Abstract
Background
Diabetic retinopathy (DR) is a chronic diabetes complication. People with Type 2 Diabetes Mellitus (T2DM) have two times the risk for dementia, suggesting it is a new chronic diabetes complication.
Objective
Evaluate the association of DR with cognitive performance in a T2DM population.
Methods
Cross-sectional study with 400 T2DM adults from whom socio-demographic, clinical, laboratory data were collected, and screening test for depression symptoms (Patient Health Questionaire-9 (PHQ-9)), Mini-Mental State Examination (MMSE), Semantic Verbal Fluency Test, Trail Making Test A and B, Word Memory test were performed. All cognitive test scores were converted into Global Cognition z-Score (GCS(z)). The association between GCS(z) < 0 with DR was performed using a multivariate binary logistic regression model adjusted for age ≥ 65 years, school years ≤ 6 years, DM duration ≥ 10 years, depression symptoms score > 9 at PHQ-9, arterial hypertension, physical activity, diabetic retinopathy, macular edema, and cardiovascular disease.
Results
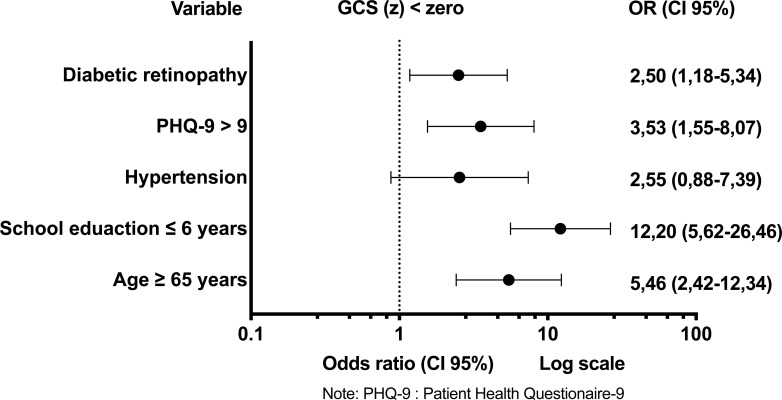
After exclusions, the 251 eligible patients were 56.6% female, with a mean age of 61.1 (±9.8) years, DM duration of 12.6 (±8.9) years, and 7.6 (±4.2) years of school education. DR prevalence was 46.5%. Multivariate Logistic Regression Model showed an association between DR and GCS(z) < 0, with odds ratio (CI95%) of 2.50 (1.18-5.34), adjusted for age, low education level, arterial hypertension and depression symptoms (OD and CI95% respectively: 5.46(2.42-12.34); 12.19 (5.62-26.46); 2.55 (0.88-7.39); 3.53 (1.55-8.07)).
Conclusion
In this T2DM population, having DR increased the chance for worse cognitive performance even when adjusted for age, low education level, presence of arterial hypertension, and depression symptoms.
Keywords: Diabetic retinopathy, cognitive dysfunction, type 2 diabetes mellitus, risk factors, dementia, cognitive decline
1. INTRODUCTION
Type 2 Diabetes Mellitus has long-term micro and macrovascular complications related to DM duration, glycemic control, and comorbidities. Among these complications, diabetic retinopathy (DR) is, according to the World Health Organization, responsible for 4.8% of blindness cases in the world. A meta-analysis of 35 studies with 22,896 people with Type 1 and 2 DM, evaluated between 1980 and 2008, in the United States of America, Australia, Europe,and Asia, found a global prevalence of DR of 34.6% and critical loss of visual acuity of 10.2%. For type 2 DM alone, DR prevalence was 25.2%, and critical loss of visual acuity was 6.9% [1-4]. Longitudinal studies identified that the risk for dementia is greater in the population with diabetes [5-12]. This risk is 1.73 for all types of dementia when compared to people without diabetes [12, 13]. The mechanisms by which DM acts as a casual or accelerating factor in the dementia process, whether vascular dementia or Alzheimer’s dementia, are not yet fully known. Several pathogenic mechanisms have been postulated, among them: chronic hyperglycemia and its enzymatic glycation end products, insulin resistance, glycemic variability, hypoglycemia, DM microvascular disease, cardiovascular disease and its risk factors, in addition to inflammation, oxidative stress, and metabolism of amyloid peptides, amylin and tau protein [14-16]. Microvascular dysfunction is well recognized as part of DM complications, including cerebral microvascular dysfunction. The genesis of microvascular dysfunctions is associated with arterial hypertension, hyperglycemia, obesity, and insulin resistance [17]. Among the classic DM microvascular complications, DR has been identified in cross-sectional and longitudinal studies as a risk factor for the presence of dementia and cognitive dysfunction in patients with Type 1 and 2 DM [18-21]. It seems logical to think that micro and macrovascular alterations that occur in other parts of the body happen similarly in the brain. According to this logic, the retinal microvascular evaluation would mirror the brain microvasculature since the retina and the brain have very similar anatomy, embryology, and physiology. A review of studies that evaluated the association between retinal vascular alterations and dementia or cognitive deterioration found an association between retinal alterations and these outcomes, as well as with the alterations in brain imaging. This association was greater as the retinal alterations were more severe and suggested vascular pathophysiology, but the effect size was modest, possibly due to the concurrence of other associated risk factors [22]. On the other hand, another populational study showed an association between retinal neurodegeneration and the presence of cerebral atrophy and not with cerebral vascular alterations, suggesting that this association may be due to neuronal degeneration and not to vascular dysfunction [23]. Therefore, the mechanisms of these associations are not yet fully clear, and further studies are necessary to better understand the pathophysiology of this association and identify whether vascular or retinal neurodegeneration markers can be used as predictors of cognitive dysfunction in this population, considering that until now, there is still no specific biomarker or set of biomarkers, as well as no imaging exam to determine future risk of minimal cognitive dysfunction (MCD) and dementia or to determine the ones who will have a worse prognosis [24]. This study aimed to assess the association of DR and macular edema with cognitive performance in a population of T2DM patients in an upper-middle-income country.
2. MATERIALS AND METHODS
2.1. Study Design and Sample
A cross-sectional study was conducted in a tertiary hospital in Southern Brazil, from September 2017 to December 2020, with patients over 18 years old with T2DM of both genders, randomly recruited according to their attendance at their routine appointments. Patients with T2DM were considered those who did not need insulin in the first 3 years of the disease and had no history of ketonuria or ketonemia at diagnosis [25]. Patients who were using medicines that alter cognition (benzodiazepines, hypnotics, antipsychotics, tricyclic antidepressants, anticonvulsants, anticholinergics, and antihistamines) were excluded, as well as those who were unable to perform the cognitive tests due to illiteracy, vision or hearing impairment. Those with a previous diagnosis of dementia of any etiology, stroke, traumatic brain injury, Parkinson's disease, schizophrenia, or any other situation that affects cognition were also excluded, as well as the ones that met dementia criteria at the MMSE test. The study was approved by the Research Ethics Committee and conducted following the principles of the Declaration of Helsinki [26].
2.2. Data Collection
Participants answered a questionnaire containing demographic data (age, gender, race, marital status, and school education), lifestyle data (physical activity, alcohol consumption, smoking), and medical history [DM onset age, acute complications (severe hypoglycemia) and diabetes chronic (retinopathy, neuropathy, diabetes kidney disease, cardiovascular disease)], comorbidities and use of medication. Additional information was captured from medical records. It was considered physically active participants who met the criteria of at least 150 minutes of moderate or 75 minutes of intensive aerobic exercise per week. Severe hypoglycemia was defined as that in which the patient needed help from others for treatment and/or had a decrease in consciousness level, with improvement in symptoms after treatment [27]. The following data was collected from the physical examination: Body Mass Index (BMI), Abdominal and Neck Circumference, Systolic Blood Pressure (SBP), and Diastolic Blood Pressure (DBP). Arterial hypertension diagnosis was defined as SAP ≥ 140 mmHg and DBP ≥ 90 mmHg or also if using antihypertensive medication [28]. Retina clinical examination was performed at the ophthalmologic clinic by retinal mapping under drug-induced mydriasis by indirect binocular ophthalmoscopy and slit-lamp biomicroscopy and, when indicated, by fluorescein angiography and optical coherence tomography classified as no diabetic retinopathy (DR), non-proliferative DR, proliferative DR, and macular edema [25, 29, 30]. Diabetic neuropathy was considered in the presence of clinical symptoms and signs compatible with peripheral sensory-motor neuropathy according to the guidelines of the Brazilian Diabetes Society, based on peripheral neurological clinical examination [25]. Laboratory tests and complementary tests data were retrieved from medical records: glycated hemoglobin a1c (HBA1c), fasting glucose, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL) cholesterol, triglycerides, thyroid-stimulating hormone, free thyroxine, B12 vitamin, creatinine, and albumin-to-creatinine ratio (ACR). Creatinine value was used to calculate the estimated glomerular filtration rate (eGFR) adjusted for age and gender using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula [25, 27, 31, 32]. DM kidney disease was considered in the presence of eGFR < 60ml/min/1.73m2 or ACR > 30 mg/g persistently elevated for more than 3 months, following the recommendations of KDIGO (Kidney Disease: Improving Global Outcomes) and the guidelines of the Brazilian Diabetes Society [25, 33]. Diagnosis of dyslipidemia was based on the Guidelines of the Brazilian Cardiology Society (LDL ≥100 mg/dl if intermediate cardiovascular risk, ≥ 70 mg/dl if high cardiovascular risk; HDL ≤ 45 mg/dl; Triglycerides ≥ 150 mg/dl or using lipid lowering medication) [34].
2.3. Cognitive and Depression Symptoms Assessment
The cognitive tests performed, validated in Portuguese, were: Trail Making Test A and B to assess sustained attention, mental flexibility, executive function, spatial/visual organization, and processing speed [35-38]; Semantic Verbal Fluency test to assess semantic memory storage capacity, ability to retrieve information from memory, and processing of executive functions [37-40]; CERAD (The Consortium to Establish a Registry for Alzheimer’s Disease) Word Memory Test to assess memory [38-40] and Mini-Mental State Examination, to screen patients with dementia and make a global assessment of cognition, covering aspects of orientation, memory, attention, calculation, language, and comprehension [32, 35-41]. The cutoff value of this test varies according to education level, and scores with values below the cutoff corrected by the education level in the Brazilian population were used to indicate the risk of dementia [42-47] (Supplementary file 1 (3MB, zip) ). The PHQ-9 (Patient Health Questionaire-9), validated in Portuguese, was carried out and values above 9 were considered as a risk of a diagnosis of major depression (Supplementary file 2 (3MB, zip) ) [48, 49].
2.4. Statistical Analysis
To uniformly analyze the set of different cognitive tests, the results of all tests were transformed into Z scores, added, and divided by the total number of tests. This result created a continuous variable called Global Cognitive Score [GCS(z)]. A GCS(z) < 0 at baseline means a worse cognitive performance compared to the group. The association estimation of GCS(z) < 0 with DR adjusted for risk factors for cognitive dysfunction was performed using backward conditional multivariate binary logistic regression. The categorized variables chosen to be included in the model were: age 65 years (yes/no), school education 6 years (yes/no), gender (female/male), physically activity (yes/no), smoking (current or previous/never) alcoholism (current or previous/never), severe hypoglycemia (yes/no), DM 10 years (yes/no), PHQ-9 > 9 (yes/no), arterial hypertension (yes/no), depression/anxiety diagnosis (yes/no), cardiovascular disease (yes/no), use of insulins (yes/no), use of statins (yes/no), hypothyroidism (yes/no), any DR (yes/no), macular edema (yes/no), diabetic neuropathy (yes/no), DM kidney disease(yes/no), BMI ≥ 30 kg/m2 (yes/no), eGFR < 60 ml/min/1.73 m2 (yes/no) and HBA1c ≥ 7%(yes/no). Variables with p < 0.25 in the univariate binary regression analysis were used in the final model [44]. For all other tests, the significance level used was 5% (SPSS version 22. IBM Corporation, Armonk, NY®) [50].
3. RESULTS
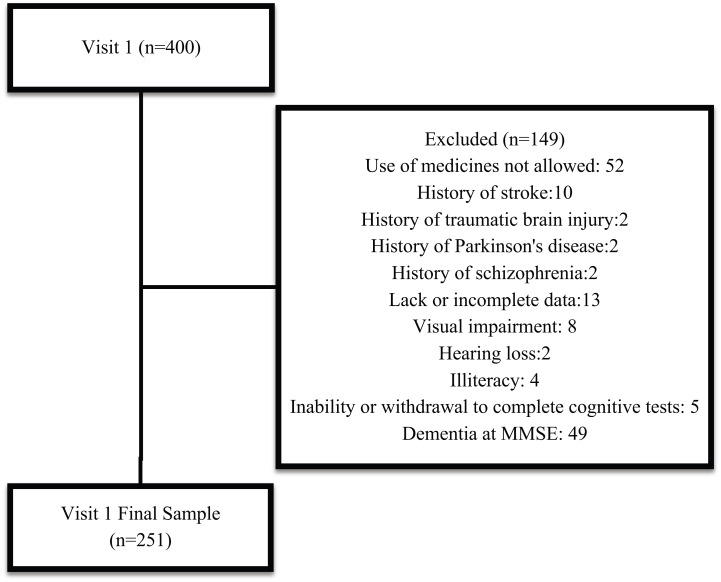
Among the 400 patients evaluated, 149 were excluded (Fig. 1).
Fig. (1).
Sample flux diagram.
The final sample consisted of 251 patients, 56,6% female, with mean age of 61,1 (±9,8) years, 12,6 (±8,9) years of DM duration, and 7,6 (±4,2) years of school education. Two hundred and six patients (82,4%) were hypertensive and 89% had dyslipidemia. The prevalence of at least one microvascular complication was 54%, with DR being prevalent in 46,5%. Cardiovascular disease was present in 35,2%. In the screening for depression symptoms by the PHQ-9, 37,1% had a score compatible with the risk of major depression and 21,3% had presented at least one episode of severe hypoglycemia in the previous year. DBP value was controlled in approximately 36% of the patients and SBP in 65% (Table 1). Data from laboratory tests were within normal ranges, except for lipid profile, fasting glucose, and HBA1c, with only 30% having HBA1c < 7%. LDL cholesterol levels were within expectations in about 21% of patients and HDL cholesterol in 40%, according to individual stratification of individual risk (Table 1). The prevalence of GCS(z) < 0 was 46,6% and the summary of cognitive tests and GCS(z) results are described in Table 1 (Supplementary Files 3 (3MB, zip) and 4 (3MB, zip) ).
Table 1.
Sample characteristics.
| Characteristics | Mean ± SD / Median (IQR)*/% |
|---|---|
| Age (years) | 61.1 ± 9.8 |
| School education (years) | 7.6 ± 4.2 |
| DM duration (years) | 12.6 ± 8.9 |
| Female (%) | 56.6 |
| Physically Active (%) | 27.9 |
| Smoker/Former Smoker (%) | 47.1 |
| Alcoholic/Former alcoholic (%) | 29.5 |
| Diastolic Blood Pressure (mmHg) | 80.4 ±10.8 |
| Systolic Blood Pressure (mmHg) | 131.2 ± 17.8 |
| BMI (Kg/m2) | 30.8 ± 5.3 |
| Arterial hypertension (%) | 82.4 |
| Dyslipidemia (%) | 89.0 |
| Hypothyroidism (%) | 26.1 |
| Cardiovascular disease (%) | 35.2 |
| Diabetic Retinopathy (%) | 46.5 |
| Macular edema (%) | 13.8 |
| Diabetic Neuropathy (%) | 16.1 |
| Diabetic kidney disease (%) | 54.0 |
| Severe hypoglycemia (%) | 21.3 |
| Depression/Anxiety (%) | 22.8 |
| PHQ-9 score > 9 (%) | 37.1 |
| Insulin Use (%) | 58.8 |
| Statins Use (%) | 76.2 |
| eGFR (ml/min/1.73m2) | 84.8(36.3) |
| HbA1c (%) | 8.0(2.4) |
| ACR (mg/g creatinine)* | 21.4(80.6) |
| MMSE (score)* | 27.2 ± 2.0 |
| Verbal fluency (score) | 16.5 ± 4.9 |
| TMT A (seconds) | 56.3 ± 27.9 |
| TMT B (seconds) | 162.8 ± 107.7 |
| Immediate Memory (score) | 16.3 ± 4.5 |
| Recall Memory (score) | 5.1 ±1.9 |
| Recognition Memory (score) | 8.1 ± 2.0 |
| GCS(z) (score) | -0.015 ± 0.671 |
| GCS(z) < 0 (%) | 46.6 |
Note: SD: Standard deviation: * IQR: Interquartile range
DM: Diabetes Mellitus: BMI: Body Mass Index: PHQ-9: Patient Health Questionnaire-9, eGFR: estimated Glomerular Filtration Rate, ACR: Albumin-to-creatinine ratio, TSH: thyroid-stimulating hormone, MMSE: Mini-Mental State Exam, TMT A and B: Trail making test A and B: GCS(z), Global Cognitive score (z).
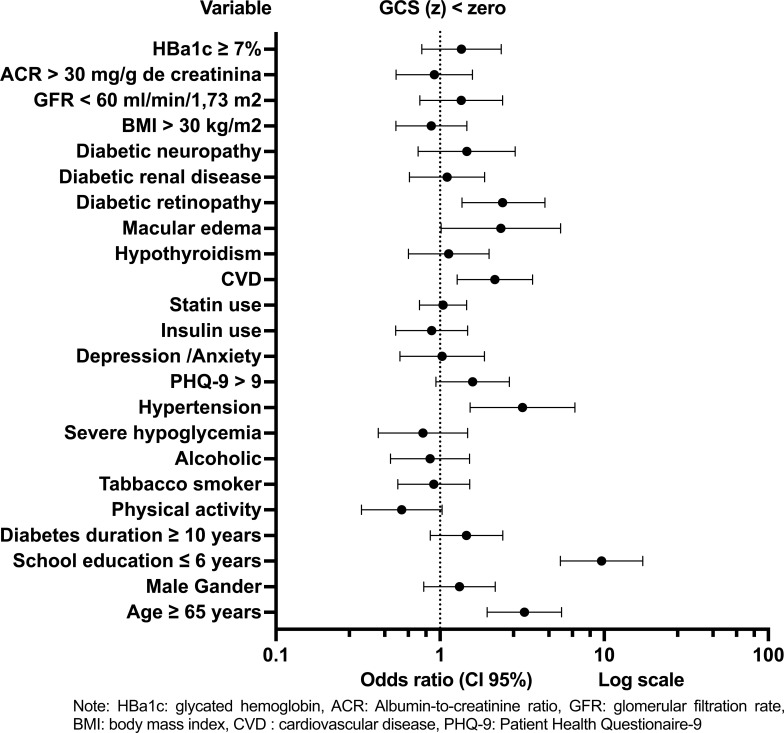
In the univariate binary logistic regression model, variables identified as significantly associated with GCS(z) < 0 and included in the multiple regression model were: age ≥ 65 years, school education 6 years, DM duration ≥ 10 years, physical activity, score on the PHQ-9 questionnaire > 9, arterial hypertension, cardiovascular disease, DR, and macular edema (Fig. 2) (Supplementary Files 5 (3MB, zip) ).
Fig. (2).
Predictive factors for Global Cognitive Score < 0 Univariate Logistic Regression.
The multivariate logistic regression model showed an association between DR and GCS(z) < 0, with odds ratio (OD) and 95% confidence interval (CI95%) of 2,50 (1,18-5,34); even after adjustment for age ≥ 65 years, school education years ≤ 6, arterial hypertension and depression symptoms [OD and CI95% respectively: 5,46(2,42-12,34); 12,19(5,62-26,46); 2,55(0,88-7,39); 3,53(1,55-8,07)] (Fig. 3) (Supplementary File 6 (3MB, zip) ).
Fig. (3).
Predictive factors for global cognitive score < 0. Multivariate logistic regression.
4. DISCUSSION
In this sample, DR was associated with worse cognitive performance, regardless of advanced age, low education level, arterial hypertension, and depression symptoms.
Age and schooling are well-known and expected MCD and dementia risk factors [51-54]. In this sample, schooling was the risk factor that most contributed to a chance of scoring below average on the GCS(z), followed by age. The Brazilian Longitudinal Study of Adult Health (ELSA-Brazil) identified that education, more than age, interferes with the results of the cognitive tests in one Brazilian population. Another regional study that assessed cognitive performance in a population of DM patients found the same association between the MMSE results, education, and age [47]. In the last Dementia Prevention, Intervention and Care Commission report, published in the Lancet magazine in 2020, school education lower or equal to 4 years was identified as a relative risk of 1.6 for the development of dementia and predicted that if only this risk factor could be eliminated, the prevalence of dementia would be reduced by 7% [55]. These findings reinforce the need to assess the results of cognitive tests according to education and age, or at least adjust them for these variables.
The retina and the brain have the same embryological origin and similar pathophysiological and aging mechanisms. Therefore, it can be an easily accessible source of information for cerebral neurodegenerative processes. Communication between the brain and the retina occurs through retinal ganglion cells, connecting with the cortex through the optic nerve. The blood-brain and blood-retinal barriers regulate the supply of oxygen and glucose to the neurons and prevent the exposure of the central nervous system to pathogens and toxic substances, thus protecting the central nervous system and retina microenvironment, respectively. Among other functions, the barrier tries to protect neurons from inflammatory cytokines commonly circulating in patients with diabetes and its comorbidities [56, 57]. The production or activation of inflammatory cytokines at the brain level can lead to insulin action resistance in the brain, resulting in deterioration of brain processes such as neuron survival, dendritic plasticity, synaptic function, learning, and memory [58-60].
Optical coherence tomography (OCT) is a non-invasive exam that allows the measurement of retinal thickness and its components. The retinal nerve fiber layer is the innermost part of the retina and is formed by retinal ganglion cells and alterations in this region are associated with neurodegeneration [20, 61, 62]. The Rotterdam cohort study found a correlation between neurodegenerative retinal alterations detected in OCT with brain atrophy, but not with micro-hemorrhages or lacunar infarcts in the nuclear magnetic resonance [23]. Retinal glial, neural, and microvascular dysfunction are interdependent for the development of diabetic retinopathy. A thinner retinal nerve fiber layer thickness is present even with minimal DR in T1DM, possibly anteceding the retina's vascular deterioration [15, 63]. Other studies have observed an association between corneal neurodegeneration findings with cognitive alterations and imaging findings related to MCD and dementia [64, 65].
Retinal microcirculation can also be non-invasively visualized using retinal arteriography and its alterations have also been associated with cognitive decline in Type 1 and 2 DM populations with long-term duration [18, 20, 21, 66-68]. Therefore, both, microcirculation and neurodegenerative retinal and corneal alterations are associated with cognitive changes and dementia, but the mechanisms supporting this association are not fully elucidated.
Visual loss on its own may also be a risk factor or be associated with MCD and dementia. A meta-analysis of prospective studies in the general population showed that moderate and severe loss of visual acuity is associated with cognitive dysfunction. However, evidence quality is low and other studies disagree on this topic. Therefore, it is still necessary to confirm this possible association [69-74].
Recently a systematic review and meta-analysis of twenty-two studies, including cross-sectional and cohort studies showed a similar association between DR and cognitive impairment. In this study, the presence of DR reflected a higher cognitive dysfunction with OR=2,45(95%CI:1,76-3,41) and HR=1,34(95%CI:1,10-1,62). The pooled OR was 2,38 and 3,11 for Asia and Oceania respectively, and there was no association in North America and with T1DM. There was no study from South America. They also found that DR severity showed a positive correlation with cognitive impairment [75]. One other review and meta-analysis evaluated the association between DR and cerebral small vessel disease with any type of cognitive dysfunction and found an association between DR and structural abnormalities in the brain and impaired cognitive function [76].
Ophthalmologic evaluation is already part of routine screening for complications related to DM and is recommended at diagnosis and once a year thereafter. Further exploring the association of retinal alterations in the DM population and the ability to potentially predict present and future cognitive performance may be a useful tool for selecting higher-risk patients for evaluation and follow-up.
One limitation of this study is the absence of assessment of biomarkers, retinal neurodegeneration images, and brain images to correlate with clinical data. Most patients only underwent retinal angiography, and none had brain images. However, we reproduced data from a real-life scenario in public health setting that could be generalized for similar health services in developing countries. In addition, these findings should be monitored to confirm their long-term role.
CONCLUSION
There are few studies in Brazil evaluating risk factors related to cognitive performance and dementia in the population with DM, and none of them has explored the presence of retinopathy as a risk factor or associated factor. This study showed this association even after adjustment for other important risk factors. The association between DR and MCD in this population is an interesting field to be explored, as it has the potential to select patients at risk through simple and non-invasive exams during the evaluation of a complication that is already part of the medical routine of people with DM, avoiding more costs and more procedures. The evolution of science towards finding biomarkers and/or image markers to help predict cognitive evolution in middle-aged patients with T2DM should be valuable for assessing cognition in this population. Neurodegeneration and retina vascular markers may be promising for this function. Meanwhile, seeking clinical markers that can help in the early identification of higher-risk patients for cognitive alterations may be useful to try to delay cognitive decline in this population.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- HBA1c
Glycated hemoglobin
- DM
Diabetes mellitus
- T2DM
Type 2 Diabetes mellitus
- MCD
Minimal cognitive decline or dysfunction
- CVD
Cardiovascular disease
- GCS(z)
Global cognitive score
- HDL
High-density lipoprotein
- BMI
Body mass index
- LDL
Low-density lipoprotein
- MMSE
Mini-Mental State Examination
- DBP
Diastolic blood pressure
- SBP
Systolic blood pressure
- PHQ-9
Patient Health Questionaire-9
- ACR
Albumin-to-creatinine ratio
- DR
Diabetic Retinopathy
- eGFR
estimated Glomerular filtration rate
- OCT
Optical coherence tomography
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study protocol was reviewed and approved by the Research Ethics Committee of the Catholic University of Paraná, Brazil.
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. All humans research procedures followed were in accordance with the standards set forth in the Declaration of Helsinki principles.
CONSENT FOR PUBLICATION
Written informed consent was obtained from each participant before enrolment in the study.
STANDARDS OF REPORTING
STROBE guidelines were followed in this study.
AVAILABILITY OF DATA AND MATERIALS
Most data generated or analyzed during this study are included within the article and its supplementary information file. Any additional data is available from the corresponding author [CPB] on reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest financial or otherwise.
SUPPLEMENTARY MATERIALS
Supplementary material is available on the publisher’s website along with the published article.
REFERENCES
- 1.Prince M.J., Wimo A., Guerchet M.M., Ali G.C., Wu Y-T., Prina M. World Alzheimer Report 2015 - The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. London: Alzheimer's Disease International. 2015.
- 2.Vos T., Lim S.S., Abbafati C., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. nternational Diabetes Federation. IDF Diabetes Atlas 9th ed. 2019. Available from: https://www.diabetesatlas.org. [Google Scholar]
- 4.Yau J.W.Y., Rogers S.L., Kawasaki R., et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ott A., Stolk R.P., van Harskamp F., Pols H.A.P., Hofman A., Breteler M.M.B. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53(9):1937–1942. doi: 10.1212/WNL.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 6.Peila R., Rodriguez B.L., Launer L.J. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51(4):1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 7.Rawlings A.M., Sharrett A.R., Schneider A.L.C., et al. Diabetes in midlife and cognitive change over 20 years: A cohort study. Ann. Intern. Med. 2014;161(11):785–793. doi: 10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts R.O., Knopman D.S., Geda Y.E., et al. Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimers Dement. 2014;10(1):18–26. doi: 10.1016/j.jalz.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes D.E., Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munshi M.N. Cognitive dysfunction in older adults with diabetes: What a clinician needs to know. Diabetes Care. 2017;40(4):461–467. doi: 10.2337/dc16-1229. [DOI] [PubMed] [Google Scholar]
- 11.Cukierman T., Gerstein H.C., Williamson J.D. Cognitive decline and dementia in diabetes-systematic overview of prospective observational studies. Diabetologia. 2005;48(12):2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J., Chen C., Hua S., et al. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res. Clin. Pract. 2017;124:41–47. doi: 10.1016/j.diabres.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Gudala K., Bansal D., Schifano F., Bhansali A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J. Diabetes Investig. 2013;4(6):640–650. doi: 10.1111/jdi.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biessels G.J., Staekenborg S., Brunner E., Brayne C., Scheltens P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006;5(1):64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 15.Simó R., Ciudin A., Simó-Servat O., Hernández C. Cognitive impairment and dementia: A new emerging complication of type 2 diabetes-The diabetologist’s perspective. Acta Diabetol. 2017;54(5):417–424. doi: 10.1007/s00592-017-0970-5. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira S.T., Clarke J.R., Bomfim T.R., De Felice F.G. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimers Dement. 2014;10(1) Suppl.:S76–S83. doi: 10.1016/j.jalz.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 17.van Sloten T.T., Sedaghat S., Carnethon M.R., Launer L.J., Stehouwer C.D.A. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020;8(4):325–336. doi: 10.1016/S2213-8587(19)30405-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heringa S.M., Bouvy W.H., van den Berg E., Moll A.C., Kappelle L.J., Biessels G.J. Associations between retinal microvascular changes and dementia, cognitive functioning, and brain imaging abnormalities: A systematic review. J. Cereb. Blood Flow Metab. 2013;33(7):983–995. doi: 10.1038/jcbfm.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crosby-Nwaobi R.R., Sivaprasad S., Amiel S., Forbes A. The relationship between diabetic retinopathy and cognitive impairment. Diabetes Care. 2013;36(10):3177–3186. doi: 10.2337/dc12-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fickweiler W., Wolfson E.A., Paniagua S.M., et al. Association of cognitive function and retinal neural and vascular structure in type 1 diabetes. J. Clin. Endocrinol. Metab. 2021;106(4):1139–1149. doi: 10.1210/clinem/dgaa921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Exalto LG, Biessels GJ, Karter AJ, Huang ES, Quesenberry CP, Whitmer RA. Severe diabetic retinal disease and dementia risk in type 2 diabetes. J Alzheimers Dis. 2014;42 Suppl 3(03):S109-17. doi: 10.3233/JAD-132570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng D., Zhao X., Yang S., Wang G., Ning G. Association between diabetic retinopathy and cognitive impairment: A systematic review and meta-analysis. Front. Aging Neurosci. 2021;13:692911. doi: 10.3389/fnagi.2021.692911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutlu U., Bonnemaijer P.W.M., Ikram M.A., et al. Retinal neurodegeneration and brain MRI markers: The Rotterdam study. Neurobiol. Aging. 2017;60:183–191. doi: 10.1016/j.neurobiolaging.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Gasecka A., Siwik D., Gajewska M., et al. Early biomarkers of neurodegenerative and neurovascular disorders in diabetes. J. Clin. Med. 2020;9(9):2807. doi: 10.3390/jcm9092807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diretrizes da Sociedade Brasileira de Diabetes Mellitus 2017– 2018. In. São Paulo, Brasil: Clannad Editora Científica; 2017. Available from: https://diabetes. orgbr/ebook/ diretrizes-dasociedade- brasileira-de-diabetes-2017- 2018/
- 26.World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 27.Seaquist E.R., Anderson J., Childs B., et al. Hypoglycemia and diabetes: A report of a workgroup of the American diabetes association and the endocrine society. Diabetes Care. 2013;36(5):1384–1395. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barroso W.K.S., Rodrigues C.I.S., Bortolotto L.A., et al. Brazilian Guidelines of Hypertension - 2020. Arq. Bras. Cardiol. 2021;116(3):516–658. doi: 10.36660/abc.20201238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ting D.S.W., Cheung G.C.M., Wong T.Y. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: A review. Clin. Exp. Ophthalmol. 2016;44(4):260–277. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson C.P., Ferris F.L., III, Klein R.E., et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 31.Zanocco J.A., Nishida S.K., Passos M.T., et al. Race adjustment for estimating glomerular filtration rate is not always necessary. Nephron Extra. 2012;2(1):293–302. doi: 10.1159/000343899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.KDIGO 2020 Clinical practice guideline for diabetes management in chronic kidney disease. Nephrology and Dialysis. 2021;23(2) Suppl.:9–121. doi: 10.1016/j.kint.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Mastrocola L.E., Amorim B.J., Vitola J.V., et al. Update of the Brazilian Guideline on Nuclear Cardiology - 2020. Arq. Bras. Cardiol. 2020;114(2):325–429. doi: 10.36660/abc.20200087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campanholo K.R., Romão M.A., Machado M.A.R., et al. Performance of an adult Brazilian sample on the Trail Making Test and Stroop Test. Dement. Neuropsychol. 2014;8(1):26–31. doi: 10.1590/S1980-57642014DN81000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamdan A.C., Hamdan E.M.L.R. Effects of age and education level on the Trail Making Test in a healthy Brazilian sample. Psychol. Neurosci. 2009;2(2):199–203. doi: 10.3922/j.psns.2009.2.012. [DOI] [Google Scholar]
- 37.Pavão Martins I., Maruta C., Freitas V., Mares I. Executive performance in older Portuguese adults with low education. Clin. Neuropsychol. 2013;27(3):410–425. doi: 10.1080/13854046.2012.748094. [DOI] [PubMed] [Google Scholar]
- 38.Nitrini R., Caramelli P., Bottino C.M de C., Damasceno B.P., Brucki S.M., Anghinah R. Diagnosis of alzheimer’s disease in Brazil: cognitive and functional evaluation. Recommendations of the scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology. Arq. Neuropsiquiatr. 2005;63(3A):720–727. doi: 10.1590/S0004-282X2005000400034. [DOI] [PubMed] [Google Scholar]
- 39.Bertolucci P.H.F., Okamoto I.H., Brucki S.M.D., Siviero M.O., Toniolo Neto J., Ramos L.R. Applicability of the CERAD neuropsychological battery to Brazilian elderly. Arq. Neuropsiquiatr. 2001;59(3-A):532–536. doi: 10.1590/S0004-282X2001000400009. [DOI] [PubMed] [Google Scholar]
- 40.Fillenbaum G.G., van Belle G., Morris J.C., et al. Consortium to establish a registry for Alzheimer’s disease (CERAD): The first twenty years. Alzheimers Dement. 2008;4(2):96–109. doi: 10.1016/j.jalz.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burns A., Brayne C., Folstein M. The mini-mental state examination, will we be using it in 2001? Int. J. Geriatr. Psychiatry. 1998;13(5):285–294. doi: 10.1002/(SICI)1099-1166(199805)13:5<285:AID-GPS753>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 42.Almeida O.P. Mini mental state examination and the diagnosis of dementia in Brazil. Arq. Neuropsiquiatr. 1998;56(3B):605–612. doi: 10.1590/S0004-282X1998000400014. [DOI] [PubMed] [Google Scholar]
- 43.Bertolucci P.H.F., Brucki S.M.D., Campacci S.R., Juliano Y.O. The Mini-Mental State Examination in a general population: Impact of educational status. Arquivos de Neuro-Psiquiatria. 1994;52(1):1–7. doi: 10.1590/S0004-282X1994000100001. [DOI] [PubMed] [Google Scholar]
- 44.Brucki S.M.D., Nitrini R., Caramelli P., Bertolucci P.H.F., Okamoto I.H. Suggestions for utilization of the mini-mental state examination in Brazil. Arq. Neuropsiquiatr. 2003;61(3B):777–781. doi: 10.1590/S0004-282X2003000500014. [DOI] [PubMed] [Google Scholar]
- 45.Kochhann R., Varela J.S., Lisboa C.S.M., Chaves M.L.F. The mini mental state examination: Review of cutoff points adjusted for schooling in a large Southern Brazilian sample. Dement. Neuropsychol. 2010;4(1):35–41. doi: 10.1590/S1980-57642010DN40100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Passos V.M.A., Giatti L., Bensenor I., Chor D., Barreto S.M. Education plays a greater role than age in cognitive test performance among participants of the Brazilian longitudinal study of adult health (ELSA-Brasil). Int. J. Epidemiol. 2015;44(Suppl. 1):i265–i5. doi: 10.1093/ije/dyv096.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alencar R.C., Cobas R.A., Gomes M.B. Assessment of cognitive status in patients with type 2 diabetes through the mini-mental status examination: A cross-sectional study. Diabetol. Metab. Syndr. 2010;2(1):10. doi: 10.1186/1758-5996-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monteiro S., Torres A., Pereira A., Albuquerque E., Morgadinho R. Preliminary validation study of a Portuguese version of the patient health questionnaire (PHQ-9). Eur. Psychiatry. 2013;28(S1):1. doi: 10.1016/S0924-9338(09)71489-0. [DOI] [Google Scholar]
- 49.Santos I.S., Tavares B.F., Munhoz T.N., et al. Sensibilidade e especificidade do patient health questionnaire-9 (PHQ-9) entre adultos da população geral. Cad. Saude Publica. 2013;29(8):1533–1543. doi: 10.1590/S0102-311X2013001200006. [DOI] [PubMed] [Google Scholar]
- 50.Hosmer D.W., Lemeshow S. Applied Logistic Regression. New York, Toronto: Wiley; 2000. p. 383. [DOI] [Google Scholar]
- 51.Gregg E.W., Sattar N., Ali M.K. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016;4(6):537–547. doi: 10.1016/S2213-8587(16)30010-9. [DOI] [PubMed] [Google Scholar]
- 52.Petersen R.C., Lopez O., Armstrong M.J., et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126–135. doi: 10.1212/WNL.0000000000004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langa K.M., Levine D.A. The diagnosis and management of mild cognitive impairment: A clinical review. JAMA. 2014;312(23):2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caamaño-Isorna F., Corral M., Montes-Martínez A., Takkouche B. Education and dementia: A meta-analytic study. Neuroepidemiology. 2006;26(4):226–232. doi: 10.1159/000093378. [DOI] [PubMed] [Google Scholar]
- 55.Livingston G., Huntley J., Sommerlad A., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Díaz-Coránguez M., Ramos C., Antonetti D.A. The inner blood-retinal barrier: Cellular basis and development. Vision Res. 2017;139:123–137. doi: 10.1016/j.visres.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blazes M., Lee C.S. Understanding the brain through aging eyes. Advances in Geriatric Medicine and Research. 2021;3(2):e210008. doi: 10.20900/agmr20210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao W-Q., Chen H., Quon M.J., Alkon D.L. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. 2004;490(1-3):71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 59.Ferrario CR, Reagan LP. Insulin-mediated synaptic plasticity in the CNS: Anatomical, functional and temporal contexts. Neuropharmacology. 2018;136(Pt B):182-91. doi: 10.1016/j.neuropharm.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiu S-L., Chen C-M., Cline H.T. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58(5):708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ko F., Muthy Z.A., Gallacher J., et al. Association of retinal nerve fiber layer thinning with current and future cognitive decline: A study using optical coherence tomography. JAMA Neurol. 2018;75(10):1198–1205. doi: 10.1001/jamaneurol.2018.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khawaja A.P., Chan M.P.Y., Yip J.L.Y., et al. Retinal nerve fiber layer measures and cognitive function in the EPIC-norfolk cohort study. Invest. Ophthalmol. Vis. Sci. 2016;57(4):1921–1926. doi: 10.1167/iovs.16-19067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Dijk H.W., Verbraak F.D., Kok P.H.B., et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest. Ophthalmol. Vis. Sci. 2010;51(7):3660–3665. doi: 10.1167/iovs.09-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ponirakis G., Al Hamad H., Sankaranarayanan A., et al. Association of corneal nerve fiber measures with cognitive function in dementia. Ann. Clin. Transl. Neurol. 2019;6(4):689–697. doi: 10.1002/acn3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Janahi E., Ponirakis G., Al Hamad H., et al. Corneal nerve and brain imaging in mild cognitive impairment and dementia. J. Alzheimers Dis. 2020;77(4):1533–1543. doi: 10.3233/JAD-200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ponirakis G., Elsotouhy A., Al Hamad H., et al. Association of cerebral ischemia with corneal nerve loss and brain atrophy in MCI and dementia. Front. Neurosci. 2021;15:690896. doi: 10.3389/fnins.2021.690896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee C.S., Larson E.B., Gibbons L.E., et al. Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimers Dement. 2019;15(1):34–41. doi: 10.1016/j.jalz.2018.06.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheung C.Y., Ikram M.K., Chen C., Wong T.Y. Imaging retina to study dementia and stroke. Prog. Retin. Eye Res. 2017;57:89–107. doi: 10.1016/j.preteyeres.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 69.Naël V., Pérès K., Dartigues J-F., et al. Vision loss and 12-year risk of dementia in older adults: The 3C cohort study. Eur. J. Epidemiol. 2019;34(2):141–152. doi: 10.1007/s10654-018-00478-y. [DOI] [PubMed] [Google Scholar]
- 70.Shang X., Zhu Z., Wang W., Ha J., He M. The association between vision impairment and incidence of dementia and cognitive impairment: A systematic review and meta-analysis. Ophthalmology. 2021;128(8):1135–1149. doi: 10.1016/j.ophtha.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 71.Chen S.P., Bhattacharya J., Pershing S. Association of vision loss with cognition in older adults. JAMA Ophthalmol. 2017;135(9):963–970. doi: 10.1001/jamaophthalmol.2017.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paik J-S., Ha M., Jung Y.H., et al. Low vision and the risk of dementia: A nationwide population-based cohort study. Sci. Rep. 2020;10(1):9109. doi: 10.1038/s41598-020-66002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michalowsky B., Hoffmann W., Kostev K. Association between hearing and vision impairment and risk of dementia: Results of a case-control study based on secondary data. Front. Aging Neurosci. 2019;11:363. doi: 10.3389/fnagi.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hong T., Mitchell P., Burlutsky G., Liew G., Wang J.J. Visual impairment, hearing loss and cognitive function in an older population: Longitudinal findings from the blue mountains eye study. PLoS One. 2016;11(1):e0147646. doi: 10.1371/journal.pone.0147646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu M., Mei F., Hu K., et al. Diabetic retinopathy and cognitive dysfunction: A systematic review and meta-analysis. Acta Diabetol. 2022;59(4):443–459. doi: 10.1007/s00592-021-01829-0. [DOI] [PubMed] [Google Scholar]
- 76.Chai Y-H., Zhang Y-P., Qiao Y-S., et al. Association between diabetic retinopathy, brain structural abnormalities, and cognitive impairment for accumulated evidence in observational studies. Am. J. Ophthalmol. 2022;239:37–53. doi: 10.1016/j.ajo.2022.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.
Data Availability Statement
Most data generated or analyzed during this study are included within the article and its supplementary information file. Any additional data is available from the corresponding author [CPB] on reasonable request.