Abstract
Management of lupus nephritis has evolved considerably over the past years. Here, we provide a comprehensive review of clinical trials that form the basis for the Kidney Disease: Improving Global Outcomes and EULAR/ERA-EDTA updated guidelines and present day trials that will change the landscape of lupus nephritis therapy in years to come. In addition, we highlight the issues related to cost of therapy, resistant disease, and downstream adverse effects of specific therapies.
Keywords: glomerular disease, lupus nephritis, systemic lupus erythematosus
Introduction
Lupus nephritis (LN) occurs in approximately 40% of patients with SLE, with 5%–15% of these patients progressing to ESKD within 10 years.1,2 Risk factors for progressive kidney disease include clinical parameters (proteinuria, glomerular filtration rate, complement levels, anti-dsDNA titer, presence of antiphospholipid antibodies), kidney biopsy classification (including measures of activity and chronicity), and nonadherence to therapy.3–6 Proteinuria is currently the best clinical prognostic biomarker, with studies indicating that a cutoff of 0.7–0.8 g/24 hours at 1 year is predictive of a good long-term renal prognosis.7 More recently, studies have investigated the association of novel biomarkers with kidney disease progression (see Emerging Biomarkers below).
There has also been significant progress in identifying antigenic targets within the kidney, which differ according to the histopathologic classification and may also correlate with prognosis. Anti-dsDNA and antihistone antibodies with an IgG2 isotype and antibodies to annexin A1 and α-enolase have been associated with proliferative LN.8,9 In membranous LN, exostosin 1 and 2 antigens have been identified in approximately 30% of subjects and are associated with a better prognosis.10–12 Other newly identified antigens in membranous LN include neural cell adhesion molecule 113 and TGF-β receptor 3 (TGFβR3).14
These new molecular insights into LN provide hope that better and more specific therapies for LN are on the way. Here, we review current standard-of-care (SOC) therapies, newly US Food and Drug Administration (FDA)–approved drugs, and select ongoing clinical trials (Figure 1). We highlight special considerations for treatment in non-European populations, cost-utility analyses of current treatment options, and important high-risk medication monitoring strategies.
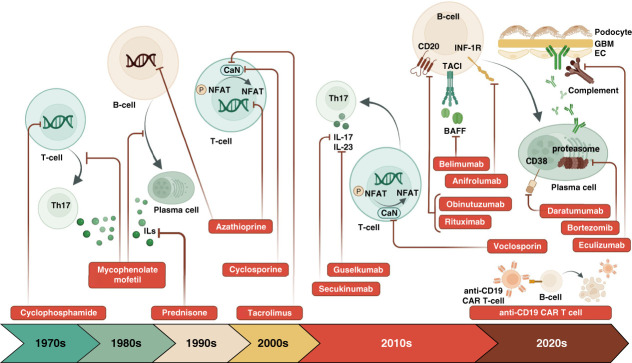
Figure 1.
Evolution of therapies for LN and their targets: standard of care, new to practice, and emerging therapies. BAFF, B-cell activating factor; CaN, calcineurin; CAR T-cell, chimeric antigen receptor T-cell; CD, cluster of differentiation; EC, endothelial cell; GBM, glomerular basement membrane; IL, interleukin; INF-1R, interferon alpha receptor 1; NFAT, nuclear factor of activated T-cells; TACI, transmembrane activator calcium modulator and cyclophilin ligand interactor; Th17, T helper 17 cell.
Current Practice and Review of Updated Guidelines
LN therapy has evolved considerably since the early 1950s when glucocorticoids (GCs) were the mainstay of treatment. In the 1970s to early 1990s, therapy with cyclophosphamide (CYC) showed improved outcomes compared with GCs alone. In a landmark 1992 National Institutes of Health (NIH) LN study, long-term CYC (2 years' duration) was associated with stable kidney function in 85% of participants compared with 65% with short-term CYC (6 months' duration) and 52% with GCs alone.15,16 Notably, the NIH study population was high risk, with 64% having kidney function impairment at study entry. As screening improved, facilitating the detection of kidney disease earlier in the SLE course, a more tolerable CYC regimen for LN was needed. In 2002, the Euro-Lupus Nephritis Trial investigated the treatment of proliferative LN in a European population with low-dose intravenous (IV) CYC (500 mg q2 weeks for six doses) versus high-dose IV CYC (0.5 g/m2 of body surface area, up to a maximum of 1500 mg/dose, every 4 weeks for six doses followed by two quarterly pulses) followed by azathioprine (AZA) in both treatment arms.17 There was no difference in treatment failure between groups after approximately 3.5 years of follow-up; furthermore, 71% of patients in the low-dose group compared with 54% in the high-dose group achieved renal remission, and approximately 30% in each group had progressive kidney disease. The 10-year long-term outcomes confirmed that the low-dose regimen followed by AZA did not differ from the high-dose group regarding death, doubling of serum creatinine, and ESKD.18 Low-dose IV CYC has been investigated in non-European populations, with similar results.19–21
In the late 1980s, mycophenolic acid (MPA), an inhibitor of inosine monophosphate dehydrogenase, and its prodrug, mycophenolate mofetil (MMF), gained popularity as an effective and tolerable antirejection medication in solid organ transplant recipients. In 2000, the Hong Kong-Guangzhou Nephrology Study Group showed that induction therapy with MMF in combination with prednisolone resulted in similar rates of remission compared with oral CYC and prednisolone (81% versus 75%, P = 1.0) and that MMF was associated with numerically fewer side effects.22 A larger, open-label, noninferiority trial comparing MMF with monthly IV CYC (0.5–1.0 g/m2) for induction therapy showed that MMF was noninferior to IV CYC and in fact more effective in inducing complete remission.23 This was followed by the Aspreva Lupus Management Study (ALMS) trial in 2009 that compared MMF versus high-dose IV CYC in a multinational, multiethnic cohort and showed no difference in remission induction between the two groups (53% with IV CYC versus 56% with MMF, P = 0.58).24 In 2011, the ALMS study group published their 36-month, randomized, controlled trial comparing MMF with AZA for maintenance therapy and found that MMF was superior to AZA for the primary end point of time to treatment failure (16.4% with MMF versus 32.4% with AZA).25 The MAINTAIN Nephritis trial, an investigator-initiated study, also investigated AZA versus MMF for maintenance therapy and found no statistically significant difference in the primary outcome of time to renal flare.26 On the basis of the findings from these studies, the 2011 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommended first-line induction therapy for LN classes III and IV with either CYC or mycophenolate and maintenance therapy with AZA or MMF and low-dose oral corticosteroids (category 1B recommendation).27
The role of calcineurin inhibitors (CNIs) in lupus has been largely in combination with other SOC therapy. Zhang et al. showed that multitarget therapy consisting of tacrolimus (4 mg/d), MMF (1 g/d), and GCs was superior to IV CYC with GCs as induction therapy for LN in a predominantly Chinese population (complete remission of 45.9% versus 25.6%, P < 0.001).28 The follow-up maintenance study compared multitarget therapy consisting of tacrolimus (2–3 mg/d), MMF (0.5–0.75 g/d), and prednisone 10 mg/day with AZA and prednisone (10 mg/d) and demonstrated similar primary outcome of renal relapse rates (5.4% versus 7.6%, P = 0.74), but significantly more adverse events in the AZA versus multitarget group (44.4% versus 16.4%, P < 0.01).29
Rituximab, a chimeric anti-CD20 monoclonal antibody, has been investigated in combination with MMF for induction treatment of proliferative LN (Lupus Nephritis Assessment with Rituximab, LUNAR trial) and as a steroid-sparing agent (RITUXILUP study).30,31 The LUNAR trial was a randomized, double-blind, placebo-controlled phase III trial that compared rituximab (1000mg q2 weeks at months 0 and 6) and MMF with placebo and MMF and did not show a significant difference in renal response at 52 weeks between groups (56.9% versus 45.8%, P = 0.18).30 RITUXILUP, a single-center, prospective, observational study of rituximab in 50 consecutive patients with LN class III, IV, or V, suggested that steroid avoidance is safe in those who received rituximab, preparing the way for larger, randomized, and placebo-controlled studies.31
A 2018 Cochrane review of 74 studies (67 on induction therapy and nine on maintenance therapy) concluded that MMF provides equivalent disease remission compared with IV CYC and may avoid drug-related toxicity, supporting its use as first-line induction therapy. For maintenance therapy, although AZA is associated with higher risk of disease relapse compared with MMF, the differential effect on ESKD and mortality is unclear due to very low-certainty evidence.32
On the basis of these studies, the 2019 EULAR/ERA-EDTA guidelines33 recommend treatment of active class III or IV LN with induction therapy consisting of either MMF/MPA or low-dose IV CYC as first-line agents alongside GCs. Notably, lower-dose steroids may be as efficacious as higher dose; thus, the task force recommends a total IV methylprednisolone dose of 500–2500 mg followed by oral prednisone at 0.3–0.5 mg/kg per day, with a reduction to ≤7.5 mg/d by 3–6 months. For maintenance therapy, the task force recommends MMF in those who received MMF for induction and either MMF or AZA in those who received IV CYC for induction. Length of therapy should take into account the specific clinical situation, although the task force highlights that longer duration of therapy (5–6 years) and longer duration of remission were associated with lower frequency of relapses.
Similarly, the 2021 KDIGO glomerular disease guidelines34 recommend that in patients with active class III or IV LN, either low-dose IV CYC or MMF in conjunction with GCs is used for induction therapy. Recognizing that lower-dose GCs may be as effective as higher-dose GCs, KDIGO provides example GC regimens with an option of standard, moderate, or reduced dose schemes (discussed below). For maintenance, KDIGO now recommends MMF over AZA and includes a practice point on duration that recommends no <36 months of therapy.
For pure class V LN, KDIGO 2021 glomerular disease guidelines recommend renin–angiotensin system blockade for all patients and immunosuppressive therapy for those with nephrotic syndrome, extrarenal manifestations of SLE, or complications of proteinuria, such as thrombosis, edema, or dyslipidemia.35 This is in contrast to the EULAR/ERA-EDTA guideline recommendation for immunosuppression in patients with class V LN and either nephrotic syndrome or proteinuria >1 g/d, despite antiproteinuric therapy for a reasonable period (>3 months).33 All classes of LN are additionally managed with hydroxychloroquine per clinical practice guidelines.33,34
New to Practice
Belimumab is a monoclonal antibody that inhibits soluble human B-lymphocyte stimulator and was approved in 2011 for use in active SLE without severe renal or central nervous system involvement on the basis of two phase 3 clinical trials.36–38 This was followed by approval in December 2020 for the treatment of adults with active LN receiving standard therapy, representing the first FDA-approved therapy for active LN.
In the 2-year phase 3 Belimumab International Study in Lupus Nephritis (BLISS-LN), patients with active class III or IV (±class V) or pure class V LN (eGFR ≥30 ml/min per 1.73 m2 and urine protein to creatinine ratio ≥1 g/g) were randomized to receive IV belimumab 10 mg/kg or placebo on a background of SOC therapy.39 SOC therapy consisted of CYC-AZA (IV CYC 500 mg every 2 weeks for six infusions followed by maintenance AZA at a target dose of 2 mg/kg per day) or MMF (at a target dose of 3 g/d for 6 months followed by 1–3 g/d for the duration of the study) on the basis of the Euro-Lupus Nephritis Trial and ALMS, respectively.17,25 The BLISS-LN trial met its primary end point, with significantly more patients achieving a primary efficacy renal response with belimumab than placebo at week 104 (43% versus 32%; P = 0.03) as well as its major secondary end point of a complete renal response (CRR; 30% versus 20%; P = 0.02). Furthermore, the risk of subsequent lupus flares was reduced (14% versus 26%), and the risk of a renal-related event or death was nearly 50% lower with belimumab compared with standard therapy alone (hazard ratio, 0.51; 95% confidence interval [CI], 0.34 to 0.77; P = 0.001). Post hoc analysis did not show a benefit in the group with pure class V LN, although numbers were smaller (n = 72).39
Voclosporin is a novel CNI that has structural similarity to cyclosporin but contains a single amino acid substitution, resulting in increased calcineurin binding and a superior adverse effect profile compared with other CNIs. This includes a lower incidence of new-onset diabetes compared with tacrolimus and less risk of hypertension or nephrotoxicity at the lower doses required to produce calcineurin inhibition.40 Furthermore, voclosporin has a more predictable pharmacokinetic profile, eliminating the need for therapeutic drug-level monitoring and does not affect systemic MPA exposure, in contrast to cyclosporine, which can decrease the MPA area under the curve by approximately 40% due to a clinically significant drug–drug interaction.41,42 Voclosporin received FDA approval in January 2021 on the basis of the phase 3 AURORA-1 (Aurinia Renal Response in Active Lupus With Voclosporin) and phase 2 Aurinia Urinary Protein Reduction Active—Lupus With Voclosporin trials, representing the first oral therapy approved for LN.43,44 In the 1-year AURORA trial, patients with active class III or IV (±class V) or pure class V LN, eGFR >45 ml/min per 1.73 m2, and urine protein to creatinine ratio ≥1.5 mg/mg (≥2 mg/mg if pure class V LN) were randomized to receive oral voclosporin 23.7 mg twice daily or matching placebo for 52 weeks. All patients received SOC with MMF at a total daily dose of 2 g (doses up to 3 g/d required approval of the medical monitor) and a lower dose GC protocol. Significantly more patients in the voclosporin group than in the placebo group met the primary end point of a CRR at week 52 (41% versus 23%; odds ratio 2.65, 95% CI: 1.64 to 4.27; P < 0.0001) with a similar adverse event profile. In addition to its immunosuppressive effects, voclosporin also exerts hemodynamic and direct podocyte antiproteinuric effects, leading to a rapid reduction n proteinuria.45 There was an expected, mild early eGFR reduction in the voclosporin group, but after this, eGFR remained stable for the study duration, with low rates of study drug discontinuation due to eGFR decrease in both the voclosporin and control arms.
The results of these studies also highlight the increasing use of reduced doses of GCs in the management of LN. Such regimens that use a lower starting dose and/or a more rapid GC taper are aimed to minimize the risk of side effects associated with high cumulative GC exposure. This approach is supported by the dual effect of GCs, which manifest anti-inflammatory and immunosuppressive effects via both genomic and nongenomic mechanisms.46 Thus, IV methylprednisolone pulses (up to a cumulative dose of 1–1.5 g) can rapidly induce both anti-inflammatory and immune effects, followed by reduced dose GCs to maintain immunosuppression. In the phase 3 AURORA-1 trial, patients received IV methylprednisolone once daily on days 1 and 2 (0.5 g/d for patients >45 kg and 0.25 g/d for patients <45 kg) followed by a rapid taper of oral prednisone starting with 20–25 mg/d and reaching 2.5 mg/d at week 16.44 A retrospective analysis of 63 propensity-matched pairs of patients from the control arm of the phase 2 Aurinia Urinary Protein Reduction Active—Lupus With Voclosporin trial and both arms of the ALMS trial demonstrated similar efficacy and safety comparing conventional high-dose GCs and a lower-dose regimen, with fewer gastrointestinal side effects with lower-dose GCs.47 Similarly, the MyLupus prospective open-label study of patients with proliferative LN treated with mycophenolate suggested that reduced-dose GCs are noninferior to standard dose regimens.48 Both the 2019 EULAR/ERA-EDTA and 2021 KDIGO guidelines recommend that a regimen of reduced dose GCs after a short course of IV methylprednisolone pulses be considered during the initial treatment of active LN.33,34 In the KDIGO guideline, the reduced dose regimen consists of 0.25–0.5 g/d of IV methylprednisolone pulses for up to 3 days, followed by 0.5–0.6 mg/kg of oral prednisone (maximum 40 mg) per day, which is then tapered to ≤7.5 mg/d by 3 months and to ≤2.5 mg/d by 6 months.34 The EULAR guideline emphasizes the use of IV methylprednisolone pulses and early initiation of immunosuppressive agents to facilitate tapering oral prednisone to ≤7.5 mg/d and the eventual discontinuation of GCs.33 The increasing use of combined immunosuppressive regimens (i.e., belimumab with either MMF or low-dose CYC or CNI with mycophenolate) can further facilitate the early withdrawal of GCs and minimize the toxicities associated with high cumulative GC exposure.
Ongoing Clinical Trials
Multiple immune pathways are implicated in LN, allowing for different treatment targets for emerging therapies. These include novel therapies targeting B cells, T cells, type 1 IFN-1, the complement system, and the immunoproteasome. Novel therapies are typically evaluated as “add-on” therapies to SOC regimens that include corticosteroids in combination with MMF or CYC.
B-cell–directed therapies have had mixed success in LN as demonstrated in the LUNAR trial, RITUXILUP study, and BLISS-LN trial. As noted above, the LUNAR trial failed to demonstrate increased efficacy with the addition of rituximab to SOC, but peripheral B-cell depletion may have been suboptimal, whereas the RITUXILUP study suggested a possible role for rituximab as steroid-sparing therapy when combined with MMF.30,31 As discussed, BLISS-LN demonstrated increased efficacy over SOC therapy.39 Obinutuzumab, a fully humanized anti-CD20 antibody that more potently depletes B cells, demonstrated higher renal response rates when added to SOC compared with SOC alone in the NOBILITY phase 2 clinical trial.49 The REGENCY trial is an ongoing phase 3 clinical trial to further evaluate obinutuzumab in active proliferative LN (class III or IV±class V) with primary completion expected in 2024 (NCT04221477).
The AURORA-2 continuation study was designed to assess long-term safety and tolerability of the combination of voclosporin, MMF, and low-dose GCs over an additional 2 years of treatment after completion of AURORA-1 (NCT03597464). Preliminary results from the completed study indicate that voclosporin was well-tolerated, eGFR remained stable throughout the study, and the significant reductions in proteinuria achieved in AURORA-1 were maintained.50
Novel therapies directed at T cells include secukinumab, a monoclonal antibody against IL-17A, and guselkumab, a monoclonal antibody against IL-23, both proinflammatory T-cell cytokines. Secukinumab and guselkumab are both FDA-approved for use in psoriasis and psoriatic arthritis. The SELUNE trial is an ongoing phase 3 trial to evaluate secukinumab on top of SOC in active proliferative LN (NCT04181762). The ORCHID-LN trial is an ongoing phase 2 trial evaluating guselkumab in patients with active LN (NCT04376827).
Patients with SLE often have elevated levels of IFN-I and increased expression of IFN-stimulated genes (known as the IFN signature), which have been associated with disease activity, making IFN-I a logical target in SLE.51–53 In 2021, anifrolumab, a monoclonal antibody targeting the IFN-1 receptor, IFNAR1, became FDA-approved for the treatment of SLE. The phase 2, randomized, placebo-controlled TULIP-LN trial evaluated anifrolumab for the treatment of active LN and included a lower-dose (“basic”) regimen, intensified dosing, and placebo arm (with standard therapy consisting of MMF and GCs).54 Although the trial failed to meet the primary end point, patients in the intensified treatment arm achieved numerically higher response rates.54 Notably, the lower-dose regimen was associated with a suboptimal anifrolumab exposure (approximately 50% lower than in nonrenal SLE) and lower IFN gene signature neutralization, possibly due to higher drug clearance associated with proteinuria in active LN versus nonrenal SLE. This suggests that patients with active LN may require the intensified (higher dose) anifrolumab regimen to attain serum exposure similar to what is observed in patients with SLE without active LN and to achieve clinically meaningful responses in renal end points. The IRIS study is an ongoing phase 3 study evaluating intensified anifrolumab dosing in proliferative LN and is expected to have primary completion in 2025 (NCT05138133).
The complement system includes three activation pathways (the classical, alternative, and lectin pathways), and all three have been implicated in LN.55–57 Multiple complement inhibitors are currently being studied as adjunctive therapy to SOC, including ravulizumab, a monoclonal antibody targeting C5 (NCT04564339); iptacopan, an oral inhibitor of Factor B (NCT05268289); and ALXN2050, an oral inhibitor of Factor D (NCT05097989).
Finally, the immunoproteasome, a special class of proteosome predominantly expressed in immune cells, is a novel target for autoimmune disorders.58 A phase 2 study evaluating the addition of the immunoproteasome inhibitor KZR-616 is ongoing (NCT03393013) (Table 1).
Table 1.
Ongoing clinical trials for lupus nephritis
| Drug | Mechanism | Status |
|---|---|---|
| Obinutuzumab | Anti-CD20 | Completed phase 2 (NCT02550652); Enrolling phase 3 (NCT04221477) |
| Secukinumab | Anti-IL17A | Enrolling phase 3 (NCT04181762) |
| Guselkumab | Anti-IL23 | Enrolling phase 2 (NCT04376827) |
| Anifrolumab | Anti-IFN | Completed phase 2 (NCT02547922); Enrolling phase 3 for LN (INCT05138133) |
| Ravulizumab | C5 inhibitor | Enrolling phase 2 (NCT04564339) |
| Iptacopan | Factor B inhibitor | Enrolling phase 2 (NCT05268289) |
| ALXN2050 | Factor D inhibitor | Enrolling phase 2 (NCT05097989) |
| KZR-616 | Immunoproteasome inhibitor | Active phase 1b/2 for SLE with and without LN (NCT03393013) |
LN, lupus nephritis.
Special Considerations
Racial and Ethnic Background
Clinical outcomes according to discrete race and ethnicity categories are fraught with limitations because of misclassification and an underappreciation for race and ethnicity as a social construct. With this in mind, an analysis of the ALMS trial using self-reported race and ethnicity found that MMF versus IV CYC response rates were similar in Asian and White patients, but among patients in a combined other and Black groups, 60.4% responded to MMF versus 38.5% to IV CYC (odds ratio 2.4; 95% CI, 1.1 to 5.4, P = 0.03).59 A post hoc analysis showed that among Hispanic patients, 60.9% responded to MMF versus 38.3% to IV CYC (odds ratio 2.5; 95% CI, 1.2 to 5.1; P = 0.011).
AURORA included a racially and ethnically diverse population, including 32% Hispanic and 15% Black patients. While not powered to detect statistically significant differences between subgroups, a post hoc analysis demonstrated similar response rates in all subgroups.60
In BLISS-LN, 14% of participants were Black.39 Although response rates were lower among Black patients than in the overall population for both study arms, Black patients receiving belimumab were more likely to have a primary efficacy renal response and CRR at week 104 compared with the placebo group. BLISS-LN did not enroll significant numbers of Hispanic patients, and belimumab remains understudied in Hispanic patients with LN. Notably, nearly half of the patients enrolled in the positive BLISS-52 phase 3 trial in nonrenal SLE were Hispanic. In a post hoc analysis of a phase 3 trial of subcutaneous belimumab in moderate-to-severe SLE without severe active LN, Hispanic patients (29% of the study population) demonstrated a significantly greater SLE responder index-4 response with belimumab compared with placebo (73.8% versus 50% at week 52; P = 0.0003), a difference that was numerically greater than in non-Hispanic patients (56.3% for belimumab versus 47.7% for placebo, P = 0.04).60 Thus, while definitive conclusions regarding the superiority of belimumab and voclosporin in Black and Hispanic patients with LN cannot be made, available data suggest that the benefit of belimumab and voclosporin extends to these groups of patients.
Refractory Disease
There is no standard definition for resistant or refractory LN. Recent studies of induction therapy in LN have demonstrated a failure to achieve either a complete or partial response in 30%–63% of subjects.24,39,44 EULAR has proposed three proteinuria treatment targets: reduction in proteinuria (1) by ≥25% at 3 months, (2) by ≥50% at 6–12 months, and (3) to <0.5–0.7 g/24 hours at 12–24 months, together with a stable eGFR.33 A stricter definition of refractory LN is a failure to achieve an adequate proteinuria or eGFR response with two SOC induction regimens after 4–6 months in adherent patients with therapeutic drug levels.61 However, it is important to note that residual proteinuria may reflect segmental glomerulosclerosis rather than immunologically active disease. In cases where persistent elevation of renal biomarkers is not associated with systemic features or immunologic markers of active lupus, a kidney biopsy should be considered to determine disease activity. A kidney biopsy may also reveal a coexistent kidney disease, such as a thrombotic microangiopathy, secondary to antiphospholipid antibodies or underlying complementopathy that may require a change in treatment. When assessing refractory LN, it is important to consider medication nonadherence which has been described in up to 75% of LN patients over the first year.62 Monitoring drug levels of MPA, CNI, or hydroxychloroquine may be helpful for detecting this.
The traditional therapeutic approach to a suboptimal response to induction therapy with MMF and GCs, and endorsed by KGIDO guidelines, has been to switch to a CYC-based induction regimen, an important goal being to limit GC exposure in addition to preserving disease control. With the success of CNIs or belimumab in combination with MMF as induction agents, these are now commonly used as additive therapies when the initial response is suboptimal, although the data in refractory LN remain very limited. The addition of rituximab to MMF has been widely used for refractory LN and is supported by a multiple observational and uncontrolled studies (reviewed in Ref. 61). One systematic review of 26 studies described a complete or partial response in 74% of 300 patients.63 Obinutuzumab, which is a more potent anti-CD20 monoclonal antibody, may also prove to be an effective option for refractory patients. With these combinations, immunoglobulin levels should be monitored, and IV immunoglobulin replacement considered for hypogammaglobulinemia. In refractory patients who do not respond to the addition of anti–B-cell therapy, a range of other options have been reported in small numbers of patients. Inhibition of complement activation with eculizumab has shown promise, especially in patients with concomitant thrombotic microangiopathy (reviewed in Ref. 64). Anti–B-cell therapy with bortezomib65 or daratumumab66 may be considered. Plasma exchange seems to be of limited benefit.67 Chimeric antigen receptor T-cell therapy68 or hematopoietic stem cell therapy may be considered in difficult cases.
Emerging Biomarkers
A key aspect in the management of LN is to identify patients with active immunologic disease who are at high risk for disease progression. Currently, kidney biopsy is considered the gold standard for assessing disease activity,69 but this may not be practical in many cases, particularly for repeated assessments, and surrogate biomarkers are needed that reflect immunologic activity. Traditional biomarkers used to assess lupus activity include serum complement levels (C3, C4) and anti-dsDNA, and kidney biomarkers (hematuria, proteinuria, and serum creatinine), but these markers often do not correlate with activity on kidney biopsy.70 Novel urinary biomarkers are an attractive option as urine is readily obtainable, noninvasive, and as opposed to serum; urine biomarkers may more closely reflect activity within the kidney itself. Urinary soluble CD163, a marker of M2c-macrophage infiltration in the kidneys, is associated with histologic activity and may correlate with response to treatment.71 Urinary proteomics have also identified urinary IL-16 and TGFβ1 correlating with histologic activity.72 Other promising urinary biomarkers include tumor necrosis factor-like weak inducer of apoptosis (a proinflammatory cytokine released from monocytes), monocyte chemoattractant protein-1 (a chemokine for monocytes), neutrophil gelatinase-associated lipocalin (a marker of inflammation and tubular cell injury), vascular cell adhesion molecule (an adhesion molecule expressed on endothelial cells), and activated leukocyte cell adhesion molecule (a transmembrane glycoprotein expressed by activated T cells).73,74 Transcriptomic analyses of resident and infiltrating cells in kidney biopsy tissue have identified gene expression signatures, including type I IFN pathways, that may correlate with progression and aid in the identification of novel therapeutic targets.75 The identification of downstream urinary or serum biomarkers of specific pathways may permit a precision medicine approach targeting therapeutic agents to the individual.
Cost-Effectiveness
Concerns remain regarding the cost-effectiveness of lupus therapy. In 2009, cost-utility analysis of MMF versus CYC-AZA showed that the overall cost of medication and the subsequent management was 1.57 times higher in the MMF group.76 In 2015, cost-utility analysis of MMF versus AZA for maintenance therapy showed that over the long-term, incremental cost-effectiveness ratio of MMF compared with AZA was $6454 per quality-adjusted life year (QALY).77 A 2016 study from India showed that the cost of MMF therapy was seven times as high as CYC-AZA therapy.19 Regarding the novel agents, a 2022 US health care–based cost-effectiveness model estimated an incremental cost-effectiveness ratio of approximately $95,000/QALY for belimumab and approximately $150,000/QALY for voclosporin, each compared with its respective SOC arm. Compared with SOC, the probabilities of belimumab and voclosporin being cost-effective at a threshold of $150,000/QALY were 69% and 49%, respectively, suggesting more uncertainty in the cost-effectiveness of voclosporin and less so with belimumab.78
High-Risk Medication Monitoring
The intensive immunosuppressive regimens used to treat LN treatments increase the risk of opportunistic infections, reduced bone mineral density, malignancy, metabolic and cardiovascular disease, bone marrow suppression, and gonadal and bladder toxicity (with the use of CYC).35 All patients receiving immunosuppression should be counseled on risk of infection. General principles to reduce infections should be adhered to including limiting exposure and maintaining up-to-date vaccinations (excluding live vaccines, which are contraindicated). Data on the risk of Pneumocystis jirovecii pneumonia in immunosuppressed patients with SLE are limited, and observational data suggest that the risk in patients treated with CYC is low (0.1588%).79 Guidelines do not give clear recommendations regarding the level of immunosuppression at which to consider prophylaxis for Pneumocystis jirovecii pneumonia. The risk is highest in patients receiving prednisone ≥20 mg/d for ≥4 weeks and in patients receiving concomitant CYC. Thus, patients in these categories may be considered for prophylaxis.80
There is increased risk of avascular necrosis and osteoporosis with GC use. The American College of Rheumatology guidelines for the prevention and treatment of GC-induced osteoporosis recommend calcium and vitamin D supplementation in adults with low fracture risk and consideration of bisphosphonates in patients at moderate-to-high fracture risk.81 Given the long-term risks of prolonged steroid therapy, current guidelines recommend no or low-dose (<7.5 mg/d) GCs in the long-term maintenance of LN.33 LN is associated with a nine-fold greater risk of atherosclerotic cardiovascular disease compared with patients with SLE without nephritis.82,83 Risk-modifying strategies, including BP control and lipid management, should be implemented.
Patients receiving CYC are at risk for infertility and shared decision making should guide therapy and fertility preservation options.84,85 The risk of gonadal toxicity increases with patient age and cumulative dose.86 The risk is much lower with the Euro-Lupus CYC regimen compared with oral or NIH CYC regimens.87
In summary, LN outcomes have improved over recent decades, but there is a great need for safer and more effective therapies. MMF and IV CYC still reign as induction agents and MMF, followed by AZA, as SOC maintenance therapy. As seen in other glomerular diseases, lower doses of GCs may be as efficacious and safer than higher-dose steroids. Optimal duration of therapy remains uncertain, although a minimum of 3 years for most patients is now a widely accepted clinical practice. Recent trials show that the addition of belimumab or voclosporin to induction therapy may lead to improved kidney outcomes, but cost remains a major issue. The KDIGO 2023 clinical practice guideline for the management of LN is currently available as a public review draft and proposes a role for these agents in lupus management. Several ongoing trials investigating biologic pathways implicated in LN hold promise that effective targeted therapies may be on the way, but it remains to be seen if these therapies will be an improvement on current SOC therapy. The dream of curative targeted therapy remains elusive, but progress toward improved disease characterization, risk stratification, and the identification of disease-causing biologic pathways is cause for hope.
Disclosures
R. Avasare reports the following: Advisory or Leadership Role: Editorial Board of Glomerular Disease Journal (Karger); and Other Interests or Relationships: Member of ASN, NKF. Site PI for the NEFIGARD study, TRIDENT study. Nephrology consultant for the LAPMS clinical trial (NCT03161028). D.J. Caster reports the following: Consultancy: Aurinia, Calliditas, Chinook, GSK, and Travere; Ownership Interest: Individual stock holdings in Coca-Cola and Procter and Gamble; Research Funding: PI on Industry Sponsored Clinical Trials sponsored by Alexion, Chinook, and Travere; Honoraria: Aurinia, Calliditas, Chinook, GSK, and Travere; Advisory or Leadership Role: Lupus Foundation of America Medical Scientific Advisory Counsel; Glomerular Diseases Editorial Board; Speakers Bureau: Aurinia, Calliditas, GSK; and Other Interests or Relationships: National Institutes of Health: NIH R01 1RO1DK126777. J.A. Jefferson reports the following: Research Funding: Novartis; and Honoraria: Uptodate. A. Mitrofanova reports the following: Other Interests or Relationships: –American Society of Nephrology, member; –American Heart Association, member. The remaining author has nothing to disclose.
Funding
None.
Author Contributions
Conceptualization: Rupali Avasare.
Writing – original draft: Rupali Avasare, Dawn J. Caster, Yelena Drexler, J. Ashley Jefferson.
Writing – review & editing: Rupali Avasare, Dawn J. Caster, Yelena Drexler, J. Ashley Jefferson, Alla Mitrofanova.
References
- 1.Hanly JG O'Keeffe AG Su L, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford). 2016;55(2):252–262. doi: 10.1093/rheumatology/kev311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tektonidou MG, Dasgupta A, Ward MM. Risk of end-stage renal disease in patients with lupus nephritis, 1971-2015: a systematic review and Bayesian meta-analysis. Arthritis Rheumatol. 2016;68(6):1432–1441. doi: 10.1002/art.39594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orbai AM Truedsson L Sturfelt G, et al. Anti-C1q antibodies in systemic lupus erythematosus. Lupus. 2015;24(1):42–49. doi: 10.1177/0961203314547791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moroni G Radice A Giammarresi G, et al. Are laboratory tests useful for monitoring the activity of lupus nephritis? A 6-year prospective study in a cohort of 228 patients with lupus nephritis. Ann Rheum Dis. 2009;68(2):234–237. doi: 10.1136/ard.2008.094508 [DOI] [PubMed] [Google Scholar]
- 5.Cunha C, Alexander S, Ashby D. Hydroxycloroquine blood concentration in lupus nephritis: a determinant of disease outcome? Nephrol Dial Transplant. 2018;33(9):1604–1610. doi: 10.1093/ndt/gfx318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yap DYH, Thong KM, Yung S, Tang C, Ma BMY, Chan TM. Antiphospholipid antibodies in patients with lupus nephritis: clinical correlations and associations with long-term outcomes. Lupus. 2019;28(12):1460–1467. doi: 10.1177/0961203319879990 [DOI] [PubMed] [Google Scholar]
- 7.Mackay M Dall'Era M Fishbein J, et al. Establishing surrogate kidney end points for lupus nephritis clinical trials: development and validation of a novel approach to predict future kidney outcomes. Arthritis Rheumatol. 2019;71(3):411–419. doi: 10.1002/art.40724 [DOI] [PubMed] [Google Scholar]
- 8.Bruschi M, Sinico RA, Moroni G, Glomerular autoimmune multicomponents of human lupus nephritis in vivo: alpha-enolase and annexin AI. J Am Soc Nephrol. 2014;25(11):2483–2498. doi: 10.1681/ASN.2013090987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghiggeri GM D'Alessandro M Bartolomeo D, et al. An update on antibodies to Necleosome components as biomarkers of Sistemic lupus erythematosus and of lupus flares. Int J Mol Sci. 2019;20(22):5799. doi: 10.3390/ijms20225799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravindran A Casal Moura M Fervenza FC, et al. In patients with membranous lupus nephritis, exostosin-positivity and exostosin-negativity represent two different phenotypes. J Am Soc Nephrol. 2021;32(3):695–706. doi: 10.1681/ASN.2020081181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H Lan P Yu X, et al. Analysis of the expression of exostosins and clinicopathological features in membranous lupus nephritis in a Chinese cohort. Kidney Int Rep. 2022;7(10):2295–2298. doi: 10.1016/j.ekir.2022.07.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caza TN Storey AJ Hassen SI, et al. Discovery of seven novel putative antigens in membranous nephropathy and membranous lupus nephritis identified by mass spectrometry. Kidney Int. 2023;103(3):593–606. doi: 10.1016/j.kint.2023.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caza TN Hassen SI Kuperman M, et al. Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int. 2021;100(1):171–181. doi: 10.1016/j.kint.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caza TN Hassen SI Kenan DJ, et al. Transforming growth factor beta receptor 3 (TGFBR3)-associated membranous nephropathy. Kidney360. 2021;2(8):1275–1286. doi: 10.34067/kid.0001492021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin HA, III, Klippel JH, Balow JE. Therapy of lupus nephritis. N Engl J Med. 1986;314(10):614–619. doi: 10.1056/nejm198603063141004 [DOI] [PubMed] [Google Scholar]
- 16.Boumpas DT Austin HA III Vaughn EM, et al. Controlled trial of pulse methylprednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis. Lancet. 1992;340(8822):741–745. doi: 10.1016/0140-6736(92)92292-n [DOI] [PubMed] [Google Scholar]
- 17.Houssiau FA Vasconcelos C D'Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46(8):2121–2131. doi: 10.1002/art.10461 [DOI] [PubMed] [Google Scholar]
- 18.Houssiau FA Vasconcelos C D'Cruz D, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis. 2010;69(1):61–64. doi: 10.1136/ard.2008.102533 [DOI] [PubMed] [Google Scholar]
- 19.Rathi M Goyal A Jaryal A, et al. Comparison of low-dose intravenous cyclophosphamide with oral mycophenolate mofetil in the treatment of lupus nephritis. Kidney Int. 2016;89(1):235–242. doi: 10.1038/ki.2015.318 [DOI] [PubMed] [Google Scholar]
- 20.Mehra S, Usdadiya JB, Jain VK, Misra DP, Negi VS. Comparing the efficacy of low-dose vs high-dose cyclophosphamide regimen as induction therapy in the treatment of proliferative lupus nephritis: a single center study. Rheumatol Int. 2018;38(4):557–568. doi: 10.1007/s00296-018-3995-3 [DOI] [PubMed] [Google Scholar]
- 21.The ACCESS Trial Group. Treatment of lupus nephritis with abatacept: the abatacept and cyclophosphamide combination efficacy and safety study. Arthritis Rheumatol. 2014;66(11):3096–3104. doi: 10.1002/art.38790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan TM Li FK Tang CS, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. N Engl J Med. 2000;343(16):1156–1162. doi: 10.1056/nejm200010193431604 [DOI] [PubMed] [Google Scholar]
- 23.Ginzler EM, Dooley MA, Aranow C, Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353(21):2219–2228. doi: 10.1056/nejmoa043731 [DOI] [PubMed] [Google Scholar]
- 24.Appel GB, Contreras G, Dooley MA, Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20(5):1103–1112. doi: 10.1681/ASN.2008101028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dooley MA Jayne D Ginzler EM, et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med. 2011;365(20):1886–1895. doi: 10.1056/nejmoa1014460 [DOI] [PubMed] [Google Scholar]
- 26.Houssiau FA D'Cruz D Sangle S, et al. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis. 2010;69(12):2083–2089. doi: 10.1136/ard.2010.131995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radhakrishnan J, Cattran DC. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines—application to the individual patient. Kidney Int. 2012;82(8):840–856. doi: 10.1038/ki.2012.280 [DOI] [PubMed] [Google Scholar]
- 28.Liu Z Zhang H Liu Z, et al. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann Intern Med. 2015;162(1):18–26. doi: 10.7326/m14-1030 [DOI] [PubMed] [Google Scholar]
- 29.Zhang H Liu Z Zhou M, et al. Multitarget therapy for maintenance treatment of lupus nephritis. J Am Soc Nephrol. 2017;28(12):3671–3678. doi: 10.1681/ASN.2017030263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rovin BH Furie R Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215–1226. doi: 10.1002/art.34359 [DOI] [PubMed] [Google Scholar]
- 31.Condon MB Ashby D Pepper RJ, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis. 2013;72(8):1280–1286. doi: 10.1136/annrheumdis-2012-202844 [DOI] [PubMed] [Google Scholar]
- 32.Tunnicliffe DJ, Palmer SC, Henderson L. Immunosuppressive treatment for proliferative lupus nephritis. Cochrane Database Syst Rev. 2018;6(6):CD002922. doi: 10.1002/14651858.cd002922.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fanouriakis A Kostopoulou M Cheema K, et al. 2019 Update of the joint European league against rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA–EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79(6):713–723. doi: 10.1136/annrheumdis-2020-216924 [DOI] [PubMed] [Google Scholar]
- 34.Rovin BH Adler SG Barratt J, et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):S1–S276. doi: 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 35.Rovin BH Adler SG Barratt J, et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):S1–S276. doi: 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 36.Baker KP, Edwards BM, Main SH. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003;48(11):3253–3265. doi: 10.1002/art.11299 [DOI] [PubMed] [Google Scholar]
- 37.Navarra SV, Guzman RM, Gallacher AE. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–731. doi: 10.1016/s0140-6736(10)61354-2 [DOI] [PubMed] [Google Scholar]
- 38.Furie R, Petri M, Zamani O. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63(12):3918–3930. doi: 10.1002/art.30613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furie R, Rovin BH, Houssiau F. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383(12):1117–1128. doi: 10.1056/nejmoa2001180 [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Palmisano M, Sun D, Zhou S. Pharmacokinetic disposition difference between cyclosporine and voclosporin drives their distinct efficacy and safety profiles in clinical studies. Clin Pharmacol. 2020;12:83–96. doi: 10.2147/cpaa.s255789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Gelder T. How cyclosporine reduces mycophenolic acid exposure by 40% while other calcineurin inhibitors do not. Kidney Int. 2021;100(6):1185–1189. doi: 10.1016/j.kint.2021.06.036 [DOI] [PubMed] [Google Scholar]
- 42.van Gelder T, Huizinga RB, Lisk L, Solomons N. Voclosporin: a novel calcineurin inhibitor with no impact on mycophenolic acid levels in patients with SLE. Nephrol Dial Transplant. 2022;37(5):917–922. doi: 10.1093/ndt/gfab022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rovin BH, Solomons N, Pendergraft WF, III. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int. 2019;95(1):219–231. doi: 10.1016/j.kint.2018.08.025 [DOI] [PubMed] [Google Scholar]
- 44.Rovin BH, Teng YKO, Ginzler EM. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2021;397(10289):2070–2080. doi: 10.1016/s0140-6736(21)00578-x [DOI] [PubMed] [Google Scholar]
- 45.Faul C, Donnelly M, Merscher-Gomez S. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14(9):931–938. doi: 10.1038/nm.1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4(10):525–533. doi: 10.1038/ncprheum0898 [DOI] [PubMed] [Google Scholar]
- 47.Dall'Era M, Solomons N, Federico R, Truman M. Comparison of standard of care treatment with a low steroid and mycophenolate mofetil regimen for lupus nephritis in the ALMS and AURA studies. Lupus. 2019;28(5):591–596. doi: 10.1177/0961203319842924 [DOI] [PubMed] [Google Scholar]
- 48.Zeher M, Doria A, Lan J. Efficacy and safety of enteric-coated mycophenolate sodium in combination with two glucocorticoid regimens for the treatment of active lupus nephritis. Lupus. 2011;20(14):1484–1493. doi: 10.1177/0961203311418269 [DOI] [PubMed] [Google Scholar]
- 49.Furie RA, Aroca G, Cascino MD. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2022;81(1):100–107. doi: 10.1136/annrheumdis-2021-220920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teng YKO, Saxena A, Palmen M, Birardi V, Lisk L. FC054: voclosporin for lupus nephritis: results of the two-year aurora 2 continuation study. Nephrol Dial Transplant. 2022;37(suppl 3):gfac108.002. doi: 10.1093/ndt/gfac108.002 [DOI] [Google Scholar]
- 51.Lodi L, Mastrolia MV, Bello F. Type I interferon-related kidney disorders. Kidney Int. 2022;101(6):1142–1159. doi: 10.1016/j.kint.2022.02.031 [DOI] [PubMed] [Google Scholar]
- 52.Feng X, Wu H, Grossman JM. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54(9):2951–2962. doi: 10.1002/art.22044 [DOI] [PubMed] [Google Scholar]
- 53.Der E, Suryawanshi H, Morozov P. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat Immunol. 2019;20(7):915–927. doi: 10.1038/s41590-019-0386-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jayne D, Rovin B, Mysler EF. Phase II randomised trial of type I interferon inhibitor anifrolumab in patients with active lupus nephritis. Ann Rheum Dis. 2022;81(4):496–506. doi: 10.1136/annrheumdis-2021-221478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thanei S, Vanhecke D, Trendelenburg M. Anti-C1q autoantibodies from systemic lupus erythematosus patients activate the complement system via both the classical and lectin pathways. Clin Immunol. 2015;160(2):180–187. doi: 10.1016/j.clim.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 56.Panda AK, Parida JR, Tripathy R, Pattanaik SS, Ravindran B, Das BK. Mannose binding lectin: a biomarker of systemic lupus erythematosus disease activity. Arthritis Res Ther. 2012;14(5):R218. doi: 10.1186/ar4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song D, Guo WY, Wang FM. Complement alternative pathways activation in patients with lupus nephritis. Am J Med Sci. 2017;353(3):247–257. doi: 10.1016/j.amjms.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 58.Huber EM, Groll M. Inhibitors for the immuno- and constitutive proteasome: current and future trends in drug development. Angew Chem Int Ed Engl. 2012;51(35):8708–8720. doi: 10.1002/anie.201201616 [DOI] [PubMed] [Google Scholar]
- 59.Isenberg D, Appel GB, Contreras G. Influence of race/ethnicity on response to lupus nephritis treatment: the ALMS study. Rheumatology. 2009;49(1):128–140. doi: 10.1093/rheumatology/kep346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stohl W, Schwarting A, Okada M. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 2017;69(5):1016–1027. doi: 10.1002/art.40049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arora S, Rovin BH. Expert perspective: an approach to refractory lupus nephritis. Arthritis Rheumatol. 2022;74(6):915–926. doi: 10.1002/art.42092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feldman CH, Collins J, Zhang Z. Azathioprine and mycophenolate mofetil adherence patterns and predictors among Medicaid beneficiaries with systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2019;71(11):1419–1424. doi: 10.1002/acr.23792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weidenbusch M, Rommele C, Schrottle A, Anders HJ. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dial Transplant. 2013;28(1):106–111. doi: 10.1093/ndt/gfs285 [DOI] [PubMed] [Google Scholar]
- 64.Wright RD, Bannerman F, Beresford MW, Oni L. A systematic review of the role of eculizumab in systemic lupus erythematosus-associated thrombotic microangiopathy. BMC Nephrol. 2020;21(1):245. doi: 10.1186/s12882-020-01888-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Segarra A, Arredondo KV, Jaramillo J. Efficacy and safety of bortezomib in refractory lupus nephritis: a single-center experience. Lupus. 2020;29(2):118–125. doi: 10.1177/0961203319896018 [DOI] [PubMed] [Google Scholar]
- 66.Ostendorf L, Burns M, Durek P. Targeting CD38 with daratumumab in refractory systemic lupus erythematosus. N Engl J Med. 2020;383(12):1149–1155. doi: 10.1056/nejmoa2023325 [DOI] [PubMed] [Google Scholar]
- 67.Lewis EJ, Hunsicker LG, Lan SP, Rohde RD, Lachin JM. A controlled trial of Plasmapheresis therapy in severe lupus nephritis. N Engl J Med. 1992;326(21):1373–1379. doi: 10.1056/nejm199205213262101 [DOI] [PubMed] [Google Scholar]
- 68.Mackensen A, Müller F, Mougiakakos D. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med. 2022;28(10):2124–2132. doi: 10.1038/s41591-022-02017-5 [DOI] [PubMed] [Google Scholar]
- 69.Bajema IM, Wilhelmus S, Alpers CE. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93(4):789–796. doi: 10.1016/j.kint.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 70.Malvar A, Alberton V, Lococo B. Kidney biopsy-based management of maintenance immunosuppression is safe and may ameliorate flare rate in lupus nephritis. Kidney Int. 2020;97(1):156–162. doi: 10.1016/j.kint.2019.07.018 [DOI] [PubMed] [Google Scholar]
- 71.Mejia-Vilet JM, Zhang XL, Cruz C. Urinary soluble CD163: a novel noninvasive biomarker of activity for lupus nephritis. J Am Soc Nephrol. 2020;31(6):1335–1347. doi: 10.1681/ASN.2019121285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fava A, Rao DA, Mohan C. Urine proteomics and renal single-cell transcriptomics implicate interleukin-16 in lupus nephritis. Arthritis Rheumatol. 2022;74(5):829–839. doi: 10.1002/art.42023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stanley S, Vanarsa K, Soliman S. Comprehensive aptamer-based screening identifies a spectrum of urinary biomarkers of lupus nephritis across ethnicities. Nat Commun. 2020;11(1):2197. doi: 10.1038/s41467-020-15986-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palazzo L, Lindblom J, Mohan C, Parodis I. Current insights on biomarkers in lupus nephritis: a systematic review of the literature. J Clin Med. 2022;11(19):5759. doi: 10.3390/jcm11195759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arazi A, Rao DA, Berthier CC. The immune cell landscape in kidneys of patients with lupus nephritis. Nat Immunol. 2019;20(7):902–914. doi: 10.1038/s41590-019-0398-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tse KC, Tang CS, Lam MF, Yap DY, Chan TM. Cost comparison between mycophenolate mofetil and cyclophosphamide-azathioprine in the treatment of lupus nephritis. J Rheumatol. 2009;36(1):76–81. doi: 10.3899/jrheum.080517 [DOI] [PubMed] [Google Scholar]
- 77.Nee R, Rivera I, Little DJ, Yuan CM, Abbott KC. Cost-utility analysis of mycophenolate mofetil versus azathioprine based regimens for maintenance therapy of proliferative lupus nephritis. Int J Nephrol. 2015;2015:1–13. doi: 10.1155/2015/917567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mandrik O, Fotheringham J, Ren S. The cost-effectiveness of belimumab and voclosporin for patients with lupus nephritis in the United States. Clin J Am Soc Nephrol. 2022;17(3):385–394. doi: 10.2215/CJN.13030921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gupta D, Zachariah A, Roppelt H, Patel AM, Gruber BL. Prophylactic antibiotic usage for Pneumocystis jirovecii pneumonia in patients with systemic lupus erythematosus on cyclophosphamide: a survey of US rheumatologists and the review of literature. J Clin Rheumatol. 2008;14(5):267–272. doi: 10.1097/rhu.0b013e31817a7e30 [DOI] [PubMed] [Google Scholar]
- 80.Mecoli CA, Saylor D, Gelber AC, Christopher-Stine L. Pneumocystis jiroveci pneumonia in rheumatic disease: a 20-year single-centre experience. Clin Exp Rheumatol. 2017;35(4):671–673. PMID: 28134084. [PubMed] [Google Scholar]
- 81.Buckley L, Guyatt G, Fink HA. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69(8):1095–1110. doi: 10.1002/acr.23279 [DOI] [PubMed] [Google Scholar]
- 82.Garg S, Raval AN, Hansen KE. Association of renal arteriosclerosis with atherosclerotic cardiovascular disease risk in lupus nephritis. Arthritis Care Res (Hoboken). 2022;74(7):1105–1112. doi: 10.1002/acr.24552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hermansen ML, Lindhardsen J, Torp-Pedersen C, Faurschou M, Jacobsen S. The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatology (Oxford). 2017;56(5):709–715. doi: 10.1093/rheumatology/kew475 [DOI] [PubMed] [Google Scholar]
- 84.van Dorp W, Mulder RL, Kremer LC. Recommendations for premature ovarian insufficiency surveillance for female survivors of childhood, adolescent, and young adult cancer: a report from the international late effects of childhood cancer guideline harmonization group in collaboration with the PanCareSurFup consortium. J Clin Oncol. 2016;34(28):3440–3450. doi: 10.1200/jco.2015.64.3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377(17):1657–1665. doi: 10.1056/nejmra1614676 [DOI] [PubMed] [Google Scholar]
- 86.Manger K, Wildt L, Kalden JR, Manger B. Prevention of gonadal toxicity and preservation of gonadal function and fertility in young women with systemic lupus erythematosus treated by cyclophosphamide: the PREGO-Study. Autoimmun Rev. 2006;5(4):269–272. doi: 10.1016/j.autrev.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 87.Tamirou F, Husson SN, Gruson D, Debiève F, Lauwerys BR, Houssiau FA. Brief report: the euro-lupus low-dose intravenous cyclophosphamide regimen does not impact the ovarian reserve, as measured by serum levels of anti-Müllerian hormone. Arthritis Rheumatol. 2017;69(6):1267–1271. doi: 10.1002/art.40079 [DOI] [PubMed] [Google Scholar]