochem Biophys. 1984 Jun;231(2):378–389. doi: 10.1016/0003-9861(84)90401-6. [DOI] [PubMed] [Google Scholar]
- Witt H. T. Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The central role of the electric field. Biochim Biophys Acta. 1979 Mar 14;505(3-4):355–427. doi: 10.1016/0304-4173(79)90008-9. [DOI] [PubMed] [Google Scholar]


