Abstract
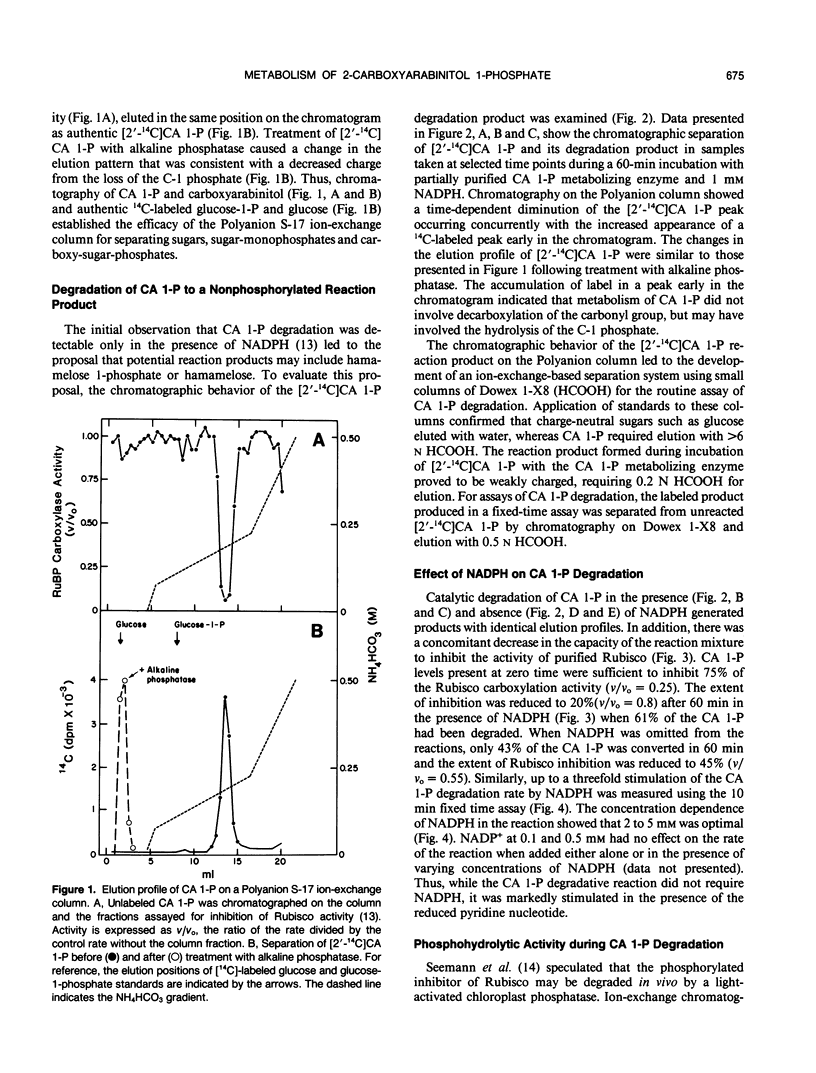
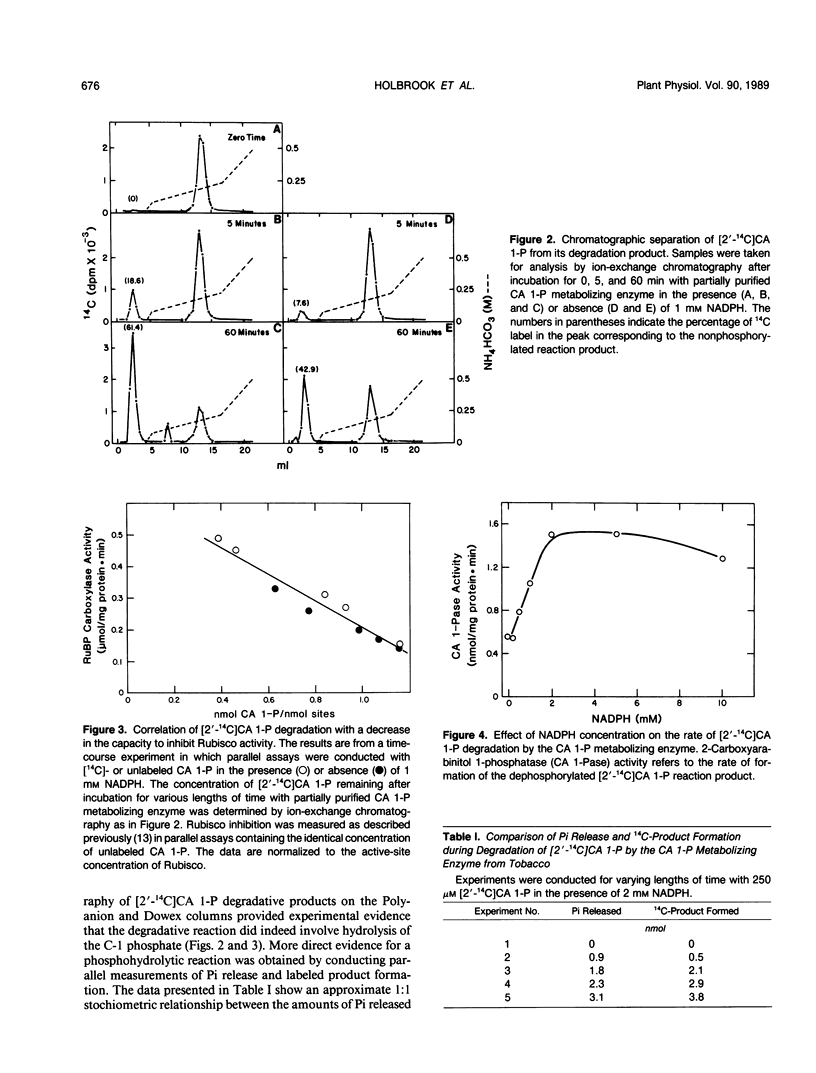
The catalytic degradation of 2-carboxyarabinitol 1-phosphate (CA 1-P), a naturally occurring inhibitor of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), was investigated by chromatographic and spectroscopic analyses of the reaction products. Carboxy-labeled [14C]CA 1-P was incubated with a partially purified tobacco (Nicotiana rustica) chloroplast protein that has been shown previously to catalyze metabolism of CA 1-P to a form incapable of inhibiting Rubisco (ME Salvucci, GP Holbrook, JC Anderson, and G Bowes [1988] FEBS Lett 231: 197-201). In the presence and absence of NADPH, ion-exchange chromatography showed a progressive conversion of [2′-14C]CA 1-P to a labeled compound which coeluted with authentic carboxyarabinitol. Parallel assays with unlabeled CA 1-P showed a concomitant decrease in the ability of reaction samples to inhibit Rubisco activity. In separate experiments, a 1:1 stoichiometry was found between the release of inorganic phosphate from [2′-14C]CA 1-P and accumulation of the 14C-labeled product. Liberation of inorganic phosphate was not observed when the tobacco enzyme was incubated with ribulose-1,5-bisphosphate, fructose-1,6-bisphosphate, glucose-1-phosphate, glucose-6-phosphate, or 6-phosphogluconate. Proton nuclear magnetic resonance spectroscopy of the labeled CA 1-P reaction product established its identity as carboxyarabinitol. We therefore propose that light-stimulated degradation of CA 1-P is catalyzed in vivo by a specific phosphatase, 2-carboxyarabinitol 1-phosphatase. Carboxyarabinitol 1-phosphatase activity was detected in the absence of NADPH, but increased threefold when 2 millimolar NADPH was present. Thus, while not required for the reaction, NADPH may play an important role in the regulation of CA 1-P degradation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Stransky H., Fürbringer M. Synthesis of hamamelose-diphosphate by isolated spinach chloroplasts. FEBS Lett. 1971 Mar 16;13(4):229–234. doi: 10.1016/0014-5793(71)80542-2. [DOI] [PubMed] [Google Scholar]
- Berry J. A., Lorimer G. H., Pierce J., Seemann J. R., Meek J., Freas S. Isolation, identification, and synthesis of 2-carboxyarabinitol 1-phosphate, a diurnal regulator of ribulose-bisphosphate carboxylase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):734–738. doi: 10.1073/pnas.84.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chifflet S., Torriglia A., Chiesa R., Tolosa S. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: application to lens ATPases. Anal Biochem. 1988 Jan;168(1):1–4. doi: 10.1016/0003-2697(88)90002-4. [DOI] [PubMed] [Google Scholar]
- Kobza J., Seemann J. R. Mechanisms for light-dependent regulation of ribulose-1,5-bisphosphate carboxylase activity and photosynthesis in intact leaves. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3815–3819. doi: 10.1073/pnas.85.11.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney C. L. A simple micro-assay for inorganic phosphate. Anal Biochem. 1976 Sep;75(1):201–210. doi: 10.1016/0003-2697(76)90071-3. [DOI] [PubMed] [Google Scholar]
- Pierce J., Andrews T. J., Lorimer G. H. Reaction intermediate partitioning by ribulose-bisphosphate carboxylases with differing substrate specificities. J Biol Chem. 1986 Aug 5;261(22):10248–10256. [PubMed] [Google Scholar]
- Pierce J., Tolbert N. E., Barker R. Interaction of ribulosebisphosphate carboxylase/oxygenase with transition-state analogues. Biochemistry. 1980 Mar 4;19(5):934–942. doi: 10.1021/bi00546a018. [DOI] [PubMed] [Google Scholar]
- Salvucci M. E., Anderson J. C. Factors affecting the activation state and the level of total activity of ribulose bisphosphate carboxylase in tobacco protoplasts. Plant Physiol. 1987 Sep;85(1):66–71. doi: 10.1104/pp.85.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Holbrook G. P. Purification and Properties of 2-Carboxy-d-Arabinitol 1-Phosphatase. Plant Physiol. 1989 Jun;90(2):679–685. doi: 10.1104/pp.90.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C. Binding of a Phosphorylated Inhibitor to Ribulose Bisphosphate Carboxylase/Oxygenase during the Night. Plant Physiol. 1985 Aug;78(4):839–843. doi: 10.1104/pp.78.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Parry M. A., Gutteridge S., Keys A. J. Species variation in the predawn inhibition of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1986 Dec;82(4):1161–1163. doi: 10.1104/pp.82.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu C. V., Allen L. H., Bowes G. Effects of Light and Elevated Atmospheric CO(2) on the Ribulose Bisphosphate Carboxylase Activity and Ribulose Bisphosphate Level of Soybean Leaves. Plant Physiol. 1983 Nov;73(3):729–734. doi: 10.1104/pp.73.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu J. C., Allen L. H., Bowes G. Dark/Light modulation of ribulose bisphosphate carboxylase activity in plants from different photosynthetic categories. Plant Physiol. 1984 Nov;76(3):843–845. doi: 10.1104/pp.76.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]


