Abstract
Females have historically been disregarded in memory research, including the thousands of studies examining roles for the hippocampus, medial prefrontal cortex, and amygdala in learning and memory. Even when included, females are often judged based on male-centric behavioral and neurobiological standards, generating and perpetuating scientific stereotypes that females exhibit worse memories compared with males in domains such as spatial navigation and fear. Recent research challenges these dogmas by identifying sex-specific strategies in common memory tasks. Here, we discuss rodent data illustrating sex differences in spatial and fear memory, as well as the neural mechanisms underlying memory formation. The influence of sex steroid hormones in both sexes is discussed, as is the importance to basic and translational neuroscience of studying sex differences.
Challenging male-centric views on memory
Learning and memory researchers have traditionally used male subjects to examine male-centric behaviors and their neurobiological mechanisms. Although some work has directly compared the sexes, such as that supporting a male advantage in spatial memory and female advantage in episodic and verbal memory [1], females have been historically omitted from preclinical and clinical memory studies [2,3]. However, considerable sex differences exist in the prevalence and severity of memory-related disorders, such as Alzheimer’s disease (AD) and post-traumatic stress disorder (PTSD) [4,5], and age-related declines in global cognition and executive function impact women earlier than they do men [6]. Therefore, discovering how sex influences memory and its underlying mechanisms may provide novel diagnostic and therapeutic targets for memory decline and memory-related disorders in both sexes (Box 1).
Box 1. Real-world implications.
Although the minutiae of sex differences research may appear trivial, such information can have meaningful clinical consequences. For example, during the coronavirus disease 2019 (COVID-19) pandemic, women with the virus exhibited lower mortality compared with men but were at greater risk for memory-related ‘brain fog’ effects, due, in part, to hormonal status [82]. Additionally, recent work on gut–brain interactions elucidated sex-specific innate and diet-induced interactions, which could manifest as differences in memory [83,84]. More historically, it has long been known that AD [4] and PTSD [5] are more prevalent and severe in women than in men, and that these differences derive from genetic, hormonal, and age-related interactions. Basic research adds to these observations by replicating clinical findings in animals, which allows insights into underlying mechanisms. For example, peripheral treatment with inflammation-inducing drugs causes greater memory deficits in female than in male mice in certain memory assessments, but the opposite outcome in others [85], effects related to sex differences in serum and brain cytokine levels, as well as brain region-specific differences in microglial activity [86]. Furthermore, in mice, depleting the microbiome reduces specific forms of fear memory in females, but not males, which is associated with sex differences in dendritic morphology [87], serotonergic and neurotrophic signaling [88], and differential neuroplasticity responses [89], each dependent upon the brain region examined. Female transgenic mice expressing human forms of AD-related genes also show greater pathology and worse memory performance over time compared with males [90], and these models have been used to develop novel treatments and preventative vaccines for the disease [91].
Furthermore, different fear conditioning and extinction paradigms have been used to show that the sexes differ in their behavioral responses to similar fear-inducing stimuli [16,48], that there are brain region-specific alterations to cell signaling and growth factor levels in the consolidation and extinction of fear memories [5,50], and that gonadal hormones strongly influence these molecular and behavioral differences [5,68]. In preclinical rodent models, tight control over different variables may be asserted by researchers, giving rise to highly specific insights into the brain, cellular, and molecular correlates of a given behavior and providing novel targets for future therapeutics. Therefore, delving into the nuances of sex differences in behavioral, brain, and molecular neuroscience will better enable the development of new, and possibly sex-specific, therapies for improving mental health.
In 2014, the National Institutes of Health (NIH) implemented a policy to ensure that new research proposals address sex as a biological variable (SABV; see Glossary) [7], which has increased the inclusion of females in memory studies [2,8]. However, male-only studies continue to greatly outnumber female-only studies, and 95% of studies in top-tier journals use statistical methods to ‘control’ for sex, rather than directly comparing the sexes [2,8]. Progress has also been slowed by recent controversies regarding brain sexual dimorphisms (Box 2) [9–11], hesitancies about experimental design (Box 3) [12–14], and fears about social equity following sex difference discoveries [15]. However, recent studies demonstrating sex differences in behavioral strategies [16], memory outcomes [17], and underlying mechanisms [18] emphasize the importance of considering SABV in these areas.
Box 2. Sex differences versus sex dimorphisms: is a brain ‘male’ or ‘female’?
In 2015, Joel and colleagues [9] published a controversial study that challenged the notion that male and female brains differ. They used neuroimaging data of gray matter volume, cortical thickness and volume, and white matter and subcortical structural volume to search for sexual dimorphisms in the brain, concluding that there is considerable overlap in connectivity and structure between male and female brains, even in regions that are typically classified as ‘male-centric’ or ‘female-centric’. Based on their analyses, the authors proposed a ‘human brain mosaic’ model, arguing against the concepts of ‘male brain’ or ‘female brain’ in favor of the view that brains comprise unique mosaics of male-typical, female-typical, and gender-neutral features. A recent related review posited that sex differences in brain region volume, connectivity, and activation during different tasks are often negligible after correcting for total brain volume, again suggesting that the brain is not sexually dimorphic [11]. Although these interpretations provide support against bias and discrimination on the basis of sex differences in brain structure, they have generated considerable criticism (e.g., [92]), notably about the features selected for analysis, the sensitivity of the methods used, and fears that such arguments may validate continuing single-sex approaches in neuroscience research. Other criticism focuses on the straw man nature of the male versus female argument, because it perpetuates misconceptions regarding sex ‘dimorphisms’, as opposed to sex ‘differences’, a topic addressed effectively by others [93].
Indeed, the idea that the brain is not overly ‘dimorphic’ (i.e., in one form or a second with almost no overlap) is not controversial at all; instead, most differences in the brain and behavior exist along a continuum, with much overlap between the sexes. Even so, cutting-edge research continues to identify sex differences within the same brain regions and cells, such as receptor composition and distribution [17,28] and molecular mechanisms [18,59,74], which may either produce sex-biased behaviors or reveal different functional routes in males and females that lead to similar behavioral outcomes in both sexes. Thus, the next frontier in understanding sex differences in the brain is likely at the cellular and molecular level, which will foster more impactful assessments of putative sex differences in function, rather than of gross structural dimorphisms that may not reflect functional outcomes.
Box 3. Experimental designs for sex differences and hormones research: a primer.
How should experiments be designed and analyzed to uncover potential sex differences in memory? Ideally, both sexes will be directly compared in the same study. If not feasible, then investigators should relate their single-sex findings to published data from the other sex (if available) and conduct future studies in the unexamined sex using the same experimental conditions. Additionally, multi-sex studies should avoid pooling the sexes in analyses or analyzing sex as a covariate because avoiding examination of sex as a discovery variable [2] precludes meaningful detection of sex differences. Preferably, researchers should use sample size-matched, sufficiently powered male and female groups, comparing the sexes directly in each analysis.
Do cyclic hormone fluctuations make it difficult to compare male and female data? In short, no. Meta-analyses of preclinical studies demonstrate that memory, anxiety, and multiple aspects of brain function are not more variable in gonadally intact females than among intact males [13,14,52]. Thus, variability is not a valid basis for female exclusion. However, hormone fluctuations may contribute to variability within a sex, as demonstrated in hippocampal gene expression [51], CA1 dendritic spine density [51,94], long-term potentiation (LTP) [72], and anxiety-like behavior in females [51]. Indeed, not categorizing females by estrous cycle phase in these studies would have led to erroneous conclusions about hormonal regulation of female neurobiology and behavior [51]. Similarly, hormone fluctuations in males should also be considered because males experience diurnal androgen fluctuations [20] that may influence brain and behavioral functions.
How might researchers measure hormonal influences on learning and memory? One approach is to monitor daily endogenous hormone fluctuations (e.g., using vaginal lavage in females or blood draws in males) during testing and retrospectively analyze data by hormone status [21]. This approach can be useful for multi-day protocols, such as the Morris water maze (MWM) or radial arm maze (RAM). However, one may also assign females to a specific cycle stage and use daily lavage to prospectively determine when each animal should be trained or tested. This approach works well with modified one- or two-day MWM protocols in which all testing can be completed within a single stage [95] or training can occur in one stage with testing the next day [33]. A similar approach can be used with one-trial learning paradigms, such as object placement (OP) and contextual fear conditioning (CFC). Disadvantages of these approaches include the stress of daily lavage and blood draws, and the lack of hormone specificity resulting from simultaneous fluctuations of multiple gonadal hormones [21].
To pinpoint contributions of specific hormones, researchers bilaterally gonadectomize subjects and administer exogenous hormones. Researchers typically gonadectomize female, but not male, rodents to eliminate estrous cycling; however, this approach reinforces ‘male’ as default, ignoring daily testosterone and corticosteroid fluctuations among males [20] and gonadectomy-induced disruptions of endocrine function in both sexes [96–98]. Thus, gonadectomy is best applied to both sexes. Acute intracranial infusions or systemic injections can determine hormone effects during particular phases of learning and memory. In single-trial learning tasks, such as OP and CFC, hormones may be delivered before or immediately after training, or before testing, to assess effects on acquisition, consolidation, or recall, respectively. However, tasks such as the MWM, RAM, and Barnes maze typically require many training days and, thus, warrant longer-term treatments via daily injections, chronic-release pellets or pumps, or the drinking water. Important long-term treatment considerations include whether to deliver a static dose chronically or to mimic the natural cycle with variable dosing. As mentioned elsewhere [22,27,99], each delivery method is subject to different potential confounds, such as restraint or injection stress. Therefore, researchers must consider delivery methods carefully with respect to their learning paradigm.
Finally, two important caveats to gonadectomy should be noted. First, cognitive brain regions synthesize sex steroids on demand [100,101]; thus, gonadectomy will not account for effects of these brain-derived hormones. Second, exogenous treatment is by nature artificial; therefore, effects must be thoughtfully interpreted relative to natural cycling.
Multiple factors influence sex differences, including genetic and hormonal variables (Box 3). Although several transgenic mouse lines, including the Four-Core Genotype (FCG) model, have enabled researchers to begin parsing chromosomal and hormonal roles in memory, the contribution of sex chromosomes has historically been difficult to assess. Thus, studies of hormonal contributions to sex differences in memory, which are far more numerous, will be the major focus of this review.
All sex steroid hormones derive from cholesterol. Progesterone is synthesized first and is converted to androgens like testosterone, which are catalyzed by aromatase into estrogens, such as 17β-estradiol (E2), or by 5α-reductase into the non-aromatizable androgen dihydrotestosterone (DHT) [19]. In female rodents, estrogen and progesterone levels fluctuate in the circulation and brain across the 4–5-day estrous cycle, peaking during proestrus followed by a steep drop during estrus which is largely maintained during metestrus and diestrus [5,19]. Males also experience modest diurnal fluctuations in androgens and corticosteroids [20]. In this review, we discuss sex steroid hormones in the context of sex differences in memory, but do not provide a systematic review of their contributions to memory formation (see instead [21,22]). Rather, the influence of sex steroid hormones is noted as an underlying factor influencing sex differences in spatial and fear memories, as well as their neural mechanisms and roles in recent challenges to pervasive male-centric sex difference dogmas in spatial and fear memory.
Sex differences in spatial memory
Decades of research have suggested that males outperform females in spatial memory tasks, such as the Morris water maze (MWM), radial arm maze (RAM), Barnes maze, and object placement (OP). However, memory outcomes in these tasks often depend on sex-preferred behavioral strategies. Males typically navigate using geometric cues (place strategy), whereas females predominantly rely on visual landmarks (cued strategy) (Figure 1A) [23]. These strategies are evident in the MWM when a visible platform moves from a previously learned location; male rats swim to the former location of the platform, whereas females swim to the platform itself [23]. However, strategy preferences are strongly influenced by hormonal status (Figure 1A), given that both sexes use place strategies when sex steroid levels are high, but cue-based strategies when levels are low [24,25]. This influence extends to other memory tests, such as a modified T-maze (Figure 1B), where infusion of E2 into the medial prefrontal cortex (mPFC) of diestrus female rats promotes a place strategy over a cue-based response strategy [26]. Importantly, many studies using these tasks have failed to account for strategy preferences and, thus, may have missed important sex-specific differences in spatial memory.
Figure 1. Sex-preferred strategy use in common spatial and fear memory tasks and key involved brain regions.
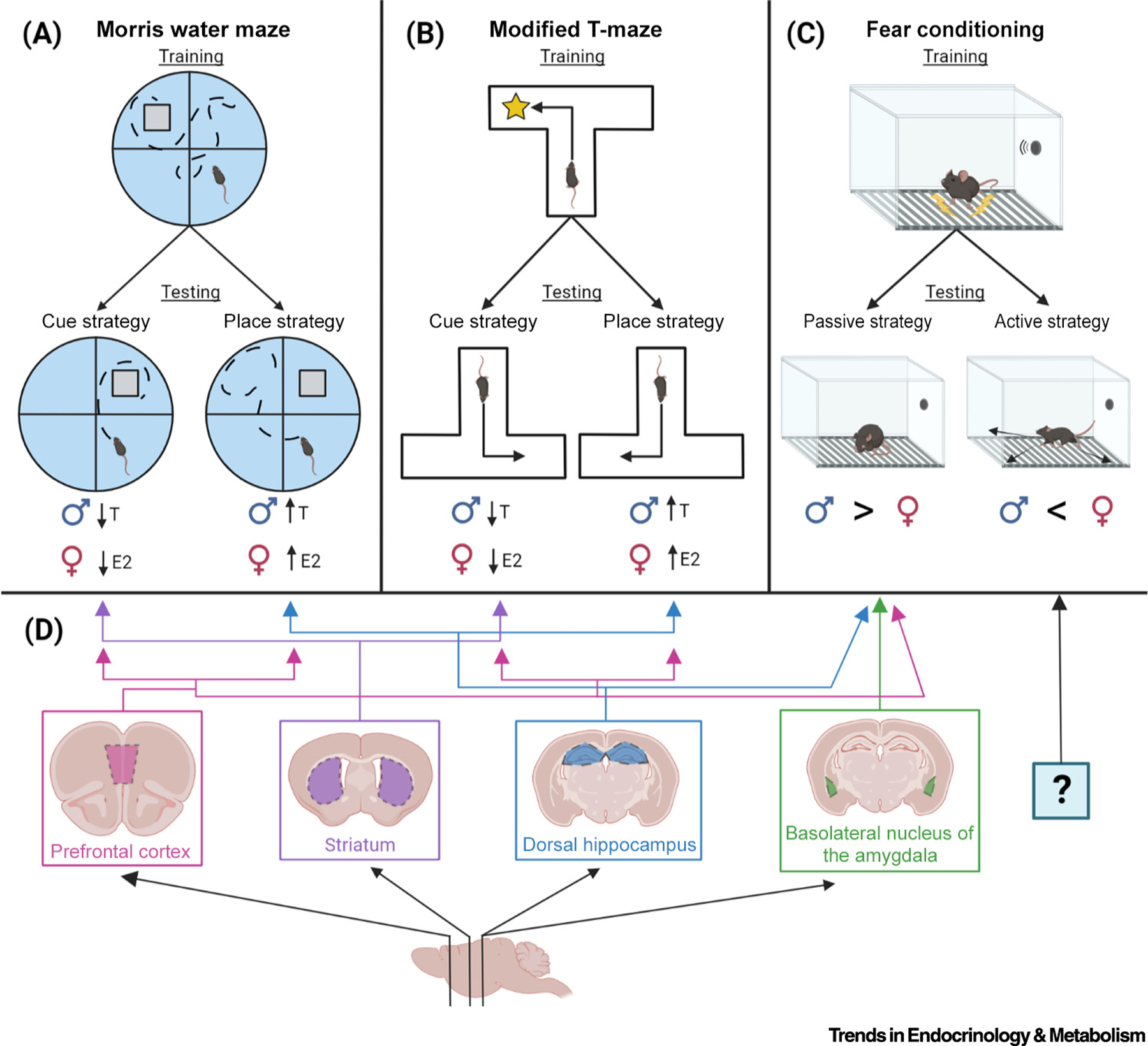
During probe tests in the Morris water maze (A) and modified T-maze (B) spatial memory tasks, male and female rodents in low-testosterone and low-estrogen states, respectively, typically use cue-based or egocentric strategies to navigate to rewarded locations (a visible platform or nutrient reinforcement). However, in high-testosterone (T) and high-estrogen (E2) states, males and females typically use allocentric spatial strategies to locate rewards based on extra-maze cognitive maps. During tone or contextual fear conditioning testing (C), male rodents typically exhibit a passive ‘freezing’ response to tone or context re-exposure, whereas females more frequently display active ‘darting’ behaviors compared with males. Memory and behavioral strategies in spatial and fear-based tasks are mediated by interactions among several brain regions, shown in (D) as cross-sectional slices from sagittal representations of the rodent brain. Brain regions such as the dorsal hippocampus and medial prefrontal cortex are involved broadly in several types of spatial and fear learning and memory, whereas others, such as the striatum and basolateral nucleus of the amygdala, mediate strategy choices in more specific tasks, namely spatial navigation and fear responding, respectively. However, as indicated by ‘?’, little is known about brain regions associated with darting behaviors in fear memory. Created with BioRender (BioRender.com).
Sex steroid hormones have long been thought to influence a form of short-term spatial memory called spatial working memory [23,27], which is often tested in the RAM, a wheel-shaped maze in which 8–12 arms radiate equidistantly from a circular central arena. Rodents retrieve food rewards from the ends of the arms, and repeated entries into previously visited arms are considered working memory errors. Males typically make fewer repeat entries compared with females [27,28]. This male advantage in spatial working memory may be reversed by neonatal gonadectomy in males [29] or neonatal treatment of females with estradiol benzoate [29] or testosterone propionate [30], suggesting strong organizational effects of circulating hormones over this type of memory.
Sex differences in a form of long-term memory called spatial reference memory are particularly influenced by experimental design. In the MWM, spatial reference memory is tested by training rodents to swim to a submerged escape platform located in a fixed position throughout testing. In the RAM, spatial reference memory is assessed by including arms that are never baited with reward and the location of which remains constant throughout testing. In both tasks, sex differences in memory depend on experimental variables. For example, fewer training sessions in the RAM initially favor better spatial reference memory in males, indicated by fewer entries into unbaited arms [28]. Although more intensive training initially favors females, males ultimately exhibit greater remote memory compared with females [31]. In the MWM, male rats display better memory for the platform location compared with females when tested immediately, 7 days, and 28 days after training, but not 24-h post-training [17]. Thus, careful consideration must be given to experimental design when comparing sexes in long-term spatial memory outcomes.
Species and hormonal status also influence observed differences. For example, female mice outperform males during MWM acquisition [32], and naturally cycling female mice in proestrus display better spatial reference memory compared with males and ovariectomized or non-proestrus females [33]. Although spatial reference memory in the MWM is improved by peripheral injection or dorsal hippocampus (DH) infusion [34,35] of E2 in ovariectomized rats, and by peripheral treatment with testosterone in gonadectomized male rats [36], the contributions of naturally cycling hormones to regulating sex differences in the MWM and RAM remain unclear.
Although swimming- and food deprivation-induced stress in the MWM and RAM, respectively, may contribute to sex differences in these tasks, differences have also been documented in the OP and Barnes maze tasks, which are minimally stressful. In OP, which tests memory for locations of previously investigated objects, male mice outperform females, although this effect diminishes over time [37] and when both sexes spend the same amount of time exploring objects during training [18]. In the Barnes maze, a circular maze in which rodents learn to find an escape hole, memory also generally favors males, although this depends highly upon age, species, strain, and behavioral strategy [32,38–40].
Exogenous sex steroid hormones affect memory in both OP and the Barnes maze. Dorsal hippocampal E2 infusion immediately after training improves OP memory consolidation in ovariectomized female, as well as intact and gonadectomized male, mice [18]. However, inhibition of hippocampal aromatase impairs OP memory in ovariectomized female mice [41] and gonadectomized, but not intact, male mice, whereas androgen receptor antagonism impairs memory among intact males [41,42], suggesting that endogenous androgens regulate OP memory consolidation in intact males, but E2 compensates for androgen loss following gonadectomy. In the Barnes maze, gonadectomy-induced spatial reference memory impairments in male rats are rescued by peripheral testosterone or E2 [40]. Additionally, loss of neuron-derived E2 in forebrain aromatase-knockout mice impairs long-term memory in the Barnes maze in both sexes [43]. Therefore, although spatial memory in non-aversive tasks typically favors males, effects may be complicated by testing parameters and hormonal milieu.
In sum, although the prevailing view has been that males exhibit superior spatial memory, numerous variables complicate this interpretation. Notably, many studies fail to consider sex differences in strategy use, perhaps missing valid female-centric methods of locating rewards in favor of male-centric responses. Additionally, although sex steroid hormones have key roles in strategy choice and memory consolidation, few studies explicitly examine these factors and, thus, more work is needed to better understand how sex influences behavioral responses and their neural underpinnings.
Sex differences in fear memory
Fear conditioning is used widely to elucidate neural mechanisms underlying learning because it models aspects of PTSD. A neutral stimulus (e.g., context or tone) is paired with an aversive stimulus (i.e., footshock), and memory is assessed by learned responding (e.g., freezing in place) when the previously neutral stimulus is re-presented (Figure 1C). Freezing is the dominant measure of fear in rodents. When returned to the conditioned context, female rodents typically freeze less compared with males [44–50], leading to a dogmatic belief that females have impaired contextual fear conditioning relative to males. This sentiment, combined with the preconceived notion that females will display greater hormone-driven variability in behavioral indices of fear [51,52], has resulted in the general exclusion of females from studies addressing mechanisms of fear learning [5]. However, recent years have seen a rapid proliferation of new fear-conditioning studies utilizing female subjects, revealing novel behavioral strategies by which females demonstrate fear memory.
Recent research has demonstrated sex differences in the ratio of passive and active responding in fear-learning paradigms [16,53]. In one seminal study, female rats displayed greater rates of active fear responding, or ‘darting’, during tone fear conditioning compared with males, which had greater proportions of passive fear responding, or ‘freezing’ (Figure 1C) [16]. Subsequent studies showed that exposure to escapable stress prior to inescapable stress reduces contextual freezing in male, but not female, rats [54] and that a safety signal during fear conditioning reduces darting behaviors in female rats [55]. Moreover, darting in both sexes depends highly on experimental conditions, as illustrated by positive correlations between darting in males and freezing in females with the number of tone–shock pairings and shock intensity, respectively [53]. Importantly, another recent study failed to find reliable sex differences in darting behaviors in mice because both sexes darted very little [56], perhaps indicating a species difference in darting behavior. Nevertheless, darting may represent an alternative, perhaps sex-specific, metric of fear learning in rodents. Future studies should determine the robustness and reliability of darting as a fear response in both sexes across species, because the possible predominance of active responding to fear-associated cues in female rodents would have important implications for the design and interpretation of studies meant to inform treatment strategies for fear-based disorders, such as PTSD.
The reliance on freezing as the primary measure of fear memory is also problematic due to inconsistencies in the literature, where some studies show that females freeze more compared with males [50,55,57,58] and others find similar levels of freezing between the sexes [48,55,59–64]. As above, these findings depend on many experimental factors. For example, freezing in contextual and tone conditioning is negatively and positively correlated with pre-shock exposure times to the context [46] and training shock intensities [53,59–61], respectively. Additionally, female rats in proestrus during testing show lower tone-associated freezing rates compared with males and non-proestrus females [57]. However, forebrain-aromatase knockout reduces contextual freezing in mice of both sexes, suggesting a key role for neuron-derived E2 in fear memory among both sexes [43]. Therefore, these and other variables must be considered carefully in fear conditioning studies with both sexes.
Sex differences are also evident in fear generalization and extinction, which, given similarities between exposure therapy in humans and fear extinction in rodents, could inform the development of sex-specific therapeutics for fear-related disorders. Female rodents generalize fear responses more compared with males, freezing equally in response to both neutral and fear-associated contexts and tones [48,57,58,63,65]. However, increased pre-exposure to the training context [58,66] and testing in the training context before a novel context [58,63] enhance contextual discrimination in female mice, eliminating or reversing sex differences. Furthermore, although both sexes extinguish freezing responses when repeatedly presented with a fear-associated context or tone in the absence of footshock, female mice extinguish less effectively than do males when given additional extinction training sessions [67]. However, this difference may reflect a lack of consideration for sex-specific strategies given that female rats extinguish darting, but not freezing, when given a safety signal in addition to a fear signal [55]. In female rats, generalization is also linked to sex hormone signaling, because females in proestrus generalize between tones [57], perhaps due to activation of estrogen receptor β (ERβ), but not ERα [68]. Additionally, ovariectomy increases the number of sessions required to extinguish fear responses, suggesting that circulating estrogens may improve extinction learning [67]. Thus, females do learn fearful stimuli and extinguish fear responses, but perhaps do so differently from males, which may reflect the higher prevalence and severity of PTSD in women compared with men.
In sum, rodent fear conditioning studies traditionally conclude that males exhibit better fear memory compared with females, which has likely led to the predominance of male-only fear studies. However, newer research suggests that females do learn fearful associations, but may simply respond differently compared with males. Novel findings of sex differences in active and passive strategy use, fear generalization, and fear extinction have revitalized the pursuit of sex effects in fear learning. A recent meta-analysis also dispels the notion of greater variability in fear and stress responding in female rodents, regardless of hormonal status [52]. Therefore, much work remains to fill gaps in understanding the neurobiology of fear learning and memory in both sexes and to fully characterize sex-specific strategies and responses. Given the translational relevance to PTSD outcomes (Box 1), this work is critical to the development of next-generation treatments for fear-related disorders.
Sex differences in neural mechanisms of memory
Among the most well-studied brain regions involved in spatial and fear memory are the DH, mPFC, and amygdala (Figure 1D). The DH is essential for the formation of contextual fear and spatial memories, effects mediated by trisynaptic plasticity between excitatory granule and pyramidal neurons of the dentate gyrus, CA1, and CA3 regions. The amygdala, particularly its basolateral nucleus (BLA), primarily mediates fear memory, whereas the mPFC contributes to the formation of both spatial and fear memories. Furthermore, balances in activity among DH, mPFC, and another region, the striatum (Figure 1D), have been associated with cued or place strategies in spatial tasks [25,69]. Within these brain regions, learning induces pre-, post-, and extra-synaptic chemical and structural changes, or synaptic plasticity. Learning rapidly triggers cell signaling, which influences transcription and translation, trafficking of neurotransmitter receptors into and out of postsynaptic membranes, and the synthesis, mobilization, and degradation of structural proteins necessary for the generation of new dendritic spines, postsynaptic protrusions at which excitatory connections are made with presynaptic terminals (Figure 2A). Coordinated increases in learning-induced postsynaptic activity among multiple neurons leads to long-term potentiation (LTP) of these synapses (Figure 2B), which produces lasting increases in neural excitability. Together, the increases in dendritic spine density and LTP, fueled by changes in gene and protein expression and degradation (Figure 2C), are considered the primary neural manifestations of learning and memory.
Figure 2. Sex differences in cellular and molecular mechanisms underlying memory.
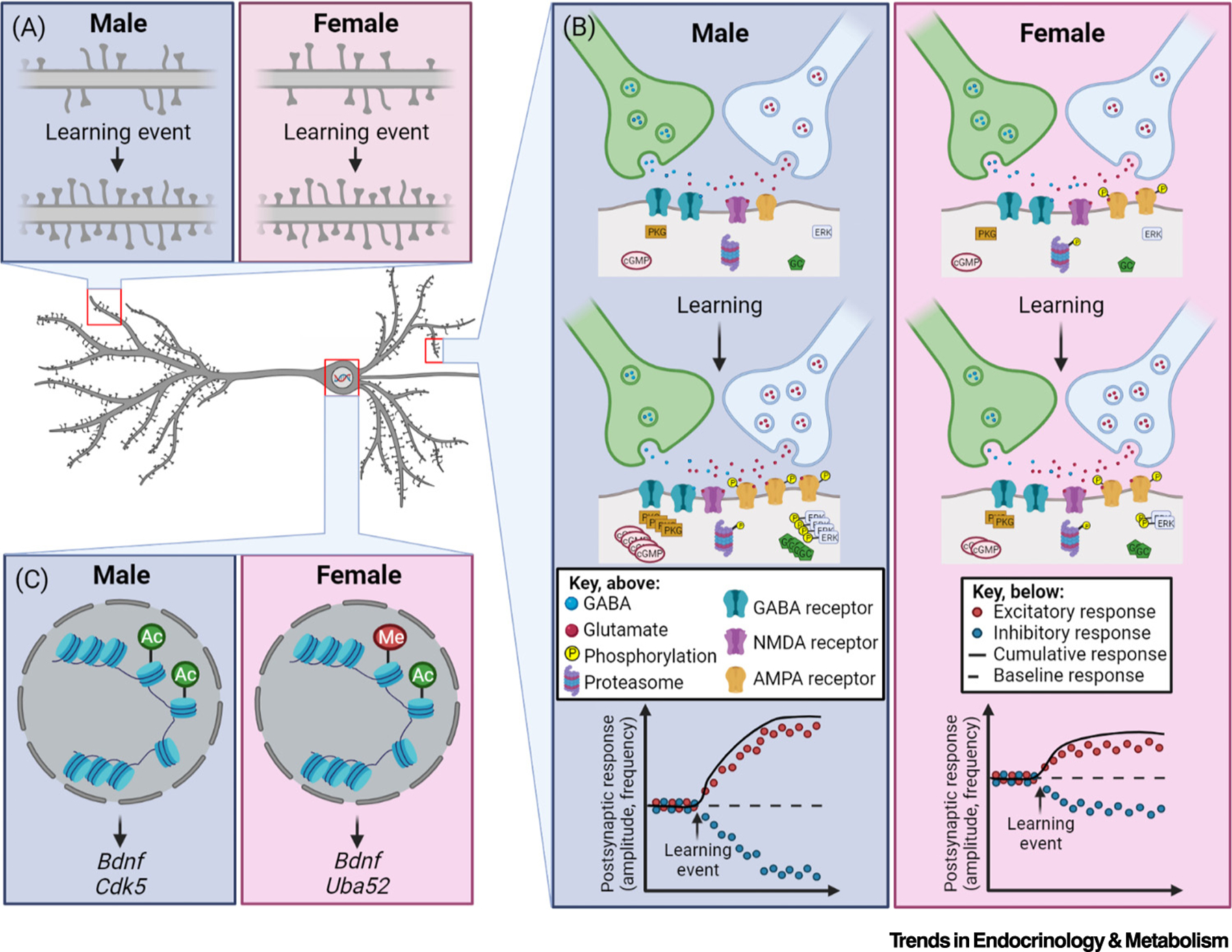
A typical pyramidal neuron, such as those in the hippocampus and medial prefrontal cortex, is shown to illustrate selected baseline and learning-induced, sex-specific changes at the cellular and molecular level in individual excitatory cells. (A) Female rodents have more large, or mushroom-type, apical dendritic spines at baseline compared with males, but learning increases all spine types in both sexes. (B) At the molecular level, females display higher levels of proteasome activity and AMPA receptor-mediated influx of cations [indicated by phosphorylation (P) groups] compared with males. In response to learning, inhibitory (GABA) tone is reduced in both sexes, whereas excitatory (glutamatergic) tone is increased. Learning also increases AMPA receptors and their phosphorylation in males, and males have greater increases in cyclic GMP (cGMP), guanylyl cyclase (GC), and protein kinase G (PKG), as well as activation of extracellular signal-regulated kinase (ERK) activity, compared with females [(B) middle]. Hippocampal proteasome activity is also increased more in males following learning, although females exhibit higher baseline proteasome activity. Initially, females have higher baseline postsynaptic electrical activity to stimuli, but males have greater increases in excitatory tone and decreases in inhibitory tone in response to learning events, leading to overall stronger potentiation in males compared with females [(B) bottom]. (C) At baseline, males have greater levels of histone acetylation, and females have higher levels of histone methylation, at individual transcription sites, leading to differential gene expression and subsequent protein expression, allowing for sex-specific responses to similar cellular stimuli. Created with BioRender (BioRender.com).
Under basal conditions, sex differences in synaptic plasticity have been observed in the aforementioned regions. For example, the dorsal CA1 of female rodents expresses greater levels compared with males of the glutamate NMDA receptor subunits GluN1 and GluN2A/B [70], as well as levels and phosphorylation of the glutamate AMPA receptor subunit GluA2 (Figure 2B) [28], sensitizing this brain region to excitation. Interestingly, expression of GluA1 and GluN1 is higher in males at synapses between the entorhinal cortex and CA1 [17], and females have higher synaptosomal GluA1 and GluA2 expression in the mPFC [71], perhaps conferring region- and circuit-specific sex differences in sensitivity to excitation. These baseline differences in protein expression imply region-specific sex differences in gene expression and protein synthesis. Intriguingly, female rats have higher basal levels of proteasome activity and, therefore, protein degradation, compared with males in the BLA, but not DH, due to epigenetic upregulation of Uba52 [59,60], indicating sex-specific roles of protein degradation in the BLA during fear learning. These differences in protein expression and degradation are concomitant with greater densities of mature, mushroom-type spines, but not overall spine densities, in the female CA1 (Figure 2A) [72], as well as overall greater spine densities in the female BLA [73]. In dorsal CA1 and mPFC, such differences culminate in greater synaptic excitability in females at baseline (Figure 2B) [28,71]. These and other sex differences in baseline synaptic plasticity could set the stage for the sex differences in learning-induced brain alterations that underlie memory formation.
Learning stimulates neural activity in distinct brain regions and circuits, and these events have been linked to sex-specific chemical and morphological changes in neurons. For example, although cellular activation elevates kinase activity in both sexes, male rat hippocampal neurons respond to stimulation by producing more protein kinase G, guanylate cyclase, cyclic GMP, and GluA1 compared with female neurons (Figure 2B) [28]. Similarly, fear extinction training in mice increases extracellular signal-regulated kinase (ERK) phosphorylation in the mPFC of both sexes, but more rapidly elevates ERK phosphorylation in the male DH, which correlates with faster extinction rates in males [67]. Fear conditioning in mice also induces histone 3 acetylation on Bdnf in DH of both sexes, but on Cdk5 only in males, promoting greater expression of the kinase CDK5 and memory retention in males [45] (Figure 2C). Additionally, neural activity induces production of immediate early genes (IEGs), such as cFos and zif268, which act as transcription factors. Male rats rely on cFos expression, whereas females rely on zif268, in dorsal CA3 to support place strategies and general spatial learning [25,69]. During fear conditioning, context pre-exposure and generalization elevate cFos expression in the dorsal CA1 and CA3 of male, and dorsal CA1 and BLA of female, mice [46,58], demonstrating important sex-specific requirements of regional activation and IEG specificity for different forms of memory.
Following learning, the sexes also differ in the maintenance of potentiated cells. For example, synaptic expression in CA1 of protein kinase Mζ (PMKζ), which regulates AMPA receptor localization at the synapse, is necessary in male but not in female rats for remote spatial memory [31]. In addition, a key element of synaptic and memory maintenance is degradation of old proteins, and its disruption in DH specifically impairs female fear recall [60], highlighting sex differences in the necessity of protein degradation to consolidate fear memories. Morphologically, learning is associated with greater spine densities in both sexes (Figure 2A), but this effect is specific to the task, circuit, and spine type (Figure 2A). These learning-induced biochemical and morphological alterations produce differential facilitation of LTP between the sexes, as evidenced by lower thresholds for both learning and LTP in the male DH compared with female DH (Figure 2B) [17,28]. Thus, although both sexes learn, encode, and maintain information through generally similar mechanisms, some underlying processes are sex specific.
Hormones directly influence cellular processes related to memory, with estrogenic signaling being the most well studied. Additionally, although E2 recruits similar factors in the cortex and hippocampus of both sexes to promote LTP, spine changes, and memory [18,43,74], E2-mediated LTP is more sensitive to calcium and protein kinase A signaling disruptions in females than in males [74]. Furthermore, E2-enhanced spatial memory relies on CREB activation via ERK and Akt signaling in ovariectomized mice, but not among gonadally intact or gonadectomized males [18], suggesting that E2 treatment produces similar electrophysiological and memory outcomes in each sex via somewhat disparate neural mechanisms. Importantly, endogenous estrogenic signaling also affects the above outcomes; dendritic spine densities are reduced in the CA1 and cortex of male and female neuronal aromatase-knockout mice [43] and rodents administered aromatase inhibitors, although females are more susceptible to E2 depletion-mediated dendritic spine loss in the CA1 and BLA compared with males [72,73,75]. Systemic, intra-mPFC, and intra-DH estrogen treatments also rescue gonadectomy-associated spine decreases in both sexes in these regions [76–80], and both endogenous and exogenous E2 promote potentiation of CA3–CA1 projections [17,43,72–75]. However, female rodents are more dependent upon the potentiating effects of E2 in the CA1 and BLA than are males [72,73,75], perhaps due to female-specific disinhibition of excitatory tone via E2 signaling, which inhibits the activity of parvalbumin-expressing GABAergic interneurons [41], leading to lower GABA release [81]. Interestingly, these effects are due to ERα-mediated activation of inositol triphosphate signaling in the female, but not male, DH [81]. Evidently, thememory-mediating effects of E2 can differ by sex, brain region, cell type, and ER localization, underscoring the need to fully explore these neural mechanisms in both sexes.
Overall, numerous sex differences have been reported in the neurobiological factors underlying memory under basal conditions, following learning, and related to endogenous and exogenous estrogen signaling. These differences may be region, circuit, and cell specific, and, therefore, must be examined in greater detail, which would provide vital new information about the neurobiology of learning and memory in both sexes, as well as new therapeutic targets for memory-related disorders.
Concluding remarks and future perspectives
Although most research into the neurobiology of learning and memory has been conducted in males only, mandates from the NIH and other funding agencies to incorporate SABV into preclinical and clinical research have begun to provide a more holistic understanding of learning processes. However, even with these policy changes, considerable ground remains to be covered, given that the field continues to conduct male-only- and sex-as-a-covariate-biased studies instead of sex-equivalent- and sex-as-a-discovery-variable-focused research. Given recent and historical findings of male- and female-favored behavioral responses, even within the same task and in response to the same stimuli, greater inclusion of females in learning and memory research will also require seismic shifts toward more sex-appropriate measures of memory. Furthermore, repeated recent findings of convergent and latent sex differences in mechanisms underlying memory-related processes warrant caution and more rigorous studies appropriately designed and powered to detect putative sex differences and determine how they might contribute to disparate risk of memory-related disorders, such as AD and PTSD (Box 1; see Outstanding questions). Although more work is sorely needed, such research will significantly advance our understanding of brain function and could lead to innovative new treatments for memory loss in both women and men.
Outstanding questions.
In learning and memory paradigms besides those testing spatial and fear memory, are potential learning-related responses being ignored in favor of male-centric or female-centric behaviors?
What are the relative contributions of sex chromosomes and hormones to producing sex differences in memory and underlying neurobiology?
What aspects of cellular and subcellular function lead to sex differences in memory and do these vary by brain region or type of memory?
Do the roles of brain-synthesized sex steroid hormones in mediating memory differ by sex, type of memory, or brain region?
How might neurons interact with non-neuronal cells in the brain and periphery to produce sex differences in memory?
Highlights.
Although male rodents are thought to exhibit better spatial and fear memories compared with females, the sexes often respond differently to learning stimuli, resulting in mischaracterization of female memory abilities.
Responses to learning stimuli are influenced by sex steroid hormones and sex. High hormone levels are associated with place strategies in spatial tasks. In fear tasks, males use passive strategies, whereas females use passive and active strategies.
The sexes differ in neural mechanisms underlying memory formation, as indicated by basal, learning-induced, and hormone-driven brain region- and cell type-specific sex differences in cellular activity and morphology.
Better understanding of how sex differences in neural function contribute to sex differences in memory is vital and will lead to next-generation therapies for memory disorders in both sexes.
Acknowledgments
Sex differences and hormone-related research in the Frick laboratory has received current and past support from the NIH (R01AG22525, R01MH107886, 2R15GM118304-02, R43AG079715, R03MH65460, F31MH118822, and F32MH118782), the Alzheimer’s Association (IIRG-03-6051, SAGA-17-419092, ABA-22-973796), the University of Wisconsin System, the University of Wisconsin-Milwaukee Research Foundation, and the University of Wisconsin-Milwaukee Office of Undergraduate Research, Office of Research, and College of Letters and Science. The authors would like to thank members of the Frick laboratory past and present whose data were referenced in this review, particularly those who have studied sex differences as part of their research.
Glossary
- 17β-estradiol
the most potent and ubiquitous estrogen synthesized before menopause and outside of pregnancy.
- Active fear responding
darting or flight-like responses to fearful stimuli
- Aromatase
biosynthetic enzyme responsible for converting androgens into estrogens
- Basolateral nucleus of the amygdala
hub of the fear brain circuit where fear signals are integrated and processed as a whole experience and consolidated into one memory
- Cued strategy
navigation strategy in which specific objects in the environment are used as landmarks to locate a goal
- Dendritic spines
knobby protuberances on excitatory dendrites that constitute the most common postsynaptic localization for excitatory synapses
- Dorsal hippocampus
dorsal portion of the hippocampus with a well-established role in mediating the formation of spatial, contextual, and recognition memories
- Fear conditioning
process by which fear responses, such as freezing or darting, are elicited by repeatedly pairing an aversive stimulus, such as a footshock, with a neutral stimulus (e.g., tone) or context
- Fear extinction
process by which the fear response is reduced over time through multiple re-exposures to a fear-associated stimulus or context without concurrently presenting footshocks
- Fear generalization
associating a fearful stimulus with multiple other stimuli, such that fear learning in one context elicits fear behaviors in a unique, yet similar, context
- Four-Core Genotype (FCG) model
genetically XX or XY mice in which ovaries or testes can be expressed by regulating the testes-determining gene
- Immediate early genes
genes that are activated rapidly after cellular stimulation, often acting as transcription factors for other genes that promote neuronal plasticity
- Long-term potentiation
strong excitatory stimulation triggers postsynaptic signaling that produces a prolonged increase in excitatory postsynaptic physiological responses
- Medial prefrontal cortex
anterior midline portion of the prefrontal cortex that mediates working and long-term memory in numerous domains (e.g., spatial, recognition, or fear), as well as judgement and decision-making processes
- Memory consolidation
process of transferring memory from short-term to long-term storage. Cellular activity, inter- and intraregion connectivity, protein synthesis and activation, and dendritic remodeling are all associated with consolidation
- Passive fear responding
freezing or stationary responses to fearful stimuli
- Place strategy
spatial navigation strategy in which geometric and relational extra-maze cues, such as environment shape or spatial relations among environmental cues, are used to locate a goal
- Reference memory
form of long-term memory in which previously learned information that does not change over time guides future behavior
- Remote memory
very long-term memory system in which information learned long before recall guides future behavior
- Sex as a biological variable (SABV)
part of the 2014 NIH mandate requiring funded research to consider sex as a biological variable in grant proposals. Proposals need not include both sexes but must consider how sex may affect experimental variables
- Synaptic plasticity
changes in cellular activity, signaling cascades, and cellular morphology in neurons that make postsynaptic cells respond more effectively to future presentations of the same stimulus; often considered the biological manifestation of learning
- Working memory
form of short-term memory in which newly learned information guides future behavior
Footnotes
Declaration of interests
K.M.F. is a co-founder and the chief scientific officer of Estrigenix Therapeutics, Inc., a company that aims to improve women’s health by developing safe, clinically proven treatments for the mental and physical effects of menopause. A.W.F. has no conflicts of interests to declare.
References
- 1.Yuan L et al. (2019) Gender differences in large-scale and small-scale spatial ability: a systematic review based on behavioral and neuroimaging research. Front. Behav. Neurosci 13, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rechlin RK et al. (2022) An analysis of neuroscience and psychiatry papers published from 2009 and 2019 outlines opportunities for increasing discovery of sex differences. Nat. Commun 13, 2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geller SE et al. (2018) The more things change, the more they stay the same: a study to evaluate compliance with inclusion and assessment of women and minorities in randomized controlled trials. Acad. Med 93, 630–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Launer L et al. (1999) Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. Neurology 52, 78–84 [DOI] [PubMed] [Google Scholar]
- 5.Lebron-Milad K and Milad MR (2012) Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol. Mood Anxiety Disord 2, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine DA et al. (2021) Sex differences in cognitive decline among US adults. JAMA 4, e210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clayton J (2016) Studying both sexes: a guiding principle for biomedicine. Fed. Am. Soc. Exp. Biol 30, 519–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Will T et al. (2017) Problems and progress regarding sex bias and omission in neuroscience research. eNeuro 4, e0278–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joel D et al. (2015) Sex beyond the genitalia: the human brain mosaic. Proc. Natl. Acad. Sci. U. S. A 112, 15468–15473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joel D et al. (2018) Analysis of human brain structure reveals that the brain ‘types’ typical of males are also typical of females, and vice versa. Front. Hum. Neurosci 12, 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eliot L et al. (2021) Dump the ‘dimorphism’: comprehensive synthesis of human brain studies reveals few male-female differences beyond size. Neurosci. Biobehav. Rev 125, 667–697 [DOI] [PubMed] [Google Scholar]
- 12.Prendergast BJ et al. (2014) Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev 40, 1–5 [DOI] [PubMed] [Google Scholar]
- 13.Becker JB et al. (2016) Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol. Sex Differ 7, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz A et al. (2017) Similar reliability and equivalent performance of female and male mice in the open field and water-maze place navigation task. Am. J. Med. Genet 175, 380–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maney DL (2016) Perils and pitfalls of reporting sex differences. Philos. Trans. R. Soc. B Biol. Sci 371, 1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruene TM et al. (2015) Sexually divergent expression of active and passive conditioned fear responses in rats. eLife 4, e11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi X et al. (2016) Sex differences in long-term potentiation at temporoammonic-CA1 synapses: potential implications for memory consolidation. PLoS ONE 11, e0165891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koss WA et al. (2018) Sex differences in the rapid cell signaling mechanisms underlying the memory-enhancing effects of 17β-estradiol. eNeuro 5 ENEURO.0267–18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui J et al. (2013) Estrogen synthesis and signaling pathways during ageing: from periphery to brain. Trends Mol. Med 19, 197–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harden KP et al. (2016) Diurnal coupling between testosterone and cortisol from adolescence to older adulthood. Psychoneuroendocrinology 73, 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frick KM et al. (2015) Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn. Mem 22, 472–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamson DK et al. (2016) Sex hormones and cognition: neuroendocrine influences on memory and learning. Compr. Physiol 6, 1295–1337 [DOI] [PubMed] [Google Scholar]
- 23.Yagi S and Galea LAM (2018) Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 44, 200–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spritzer MD et al. (2013) Testosterone influences spatial strategy preferences among adult male rats. Horm. Behav 63, 800–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yagi S et al. (2017) Sex and estrous cycle differences in immediate early gene activation in the hippocampus and the dorsal striatum after the cue competition task. Horm. Behav 87, 69–79 [DOI] [PubMed] [Google Scholar]
- 26.Almey A et al. (2014) Medial prefrontal cortical estradiol rapidly alters memory system bias in female rats: ultrastructural analysis reveals membrane-associated estrogen receptors as potential mediators. Endocrinology 155, 4422–4432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koss WA and Frick KM (2017) Sex differences in hippocampal function. J. Neurosci. Res 95, 539–562 [DOI] [PubMed] [Google Scholar]
- 28.Monfort P et al. (2015) Gender differences in spatial learning, synaptic activity, and long-term potentiation in the hippocampus in rats: molecular mechanisms. ACS Chem. Neurosci 6, 1420–1427 [DOI] [PubMed] [Google Scholar]
- 29.Williams CL et al. (1990) Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav. Neurosci 104, 84–97 [DOI] [PubMed] [Google Scholar]
- 30.Roof RL (1993) Neonatal exogenous testosterone modifies sex difference in radial arm and Morris water maze performance in prepubescent and adult rats. Behav. Brain Res 53, 1–10 [DOI] [PubMed] [Google Scholar]
- 31.Sebastian V et al. (2013) PKMζ differentially utilized between sexes for remote long-term spatial memory. PLoS ONE 8, e81121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolmogorova D et al. (2019) Pubertal immune stress transiently alters spatial memory processes in adulthood. Psychoneuroendocrinology 102, 261–272 [DOI] [PubMed] [Google Scholar]
- 33.Frick KM and Berger-Sweeney J (2001) Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav. Neurosci 115, 229–237 [DOI] [PubMed] [Google Scholar]
- 34.Sandstrom NJ and Williams CL (2001) Memory retention is modulated by acute estradiol and progesterone replacement. Behav. Neurosci 115, 384–393 [PubMed] [Google Scholar]
- 35.Packard MG and Teather LA (1997) Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol. Learn. Mem 68, 172–188 [DOI] [PubMed] [Google Scholar]
- 36.Spritzer MD et al. (2011) Effects of testosterone on spatial learning and memory in adult male rats. Horm. Behav 59, 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frick KM and Gresack JE (2003) Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav. Neurosci 117, 1283–1291 [DOI] [PubMed] [Google Scholar]
- 38.Barrett GL et al. (2009) The chronology of age-related spatial learning impairment in two rat strains, as tested by the Barnes maze. Behav. Neurosci 123, 533–538 [DOI] [PubMed] [Google Scholar]
- 39.O’Leary OF et al. (2009) Chronic fluoxetine treatment increases expression of synaptic proteins in the hippocampus of the ovariectomized rat: role of BDNF signalling. Psychoneuroendocrinology 34, 367–381 [DOI] [PubMed] [Google Scholar]
- 40.Locklear MN and Kritzer MF (2014) Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Horm. Behav 66, 298–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernández-Vivanco A et al. (2022) Sex-specific regulation of inhibition and network activity by local aromatase in the mouse hippocampus. Nat. Commun 13, 3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koss WA and Frick KM (2019) Activation of androgen receptors protects intact male mice from memory impairments caused by aromatase inhibition. Horm. Behav 111, 96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu Y et al. (2019) Neuron-derived estrogen regulates synaptic plasticity and memory. J. Neurosci 39, 2792–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maren S et al. (1994) Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res 661, 25–34 [DOI] [PubMed] [Google Scholar]
- 45.Sase AS et al. (2018) Sex-specific regulation of fear memory by targeted epigenetic editing of Cdk5. Biol. Psychiatry 85, 623–634 [DOI] [PubMed] [Google Scholar]
- 46.Colon LM and Poulos AM (2020) Contextual processing elicits sex differences in dorsal hippocampus activation following footshock and context fear retrieval. Behav. Brain Res 393, 112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Day HLL et al. (2016) Sex differences in discriminating between cues predicting threat and safety. Neurobiol. Learn. Mem 133, 196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lynch J et al. (2013) Sex differences in the generalization of fear as a function of retention intervals. Learn. Mem 20, 628–632 [DOI] [PubMed] [Google Scholar]
- 49.Colon L et al. (2018) Sexual differentiation of contextual fear responses. Learn. Mem 25, 230–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gresack J et al. (2009) Sex differences in contextual fear conditioning are associated with differential ventral hippocampal extracellular signal-regulated kinase activation. Neuroscience 159, 451–467 [DOI] [PubMed] [Google Scholar]
- 51.Rocks D et al. (2022) Why the estrous cycle matters for neuroscience. Biol. Sex Differ 13, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaluve AM et al. (2022) Female rodents are not more variable than male rodents: a meta-analysis of preclinical studies of fear and anxiety. Neurosci. Biobehav. Rev 143, 104962. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell JR et al. (2022) Darting across space and time: parametric modulators of sex-biased conditioned fear responses. Learn. Mem 29, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baratta MV et al. (2019) Controllable stress elicits circuit-specific patterns of prefrontal plasticity in males, but not females. Brain Struct. Funct 224, 1831–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greiner EM et al. (2019) Sex differences in fear regulation and reward-seeking behaviors in a fear-safety-reward discrimination task. Behav. Brain Res 368, 111903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trott JM et al. (2022) Conditional and unconditional components of aversively motivated freezing, flight and darting in mice. eLife 11, e75663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trask S et al. (2020) Decreased cued fear discrimination learning in female rats as a function of estrous phase. Learn. Mem 27, 254–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keiser AA et al. (2017) Sex differences in context fear generalization and recruitment of hippocampus and amygdala during retrieval. Neuropsychopharmacology 42, 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Devulapalli R et al. (2021) Males and females differ in the regulation and engagement of, but not requirement for, protein degradation in the amygdala during fear memory formation. Neurobiol. Learn. Mem 180, 107404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin K et al. (2021) Females, but not males, require protein degradation in the hippocampus for contextual fear memory formation. Learn. Mem 28, 248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Musaus M et al. (2021) Sex-specific linear polyubiquitination is a critical regulator of contextual fear memory formation. Front. Behav. Neurosci 15, 709392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dulka BN et al. (2021) Age-related memory impairment and sex-specific alterations in phosphorylation of the Rpt6 proteasome subunit and polyubiquitination in the basolateral amygdala and medial prefrontal cortex. Front. Aging Neurosci 13, 656944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asok A et al. (2019) Sex differences in remote contextual fear generalization in mice. Front. Behav. Neurosci 13, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Voulo M and Parsons R (2017) Response-specific sex difference in the retention of fear extinction. Learn. Mem 24, 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Day HLL et al. (2020) Sex differences in auditory fear discrimination are associated with altered medial prefrontal cortex function. Sci. Rep 10, 6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trott JM et al. (2022) Sex differences in contextual fear learning and generalization: a behavioral and computational analysis of hippocampal functioning. Learn. Mem 29, 283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuda S et al. (2015) Sex differences in fear extinction and involvements of extracellular signal-regulated kinase (ERK). Neurobiol. Learn. Mem 123, 117–124 [DOI] [PubMed] [Google Scholar]
- 68.Lynch JF et al. (2016) Hippocampal cytosolic estrogen receptors regulate fear generalization in females. Neurobiol. Learn. Mem 130, 83–92 [DOI] [PubMed] [Google Scholar]
- 69.Yagi S et al. (2016) Sex and strategy use matters for pattern separation, adult neurogenesis, and immediate early gene expression in the hippocampus. Hippocampus 26, 87–101 [DOI] [PubMed] [Google Scholar]
- 70.Brandt N et al. (2020) Sex-specific features of spine densities in the hippocampus. Sci. Rep 10, 11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knouse MC et al. (2022) Sex differences in the medial prefrontal cortical glutamate system. Biol. Sex Differ 13, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brandt N et al. (2020) Sex-specific difference of hippocampal synaptic plasticity in response to sex neurosteroids. Cereb. Cortex 30, 2627–2641 [DOI] [PubMed] [Google Scholar]
- 73.Bender RA et al. (2017) Sex-dependent regulation of aromatase-mediated synaptic plasticity in the basolateral amygdala. J. Neurosci 37, 1532–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jain A et al. (2019) Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the hippocampus. J. Neurosci 39, 1552–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang W et al. (2018) Memory-related synaptic plasticity is sexually dimorphic in rodent hippocampus. J. Neurosci 38, 7935–7951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wallace M et al. (2006) Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res 1126, 176–182 [DOI] [PubMed] [Google Scholar]
- 77.Velázquez-Zamora DA et al. (2012) Effects of selective estrogen receptor modulators on allocentric working memory performance and on dendritic spines in medial prefrontal cortex pyramidal neurons of ovariectomized rats. Horm. Behav 61, 512–517 [DOI] [PubMed] [Google Scholar]
- 78.Tuscher JJ et al. (2019) Chemogenetic suppression of medial prefrontal-dorsal hippocampal interactions prevents estrogenic enhancement of memory consolidation in female mice. eNeuro 6, e0451–18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Avila JA et al. (2017) Estradiol rapidly increases GluA2-mushroom spines and decreases GluA2-filopodia spines in hippocampus CA1. Hippocampus 27, 1224–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tuscher JJ et al. (2016) Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus. J. Neurosci 36, 1483–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tabatadze N et al. (2015) Sex differences in molecular signaling at inhibitory synapses in the hippocampus. J. Neurosci 35, 11252–11265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scully EP et al. (2020) Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol 20, 442–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Agustí A et al. (2018) Interplay between the gut-brain axis, obesity and cognitive function. Front. Neurosci 12, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hasan Mohajeri M et al. (2018) Relationship between the gut microbiome and brain function. Nutr. Rev 76, 481–496 [DOI] [PubMed] [Google Scholar]
- 85.Tchessalova D and Tronson NC (2019) Memory deficits in males and females long after subchronic immune challenge. Neurobiol. Learn. Mem 158, 60–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dockman RL et al. (2021) Sex differences in behavior, response to LPS, and glucose homeostasis in middle-aged mice. Behav. Brain Res 418, 113628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geary CG et al. (2021) Sex differences in gut microbiota modulation of aversive conditioning, open field activity, and basolateral amygdala dendritic spine density. J. Neurosci. Res 99, 1780–1801 [DOI] [PubMed] [Google Scholar]
- 88.Clarke G et al. (2013) The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 186, 666–673 [DOI] [PubMed] [Google Scholar]
- 89.Darch HT et al. (2021) Microbial memories: sex-dependent impact of the gut microbiome on hippocampal plasticity. Eur. J. Neurosci 54, 5235–5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balu D et al. (2019) The role of APOE in transgenic mouse models of AD. Neurosci. Lett 707, 134285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bakrania P et al. (2022) Discovery of a novel pseudo β-hairpin structure of N-truncated amyloid-β for use as a vaccine against Alzheimer’s disease. Mol. Psychiatry 27, 840–848 [DOI] [PubMed] [Google Scholar]
- 92.Glezerman M (2016) Yes, there is a female and a male brain: morphology versus functionality. Proc. Natl. Acad. Sci. U. S. A 113, E1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCarthy MM and Konkle ATM (2005) When is a sex difference not a sex difference? Front. Neuroendocrinol 26, 85–102 [DOI] [PubMed] [Google Scholar]
- 94.Woolley CS et al. (1990) Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci 10, 4035–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gresack JE and Frick KM (2006) Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol. Biochem. Behav 84, 112–119 [DOI] [PubMed] [Google Scholar]
- 96.Shaar CJ et al. (1975) Effects of castration and gonadal steroids on serum luteinizing hormone and prolactin in old and young rats. J. Endocrinol 66, 45–51 [DOI] [PubMed] [Google Scholar]
- 97.Wise PM and Ratner A (1980) Effect of ovariectomy on plasma LH, FSH, estradiol, and progesterone and medial basal hypothalamic LHRH concentrations in old and young Rats. Neuroendocrinology 30, 15–19 [DOI] [PubMed] [Google Scholar]
- 98.Eldridge JC et al. (1974) Effects of castration of immature rats on serum FSH and LH, and of various steroid treatments after castration. Biol. Reprod 10, 438–446 [DOI] [PubMed] [Google Scholar]
- 99.Frick KM (2015) Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm. Behav 74, 4–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kato A et al. (2013) Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front. Neural Circuits 7, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hojo Y et al. (2004) Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. U. S. A 101, 865. [DOI] [PMC free article] [PubMed] [Google Scholar]


