Abstract
Introduction:
This 10-year registry review aimed to investigate the clinical behaviour and outcomes of mixed germ cell tumours with choriocarcinoma components, a rare and aggressive subtype of testicular cancer, in Saudi Arabia. The study explores the demographic characteristics of affected patients, tumour profiles, and the mortality rate associated with this malignancy.
Methods:
Utilizing data from the Saudi Cancer Registry, the authors identified 33 cases of mixed germ cell tumours with choriocarcinoma components among 1001 testicular cancer cases recorded between 2008 and 2017. Demographic information, including age, marital status, region of residency, year of diagnosis, and 10-year survival status, were collected. Tumour factors, such as the basis of diagnosis, origin site, behaviour, grade, extension, and laterality, were also analyzed.
Results:
The majority of cases (78.8%) occurred in the young age group (18–45 years), and most tumours (97%) originated in normally descended testes. Grade IV (undifferentiated anaplastic) tumours and distant metastasis were present in 45.5% of patients. All cases exhibited malignant tumour behaviour. The overall mortality rate was 15%, with a mean time from diagnosis to death of 7.72 months (range: 0.5–21.5 months).
Conclusion:
Mixed germ cell tumours with choriocarcinoma components are rare and tend to affect younger populations. These tumours demonstrate aggressive clinical behaviour, with a significant proportion presenting with high-grade lesions and metastasis at diagnosis. The observed mortality rate underscores the poor prognosis associated with this malignancy. Our study provides essential insights into the clinical characteristics of this rare tumour subtype in the Saudi Arabian population, emphasizing the need for further research to identify prognostic factors and optimize management strategies for affected patients.
Keywords: mixed germ cell tumours, rare tumours, Saudi Arabia, testicular cancer, tumour registries
Introduction
Highlights
Rare and poor prognosis: Germ cell tumours mixed with choriocarcinoma components are rare, but they have a bad prognosis. These tumours have been poorly documented in the literature, highlighting the need for more research and understanding of their behaviour and outcomes.
Young population affected: The study found that most patients with mixed germ cell tumours and choriocarcinoma were in the young age group of 18–45 years. This suggests that this type of tumour may predominantly affect younger individuals.
Aggressive tumour characteristics: The majority of cases had grade IV undifferentiated anaplastic tumours, indicating aggressive and poorly differentiated cancer cells. Additionally, 45.5% of patients had distant metastasis at the time of diagnosis, indicating an advanced stage of the disease. These findings contribute to the poor prognosis and high mortality rate associated with these tumours.
Testicular cancers are classified into germ cell tumours (GCTs) and non-GCTs, with GCTs further divided into seminomatous (SGCTs) and non-seminomatous (NSGCTs). Despite their rarity, testicular GCTs are the most common solid tumours in men of reproductive age between 20 and 40 years old, with an incidence of ~10 in 100 000 men1.
Choriocarcinoma, comprised of cytotrophoblasts and syncytiotrophoblasts, is the most aggressive subtype of NSGCTs, exhibiting early hematogenous metastases2. The production of beta-human chorionic gonadotropin (b-HCG) hormone by choriocarcinoma is used as a marker in diagnosis, prognostic workup, formulation of appropriate treatment regimens, monitoring treatment response, and detecting potential relapse3. Choriocarcinomas typically present with haemorrhagic symptoms from sites of metastasis, such as the lungs, liver, and brain, often with normal-appearing testes4. Furthermore, clinical features such as gynaecomastia and hematogenous metastases may be observed, and serum hCG levels would typically be the highest among all germ cell tumours5. Due to their propensity to metastasize at the subclinical stage, choriocarcinomas have the poorest prognosis among testicular GCTs and are categorized in the poor prognosis subgroup per the International Germ Cell Cancer Collaborative Group (IGCCCG) classification system3,6. Additionally, risk factors associated with a poor prognosis include advanced age and an advanced tumour stage7. Choriocarcinomas appear grossly as a haemorrhagic nodule, with histology revealing haemorrhagic foci with viable tumour cells at the periphery8,9.
Pure choriocarcinoma is exceedingly rare, accounting for less than 0.3% of testicular GCTs5,6,9. More commonly, choriocarcinoma is seen as an element in ~8% of mixed GCTs6,9. Mixed GCTs with choriocarcinoma elements tend to be more aggressive and exhibit poorer outcomes than other variations of mixed GCTs10. Indeed, the histological presence of trophoblastic components or b-HCG elevations (signifying the presence of syncytiotrophoblasts) are recognized as poor prognostic indicators in patients with mixed GCTs11–15.
In Saudi Arabia, a study analyzing 1004 testicular cancer cases identified 85.2% of cases as GCTs, of which 3.6% were mixed GCTs, but their composition (choriocarcinoma or otherwise), clinical behaviour, and prognosis was not reported16. Our study provides a more in-depth analysis of mixed GCTs with choriocarcinoma components, exploring their demographic characteristics, clinical behaviour, mortality rates, and diagnosis-to-mortality intervals.
Materials and methods
Study design
This retrospective chart review included all adult patients diagnosed with mixed GCTs with choriocarcinoma components between 2008 and 2017. Data were retrieved from the Saudi Cancer Registry (SCR), which collects tumour data from all private, military, and Ministry of Health hospitals in Saudi Arabia through five regional offices (eastern, western, northern, southern, and central). The variables analyzed included the year of diagnosis, sex, age, marital status, geographical region, tumour origin site, histological subtype, tumour behaviour, grade, extent, laterality, basis of diagnosis, and survival. This work has been reported in line with the STROCSS criteria17.
Statistical analysis
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 23.0 (IBM Corporation). Categorical variables were presented as frequencies and percentages, while continuous variables were summarized as means and SD. Patients’ ages were categorized into four groups based on the WHO’s population classification: adolescents (12–17 years), young adults (18–45 years), middle-aged (46–60 years), and senile (76–90 years).
Results
Among the 1001 patients screened, 33 (3.3%) were diagnosed with mixed GCTs with choriocarcinoma components. The mean age of patients was 32.36 years (SD=16.17) (interquartile range=15–80) years, with the majority (n=26/33, 78.8%) falling into the young age group. Four patients (12.1%) were middle-aged, two (6.1%) were senile, and one (3%) was an adolescent. In terms of marital status, 42.4% (n=14/33) were married, 33.3% (n=11/33) were single, and 24.2% (n=8/33) were divorced. Regarding residence, 36.4% (n=12/33) resided in the southern region, 27.3% (n=9/33) in the central region, 18.2% (n=6/33) in the eastern region, 12.1 (n=4/33) in the western region, and 6.1% (n=2/33) in the northern region.
Table 1 presents the tumour profiles of these patients. The primary site of the tumour was in the descended testis in 97% of cases (n=32/33). Both grade IV (undifferentiated anaplastic) tumours and distant metastasis were present in 45.5% (n=15/33) of patients. Tumours were distributed relatively equally between the left and right testicles (48.5% and 45.5%, respectively). Malignant tumour behaviour was detected in all 33 patients.
TABLE 1.
Tumor profile (n=33).
| n | % | |
|---|---|---|
| Primary site | ||
| Descended testis | 32 | 97 |
| Undescended testis | 1 | 3 |
| Grade | ||
| Grade I (well differentiated) | 9 | 27.3 |
| Grade II (moderately differentiated) | 6 | 18.2 |
| Grade III (poorly differentiated) | 3 | 9.1 |
| Grade IV (undifferentiated anaplastic) | 15 | 45.5 |
| Extension | ||
| Localized | 9 | 27.3 |
| Regional: direct extension | 6 | 18.2 |
| Regional: lymph node and direct extension | 3 | 9.1 |
| Distant metastasis | 15 | 45.5 |
| Lateralization | ||
| Right | 15 | 45.5 |
| Left | 16 | 48.5 |
| Bilateral | 1 | 3 |
| Base of diagnosis | ||
| Histology of primary tumour | 32 | 97 |
| Histology of metastases | 1 | 3 |
Figure 1 illustrates the incidence of mixed GCTs with choriocarcinoma components per 1 000 000 over the years, displaying an irregular pattern. The highest incidence rate was 0.25, observed in 2011, while the lowest incidence, 0.03, was observed in 2016. The overall mortality rate was 15% (n=5/33), with a mean time from diagnosis to death of 7.72 (SD=9) months, ranging from 0.5 to 21.5 months.
Figure 1.
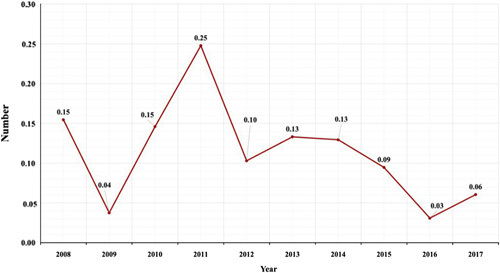
Incidence of choriocarcinoma combined with other germ cell elements per 1 000 000 throughout the year.
Discussion
Leveraging data from the SCR between 2008 and 2017, we expanded upon the prior regional study by Mohammed Abomelha, which analyzed the SCR database from 1994 to 2013 and identified 1004 testicular cancer among Saudi adults16. A steady rise in the annual incidence of testicular cancer was observed, peaking at 94 cases in 201316. Among his study population, 85.4% of testicular cancers were GCTs, with SGCTs constituting 40.7% and NSGCTs representing 44.6% of cases16. Among the NSGCTs, mixed GCTs (51.6%) were the most common, while pure choriocarcinoma was seen in 3.6% of patients16.
In terms of patient demographics, the mean age of our patients was 32 years, and 78.8% of cases occurred in individuals between 18 and 45 years, consistent with prior regional and Western data10,16,18. Li et al. recently demonstrated that an age older than or equal to 30 years is a negative predictor of outcomes in testicular choriocarcinoma patients, which may be an underlying reason for the advanced presentation and aggressive course of our patients7. Our results suggest an increasing testicular cancer incidence in Saudi Arabia, which echoes the findings of Abomelha’s study16. This trend is consistent with recent global data indicating a rise in testicular cancer incidence19. Nevertheless, we found the incidence of choriocarcinoma-containing mixed GCTs to be irregular, peaking in 2013 at 0.25.
Regarding tumour features, nearly all cases (32/33) of mixed GCTs with choriocarcinoma were unilateral without a specific predilection regarding laterality, in line with prior data20. Our results also corroborate previous studies suggesting that choriocarcinoma in mixed GCTs is a risk factor for an aggressive tumour phenotype characterized by high grade and metastasis at presentation12,21. Of the 1001 testicular cancer cases in our study, 3.3% were mixed GCTs with a choriocarcinoma component, of which almost half (45.5%) were grade IV anaplastic lesions and metastatic at the time of diagnosis, resulting in a mortality rate of 15% (5/33 patients). A study by Alvarado-Cabrero et al. observed a higher mortality rate than our findings, with all six patients with pure choriocarcinoma and five out of nine patients with choriocarcinoma-predominant mixed GCT succumbing to the disease during a median follow-up of 27 months20. To explain this, various risk factors may have differed between our studies, including the degree of b-HCG elevation and extrapulmonary metastases, recognized as poor prognostic factors in GCTs22,23. Interestingly, Hassan et al. observed that a small component of choriocarcinoma (<5%) in mixed GCTs does not exhibit the aggressive phenotype of pure choriocarcinoma or choriocarcinoma-predominant (>50%) mixed GCTs24. Hence, the degree of choriocarcinoma present within mixed GCTs may also explain discrepant findings on the outcomes of these tumours, perhaps related to a threshold amount of choriocarcinoma in the mixed GCT above which there is a poor prognosis9.
Limitations
It is essential to acknowledge the limitations of our study. Potential under-reporting, a common challenge in case registries worldwide, may have affected the accuracy of our data. Additionally, the registry did not originally collect essential parameters such as potential risk factor exposure, sites of metastasis, mode of treatment, and socioeconomic status. Nevertheless, to the best of our knowledge, our study is the first to report population-based national data on mixed GCTs with choriocarcinoma components from Saudi Arabia. We believe that conducting more comprehensive chart reviews over longer periods will be crucial in developing improved strategies for early diagnosis and timely management of this disease.
Conclusion
In summary, our findings align with reported data on choriocarcinoma-containing mixed GCTs, emphasizing their aggressive phenotype at presentation. Our study also provides a better understanding of this tumour’s epidemiological and clinicopathological aspects in Saudi Arabia. Our study sheds light on the clinical behaviour and outcomes of mixed germ cell tumours with choriocarcinoma components in Saudi Arabia. These tumours, while rare, tend to occur more frequently in young populations and present with an aggressive phenotype characterized by high-grade tumours and distant metastasis at diagnosis. The mortality rate among patients with mixed germ cell tumours containing choriocarcinoma was considerable, underscoring the poor prognosis associated with this malignancy. Future studies should focus on elucidating important prognostic factors to enhance the management of patients with these tumours.
Ethical approval
The study was IRB approved (approval number NRC21/223/05). Patient confidentiality was ensured, and the patients' data were collected and used by the research team only. Serial numbers were used instead of medical record numbers to ensure anonymity. Due to the retrospective nature of the study, and the use of anonymized patient data, the requirement for informed consent was waived. Ethical approval (NRC21.223.05) was obtained from King Abdullah International Medical Research Center (KAIMRC) prior to the beginning of this study. This study is a secondary analysis.
Consent
Not applicable.
Sources of funding
This research did not receive any funding from institutions in the public, commercial, or not-for-profit sectors.
Author contribution
All authors contributed to the research and/or preparation of the manuscript. M.A., A.S., and B.N.S. participated in the study design and wrote the first draft of the manuscript. A.A., Y.N., M.A., A.S., B.N.S., A.S.E., M.B., M.A.O., T.Z.A., S.A.R., B.A., R.A., M.A.L., and K.A. collected and processed the data. M.A. participated in the study design and performed the statistical analyses. M.A., A.S., and B.N.S. reviewed and finalized the manuscript. All authors read and approved the final manuscript.
Conflicts of interest disclosure
The authors declare that there are no conflicts of interest.
Research registration unique identifying number (UIN)
researchregistry9185.
Guarantor
Mohammad Alghafees.
Availability of data and material
Not applicable.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Code availability
Not applicable.
Acknowledgement
The authors thank the Saudi Cancer Registry for providing access to the data for research purposes.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 20 September 2023
Contributor Information
Abdullah Al-Khayal, Email: khayala@ksau-hs.edu.sa.
Yasser Noureldin, Email: dryasser_noor@yahoo.com.
Mohammad Alghafees, Email: Alghafees687@gmail.com.
Areez Shafqat, Email: ashafqat@alfaisal.edu.
Belal Nedal Sabbah, Email: sabbahbelal@yahoo.com;belalsabbah@gmail.com;bsabbah@alfaisal.edu.
Asem H. Elhossiny, Email: asemelhossainy@gmail.com.
Mohamad Bakir, Email: Mo7ammedbakir@gmail.com.
Mohammed Ali Omar, Email: msomar@alfaisal.edu.
Tarek Ziad Arabi, Email: tarabi@alfaisal.edu.
Saleha Abdul Rab, Email: sabdulrab@alfaisal.edu.
Bader Alsaikhan, Email: alsaikhanb@ksau-hs.edu.sa.
Reema Aldhalaan, Email: raldhalaan@alfaisal.edu.
Muhannad Alquirnas, Email: muhannadqirnas@gmail.com.
Khalid Alrabeeah, Email: rabeeahk@ksau-hs.edu.sa.
References
- 1.Albers P, Albrecht W, Algaba F, et al. Guidelines on Testicular Cancer: 2015 Update. Eur Urol 2015;68:1054–1068. [DOI] [PubMed] [Google Scholar]
- 2.Bahrami A, Ro JY, Ayala AG. An overview of testicular germ cell tumors. Arch Pathol Lab Med 2007;131:1267–1280. [DOI] [PubMed] [Google Scholar]
- 3.Rejlekova K, Cursano MC, De Giorgi U, et al. Severe complications in testicular germ cell tumors: the choriocarcinoma syndrome, Front Endocrinol 2019;10:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKendrick JJ, Theaker J, Mead GM. Nonseminomatous germ cell tumor with very high serum human chorionic gonadotropin. Cancer 1991;67:684–689. [DOI] [PubMed] [Google Scholar]
- 5.Reilley MJ, Pagliaro LC. Testicular choriocarcinoma: a rare variant that requires a unique treatment approach, Curr Oncol Rep 2015;17:2. [DOI] [PubMed] [Google Scholar]
- 6.Krag Jacobsen G, Barlebo H, Olsen J, et al. Testicular germ cell tumours in Denmark 1976-1980. Pathology of 1058 consecutive cases. Acta Radiol Oncol 1984;23:239–247. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Cai Z, Liu R, et al. Clinicopathological characteristics and survival outcomes for testicular choriocarcinoma: a population-based study. Transl Androl Urol 2021;10:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer JR. 953Choriocarcinoma, Cancer Epidemiology and Prevention 2017Oxford University Press. [Google Scholar]
- 9.Ulbright TM. Germ cell neoplasms of the testis. Am J Surg Pathol 1993;17:1075–1091. [DOI] [PubMed] [Google Scholar]
- 10.Adra N, Althouse SK, Liu H, et al. , Prognostic factors in patients with poor-risk germ-cell tumors: a retrospective analysis of the Indiana University experience from 1990 to 2014. Ann Oncol 2016;27:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logothetis CJ, Samuels ML, Selig DE, et al. Cyclic chemotherapy with cyclophosphamide, doxorubicin, and cisplatin plus vinblastine and bleomycin in advanced germinal tumors. Results with 100 patients. Am J Med 1986;81:219–228. [DOI] [PubMed] [Google Scholar]
- 12.Bosl GJ, Geller NL, Cirrincione C, et al. Multivariate analysis of prognostic variables in patients with metastatic testicular cancer. Cancer Res 1983;43:3403–3407. [PubMed] [Google Scholar]
- 13.Group TIPFS. Prognostic factors in patients with metastatic germ cell tumors who experienced treatment failure with cisplatin-based first-line chemotherapy. J Clin Oncol 2010;28:4906–4911. [DOI] [PubMed] [Google Scholar]
- 14.Adra N, Abonour R, Althouse SK, et al. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: the indiana university experience. J Clin Oncol 2017;35:1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fosså SD, Stenning SP, Gerl A, et al. Prognostic factors in patients progressing after cisplatin-based chemotherapy for malignant non-seminomatous germ cell tumours. Br J Cancer 1999;80:1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abomelha M. Adult testicular cancer: two decades of Saudi national data. Urol Ann 2017;9:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 18.Necchi A, Giannatempo P, Lo Vullo S, et al. A prognostic model including pre- and postsurgical variables to enhance risk stratification of primary mediastinal nonseminomatous germ cell tumors: the 27-year experience of a referral center. Clin Genitourin Cancer 2015;13:87–93.e1. [DOI] [PubMed] [Google Scholar]
- 19.Park JS, Kim J, Elghiaty A, et al. Recent global trends in testicular cancer incidence and mortality. Medicine (Baltimore) 2018;97:e12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarado-Cabrero I, Hernández-Toriz N, Paner GP. Clinicopathologic analysis of choriocarcinoma as a pure or predominant component of germ cell tumor of the testis. Am J Surg Pathol 2014;38:111–118. [DOI] [PubMed] [Google Scholar]
- 21.Vaeth M, Schultz HP, Von Der Maase H, et al. Prognostic factors in testicular germ cell tumours. Experiences from 1058 consecutive cases. Acta Radiol Oncol 1984;23:271–285. [DOI] [PubMed] [Google Scholar]
- 22.Patel HD, Singla N, Ghandour RA, et al. Site of extranodal metastasis impacts survival in patients with testicular germ cell tumors. Cancer 2019;125:3947–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dieckmann KP, Simonsen-Richter H, Kulejewski M, et al. Serum tumour markers in testicular germ cell tumours: frequencies of elevated levels and extents of marker elevation are significantly associated with clinical parameters and with response to treatment. Biomed Res Int 2019;2019:5030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan O, Epstein JI. The clinical significance of a small component of choriocarcinoma in testicular mixed germ cell tumor (MGCT). Am J Surg Pathol 2018;42:1113–1120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


