Abstract
Infections and inflammation during pregnancy or early life can alter child neurodevelopment and increase the risk for structural brain abnormalities and mental health disorders. There is strong evidence that TORCH infections (i.e., Treponema pallidum, Toxoplasma gondii, rubella virus, cytomegalovirus, herpes virus) alter fetal neurodevelopment across multiple developmental domains and contribute to motor and cognitive disabilities. However, the impact of a broader range of viral and bacterial infections on fetal development and disability is less well understood. We performed a literature review of human studies to identify gaps in the link between maternal infections, inflammation, and several neurodevelopmental domains. We found strong and moderate evidence respectively for a higher risk of motor and cognitive delays and disabilities in offspring exposed to a range of non-TORCH pathogens during fetal life. In contrast, there is little evidence for an increased risk of language and sensory disabilities. While guidelines for TORCH infection prevention during pregnancy are common, further consideration for prevention of non-TORCH infections during pregnancy for fetal neuroprotection may be warranted.
Keywords: neurodevelopment, maternal immune activation, perinatal complications, TORCH infections, sensory disabilities, behavioral disabilities, cognitive and intellectual disability, learning disability, speech language disorders, motor skills disorders, neurodevelopmental delay
Introduction
Brain development is a complex process that begins a few weeks after conception and continues through childhood and adolescence. In fetal life, exposure to inflammatory or infectious agents can perturb or arrest fetal brain programming with major consequences for motor abilities, cognitive function and mental health [1,2]. Strong evidence links “TORCH” infections in the mother during pregnancy to sensory and motor deficits in the child. “TORCH” is a mnemonic which currently stands for Treponema pallidum (syphilis), Toxoplasma gondii, “other” pathogens [varicella-zoster virus (VZV), parvovirus B19)], rubella virus (RuV), cytomegalovirus (CMV), herpes simplex virus (HSV), hepatitis viruses, and human immunodeficiency virus (HIV). Mother-to-child transmission of TORCH pathogens can occur either prenatally, perinatally, or postnatally through breastfeeding, but most often transmission occurs antepartum through transplacental passage of organisms. TORCH infections acquired during pregnancy are characterized by complex fetal brain injuries, which can have long-term neurologic effects on the child [3-7] and eventually in the adult. In contrast, the impact of most other “non-TORCH” infections on fetal and child neurodevelopment and neurologic deficits is poorly understood. There is a large body of work examining the risk of prenatal infection on later development of psychiatric pathology over the life course [8-10]. However, in this review, we focus on the question of whether endemic infectious diseases, like malaria, or bacterial infections (e.g., genitourinary infections) can also cause fetal brain injuries that lead to cognitive, motor or other delays or disabilities when acquired by a pregnant individual. For these and other non-TORCH infections, the impact of a maternal infection on child developmental outcomes is not generally appreciated.
The original “TORCH” paradigm was defined in 1971 by Andres Nahmias to capture four congenital infections: TOxoplasmosis, Rubella, Cytomegalovirus, and Herpes Simplex Virus [11]; the “O” later came to stand for “Other” pathogens that included parvovirus B19 and the “H” was expanded to include hepatitis B virus [12]. Traditionally, TORCH pathogens have several common characteristics including a mild illness in the infected mother, vertical transmission to the fetus, and a spectrum of several anomalies that develop in the affected fetus [13]. More recently, Zika virus (ZIKV) has also been labeled as a new “TORCH” pathogen [14-17], due to its link with complex neurologic and sensorineural injuries, microcephaly [18], and neurocognitive impairments [19]. However, there are generations within the medical and scientific workforce that associate only the specific pathogens specified by the “T”, “R”, “C”, and “H” letters with congenital anomalies and neurologic impairment. Our current understanding of the complex interactions between maternal infections and fetal/child neurodevelopment extends well beyond this simple paradigm. A broader range of bacterial and viral infections have now been studied in pregnancy with a variable focus on the different neurologic outcomes in the exposed fetus. In this review we sought to determine the spectrum of developmental differences posed by a wide spectrum of “non-TORCH” infections focusing on non-psychiatric pathology.
The objective of our narrative review was to analyze the strength of the evidence in the literature for causal relationships between non-TORCH infections and neurodevelopmental delays and disabilities in the child to highlight pathogens of concern beyond TORCH. We used a developmental domain framework seeking to classify evidence of delay or disability according to functional areas of child development. While there are many developmental frameworks and classification systems, in this review we focus on a child’s motor, cognitive, sensory, and language development.
Adverse Neurologic Outcomes Induced by TORCH Pathogens
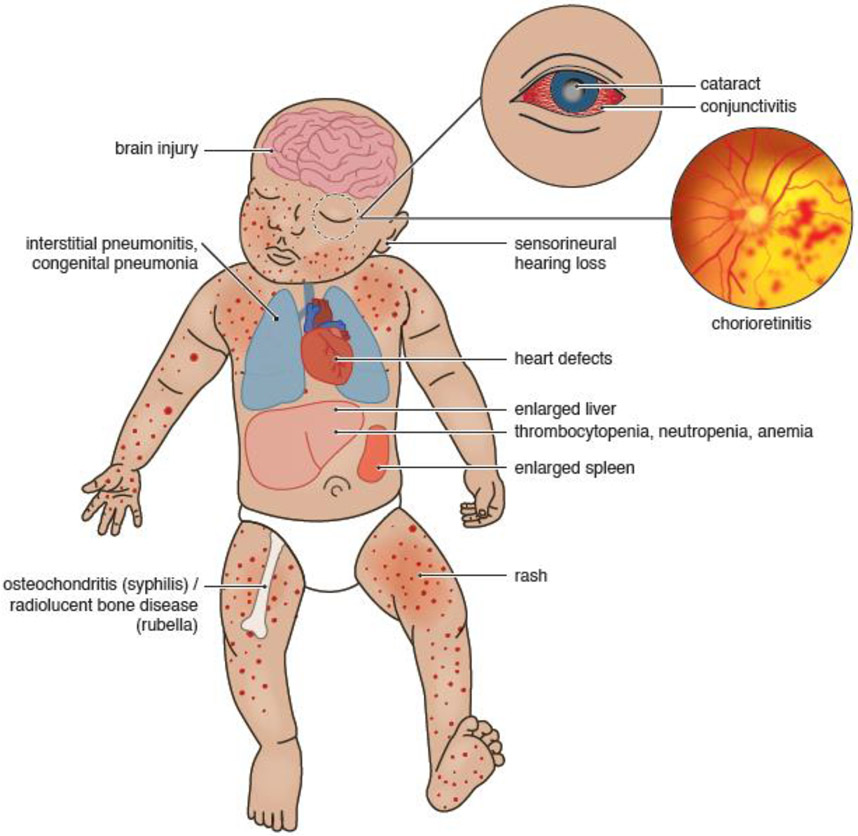
An analysis of the adverse outcomes induced by TORCH pathogens represents a starting point for consideration of neurologic outcomes potentially linked to non-TORCH pathogens. Notable clinical symptoms that are characteristic of TORCH infections at birth include fever, jaundice, low birth weight, purpura (small red or brown dots), blue/purple spots (rash), an enlarged liver, and eye injuries or cataracts (Table 1, Figure 1). Among the TORCH pathogens, T. gondii, T. pallidum, RuV, CMV, and HSV 1 and 2, HIV and ZIKV are known to increase the risk of motor delay and disability through widespread neurological damage, particularly in the motor cortex [20-25][26,27]. Studies have also established that infants exposed to maternal TORCH infections are at higher risk for cognitive, learning, and speech & language delays and disabilities [23,28-34] [22,35-51]. TORCH infections are also classically linked to eye injuries, such as congenital cataracts and chorioretinitis [52-58]. Auditory development is similarly disturbed by several TORCH infections [59-69]. A range of complex motor, learning, speech, sensory and cognitive deficits are strongly associated with classic TORCH pathogens.
Table 1.
TORCH Pathogens, Transmission Temporality, and Neonatal Outcomes
| TORCH Letter |
Pathogen (disease) |
Transmission temporality |
Strong clinical correlates of disease in neonate |
|---|---|---|---|
| T | Toxoplasma gondii (toxoplasmosis) | Greatest risk for congenital anomalies with a maternal third trimester infection | Intra-uterine growth restriction, jaundice, diffuse intracranial/intraparenchymal calcifications, chorioretinitis, hepatosplenomegaly, petechiae/purpura, chorioretinitis |
| T | Treponema pallidum (syphilis) | Greater risk of congenital disease with acquisition in 2nd trimester or later | Thrombocytopenia, maculopapular rash on palms & soles, Hutchinson’s teeth, hydrocephalus, hepatosplenomegaly, petechiae/purpura, chorioretinitis |
| O | Parvovirus B19 (Fifth’s Disease) | Greater risk of fetal anemia and non-immune hydrops in the 2nd trimester. Fetal acquisition of infection ~1-3 weeks after maternal infection. | Subcutaneous edema, hydrops fetalis, myocarditis & heart failure, retinal and corneal abnormalities, hepatosplenomegaly, petechiae/purpura, chorioretinitis |
| O | Zika virus (Congenital Zika syndrome) | Vertical transmission and fetal microcephaly can occur with maternal infection in any trimester | Newest “TORCH” pathogen due to complex and severe neurological injuries of the fetus |
| R | Rubella virus (Congenital Rubella Syndrome) | Greatest risk with first trimester infection, decreasing risk of vertical transmission as gestation progresses | Sensorineural hearing loss, cataracts, patent ductus arteriosus, pulmonary artery stenosis, myocarditis, microphthalmia, glaucoma, “blueberry muffin rash,” hepatosplenomegaly, thrombocytopenia, petechiae/purpura |
| C | Cytomegalovirus | Equal risk for congenital anomalies with maternal infection in any trimester Greater risk of congenital disease with primary infection as opposed to reactivation | Microcephaly, periventricular calcifications, sensorineural deafness, Hepatosplenomegaly, petechiae/purpura, chorioretinitis |
| H | Herpes virus simplex | Worst outcomes linked to perinatal acquisition at the time of birth. Greater risk of congenital disease with primary infection as opposed to reactivation | Skin-eye-mucus membrane lesions, fever, vesicular rash, meningoencephalitis, myocarditis, cataracts, hepatosplenomegaly, petechiae/purpura, chorioretinitis |
This table shows the connection between commonly associated “TORCH” pathogens, risk for vertical transmission depending on the time in gestation of maternal infection, and the clinical outcomes observed in the neonate. Abbreviations are shown in the table.
Figure 1. Neonatal Outcomes Associated with TORCH infections.
This figure illustrates the neonatal clinical findings associated with TORCH infections during pregnancy. TORCH infections in pregnancy are typically associated with a spectrum of congenital anomalies including congenital brain injuries, heart defects, sensorineural hearing loss, hepatosplenomegaly, liver dysfunction, conjunctivitis, chorioretinitis, lung inflammation and infection.
Maternal Immune Activation Hypothesis
Although not specific for any pathogen, there is another paradigm that requires examination when analyzing links between fetal exposure to non-TORCH pathogens and development of neurologic deficits. Many epidemiologic and animal studies suggest that non-TORCH pathogens induce fetal neurologic injury without direct infection of the fetus (Figure 2). This led to the “maternal immune activation” (MIA) hypothesis which proposed that in utero inflammation can alter fetal neurodevelopment through placental transmissions of inflammatory signals between mother and fetus. [70-74] Findings from the animal literature suggest that maternal infection and inflammation can cause generalized changes to white matter and hippocampal development, increased microglial activation as well as alterations in the development of the dopaminergic, glutamatergic, serotonergic and GABAergic neuronal systems [75-85]. Mechanisms causing abnormal oligodendrocyte development and death are also under study [86-89]. Maternal inflammation also appears to induce epigenetic changes through histone acetylation, DNA methylation and microRNA expression in a host of genes [73,90]. Although it is not yet known how maternal infection during particular gestational windows may differentially affect neurologic development, evidence from rat studies suggest that infection at different points in gestation may result in different neurological pathologies [91].
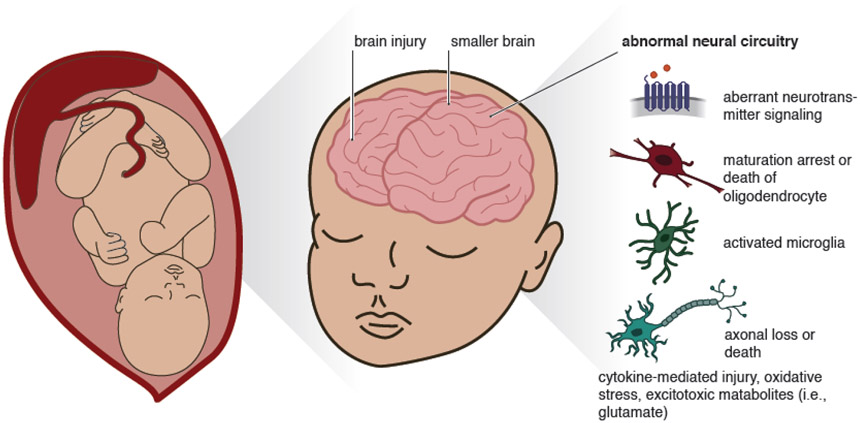
Figure 2. Fetal Brain Injury in Response to Non-TORCH pathogens.
This figure illustrates some of the mechanisms through which maternal infections caused by “non-TORCH” pathogens may alter fetal brain development. Fetal brain changes may be mediated through aberrant neurotransmitter signaling, abnormal growth or death of oligodendrocytes, microglial activation, direct neuronal injury or death from cytokines, oxidative stress, and toxic metabolites.
The MIA hypothesis raised the possibility that any infectious or inflammatory condition in the mother might be harmful to the fetal brain. Further, it allowed for the use of pathogen-associated molecular patterns, like bacterial cell wall components (e.g., Lipopolysaccharide or LPS) or viral RNA, to model the impact of common bacterial and viral inflammatory insults in animal models on fetal neurodevelopment. The MIA hypothesis also provided a broader foundation for epidemiologic studies to analyze links between systemic bacterial infections, not thought to infect the fetus, and neuropsychiatric pathology in the child. For example, epidemiological studies have investigated the impact of maternal genitourinary and respiratory infections on the long-term mental health of the child and risk of autism spectrum disorder, schizophrenia, and depression [8-10,92,93]. This framework is valuable because it suggests that a broad range of non-TORCH pathogens may also increase the risk of neurodevelopmental delay and disability.
Methods of Narrative Literature Review
We created a search strategy designed to focus on particular developmental domains rather than on general brain injury. We conducted a literature search through November 2022 using the PubMed database centering on MeSH terms “Language Disorders”, “Speech Disorders”, “Developmental Disabilities”, “Sensation Disorders”, “Intellectual Disability”, “Learning Disabilities”, “Behavioral Disorders”, and “Motor Disorders”. First, we coupled each MeSH term with the phrase “infection” or “inflammation” through the Boolean operator “AND”; next, we coupled these terms with “maternal” or “fetal” or “perinatal” or “pregnancy” or “congenital” using the Boolean operator “AND”. We also used “maternal immune activation” in combination with “cognitive”, “learning”, “language”, “dyslexia”, “dyscalculia”, “dysgraphia”, “hearing”, “vision” and “motor” using the Boolean operator “AND”. We utilized this preliminary search to identify the most prevalent neurodevelopmental conditions within each developmental domain considering primarily human studies (Table 2). Additional papers were added to the review based on references that appeared within each paper as well as using the Pubmed “cited by tool”, which revealed more recent papers. We excluded literature on psychiatric disorders and autism spectrum disorder as our intention was to focus on a set of disabilities and delays within a developmental domain framework rather than recapitulate the evidence on psychiatric pathology outcomes. We additionally excluded papers written in languages other than English. Our review focused on studies assessing correlations between abnormal neurodevelopment in the child after exposure to TORCH or non-TORCH pathogens during fetal life. As the focus of the review was on non-TORCH infections, inclusion of articles related to TORCH infections represented mainly a comparator.
Table 2.
Developmental Domains Analyzed for Injury by Non-TORCH Pathogens or Infections
| Developmental Domain |
Defining Features | Example Clinical Diagnoses in the Child |
|---|---|---|
| Motor | Deficits in movement and coordination, typically diagnosed when difficulty with motor skills affects activities of daily living | Cerebral palsy |
| Intellectual/Cognitive | Permanent limitations of cognition and intelligence, characterized by deficits in learning, logical reasoning, problem-solving, interpersonal skills, practical skills defined by intelligence quotient of less than 70, or 2 standard deviations below the median. | Cognitive impairment |
| Learning | Impairments in academic function that are not due to sensory deficits, where difficulty is experienced with reading, writing, or mathematics | Dyslexia, dyscalculia, dysgraphia, nonverbal learning disorders |
| Communication | Speech Disorders: problems with articulation and fluency when speaking Language Disorders: difficulty comprehending and using spoken or written language (form, content, function) |
Speech delay, language delay |
| Sensory | Disorders affecting somatosensory system (processing information related to vision, hearing, taste, smell and other special senses) | Sensorineural hearing loss, decreased visual acuity |
This table shows the definitions of the developmental domains that were assessed for associations with maternal non-TORCH infections. For another developmental domain framework example with age-specific milestones, see [185].
We considered the body of evidence as strong when there was consistent evidence from epidemiological studies from multiple populations with control for confounding and some evidence of increasing risk with physical proximity to the fetal compartment or severity of the infection as well as support from animal studies. We considered the body of evidence weak when there were few or conflicting epidemiological studies without confounder consideration in the design and inconsistent animal model study findings. We considered the body of evidence as moderate when there were consistent animal model findings and several epidemiological studies with confounding considerations with mostly consistent findings.
Results
Evidence Linking Maternal Non-TORCH Infections with Disabilities in Exposed Children Strong Evidence – Motor Disabilities
There is compelling evidence from both the human and animal literature that maternal infection may increase risk of motor deficits in offspring [91][98,99]. Considering extra-uterine infections first, there is moderate evidence that prenatal infections including maternal genitourinary tract infection may increase the odds of cerebral palsy (CP) in preterm and low birthweight infants [100]. A Danish population-based study found an increased risk (adjusted HR (aHR), 2.1, 95% Confidence Interval (CI) 1.4 - 3.2) of CP among infants whose mothers were diagnosed with genitourinary tract infection during pregnancy [101]. Another Danish study focusing on self-reported vaginal infections found an increased risk of both CP and spastic CP (aHR 1.52, 95% CI, 1.04 - 2.24; and aHR 1.73, 95% CI, 1.16 - 2.60, respectively), as well as maternal fever and CP (aHR, 1.53; 95% CI, 1.06 - 2.21); however these findings may be subject to significant recall bias [32]. Similarly, a case-control study in Sweden found an increased risk of CP in infants of mothers who had any infection during pregnancy (adjusted odds ratio (aOR) 2.9, 95% CI 1.7 - 4.8), severe infection during pregnancy (aOR 15.4, 95% CI 3.0 - 78.1), bacterial growth in urine during pregnancy (aOR 4.7, 95% CI 1.5 - 15.2), and antibiotic treatment in pregnancy (aOR 6.3, 95% CI 3.0 - 15.2) [104]. In a large cohort of extremely preterm infants in the United States, maternal cervical or vaginal infection during gestation was associated with increased risk of motor delay (aOR 1.7; 95% CI 1.04 - 2.7), though this may be confounded by prematurity [105]. Collectively, there is evidence from both European and U.S. cohorts that a variety of extra-uterine genitourinary infections in pregnancy are associated with an increased risk for development of motor delay and disability.
Among studies considering fetal exposure to bacterial infections or sterile inflammation in utero, there appears to be strong evidence for a causal relationship with risk of motor delay and or disability. A clinical or histopathological diagnosis of chorioamnionitis, an acute inflammation of the placental membranes, typically represents a plausible active or resolved polymicrobial bacterial infection that induced a cytokine and chemokine response reaching the fetus [106-110]. A population-based study in California examined chorioamnionitis in comparison with maternal genitourinary tract and respiratory infections and found a higher odds of CP for the combined clinical and histopathological diagnoses of chorioamnionitis (OR 3.1, 95% CI 2.9 −3.4) than for genitourinary infection (OR 1.4, 95% CI 1.3 – 1.6) and respiratory infection (OR 1.9, 95% CI 1.5 – 2.2) [111]. A meta-analysis of 26 studies found a consistent relationship between chorioamnionitis and risk of CP among full-term infants whose mother was diagnosed with clinical chorioamnionitis (RR) 4.7; 95% CI: 1.3 - 16.2) [112]. Assuming a causal role of chorioamnionitis in some proportion of cases of cerebral palsy, a nested a case-control study using a cohort of 231,582 singleton infants estimated that 11% of all cases of CP in singleton births could be attributable to chorioamnionitis [96]. As expected, an intrauterine localized infection in close proximity to the fetus was associated with a greater increase in the risk for CP than for extrauterine infections.
Considering evidence from animal studies, maternal sepsis was shown to alter strength, coordination, function and ability in the offspring in a murine model [94]. Other work in mice has demonstrated that experimentally inducing maternal inflammation with pathogen-associated molecular patterns [poly(I:C)] to mimic an infection causes motor activity and coordination deficits that seem to be more pronounced in male offspring [95]. Like the mouse model, rabbits exposed to in utero LPS had injury to both white and gray matter as well as impaired locomotion and motor deficits related to posture and feeding [96,97].
In contrast to gross motor delay and disability, there is no consistent evidence that maternal infection during gestation increases the risk of repetitive and involuntary movement disorders like Tourette syndrome or other tic disorders. While a small case-control study found that exposure to proinflammatory factors (e.g., autoimmune disease, prenatal infection) was more prevalent in mothers of children with tic disorders [113], other studies have not found a consistent association between maternal infection and tic disorders [114-116]. A large cohort study in Sweden revealed a 60% increase in hazard ratio for developing Tourette syndrome and chronic tic disorder in children exposed to prenatal maternal infection (aHR, 1.60; 95% CI, 1.23 - 2.09); however this relationship was not seen in a sibling-matched sub-analysis suggesting that heritable factors may have a causal role in both risk of tic disorders and infection [117].
Moderate Evidence – Cognitive Disabilities
Considering generalized non-TORCH infections, there is evidence from human studies that maternal infection may increase the risk of child cognitive delay and intellectual disability. Ecological studies have found an increased prevalence of intellectual disability in children and adults born during or after peaks of influenza pandemics. Takei et al. found increased risk of an intellectual disability diagnosis among patients born after peak influenza pandemic periods from 1953 to 1980 in England and Wales [130]. In their model, there was a 17% increase in births of what they deemed “mentally handicapped” individuals six months after respective seasonal peaks [130]. Another study found that male military conscripts in Norway born during the 1969-1970 influenza pandemic had reduced intelligence scores in models comparing them with males born before and after [131]. Population data from California also suggested that children of mothers who experienced a variety of infections during gestation had an increased risk of intellectual disability; this risk was highest with infection in the second trimester [132]. A large cohort study of children exposed to maternal systemic bacterial infection during pregnancy in Massachusetts and Rhode Island were found have lower IQ scores compared to children that weren’t exposed [133]. Similarly, another large cohort study of children exposed to maternal infection in utero in the United Kingdom had lower total IQ at 8 years of age compared to children not exposed to infection [134]. However, a recent population-based study in Sweden found that while maternal infection during gestation was an independent predictor of intellectual disability (aHR, 1.37 95%CI 1.23 - 1.51), this relationship was not statistically significant in a sibling model stratified on the nuclear family; this data suggested that some or all of the relationship between maternal infection and intellectual disability may have been related to familial or unmeasured confounders [135]. Conversely, in their bias analyses, the authors also studied the relationship between maternal infection in the year preceding pregnancy as a negative control and risk of intellectual disability in the child and found no statistical association [135]. Overall, the findings from this important study imply that some degree of unmeasured confounding may influence the apparent relationship between maternal infection and intellectual disability.
Evidence from maternal serum and fetal tissue reflecting systemic inflammatory responses aligns well with both animal and epidemiologic studies suggesting that maternal inflammation imparts a higher risk for child cognitive developmental delay. Several studies have found correlations between maternal inflammatory cytokines like Interleukin-6 and C-reactive Protein during pregnancy and changes in the child’s brain structural development, working memory and cognitive scores [136-138]. In a secondary analysis of a country-wide longitudinal cohort study in the United States, funisitis (inflammation of the umbilical cord) was also associated with lower intelligence quotient scores at 4 and 7 years of age [139]. Funisitis and infection of the placenta are closely linked pathologic events during an intrauterine infection.
Maternal urinary tract infections (UTI) have also been regarded as a potentially important source of inflammation during pregnancy. In the Collaborative Perinatal Project which enrolled pregnant women across the United States, follow-up studies found that among white mothers (in contrast with black mothers) UTI during pregnancy increased risk of intellectual disability by 62% in an unadjusted analysis [140]. Langridge et al. also found an increased risk of mild-to-moderate intellectual disability among children of mothers with UTI during pregnancy in Western Australia in an unadjusted analysis [141]. Evidence from a study using public insurance claims data in the United States found similar results with infants of women diagnosed with a UTI who did not take antibiotics having increased risk of intellectual disability or developmental delay (aRR 1.31, 95% CI 1.12 - 1.54) compared to women not diagnosed with UTI and compared to women diagnosed with a UTI who completed an antibiotic course (aRR 1.22 95% CI 1.02 - 1.46) [142]. A similar but unadjusted study in South Carolina had comparable results [143].
Studies in animal models support the detrimental effects of maternal immune activation on offspring cognition and memory [118,119]. Mice exposed to MIA with inflammatory antigens were found to have cognitive impairment related to disruption in the catecholaminergic, GABAergic and dopaminergic systems [120-124]. Rats with similar inflammatory prenatal exposure had differences in working memory and other cognitive domains compared to non-exposed rats [125-129]. Another study among pregnant rhesus monkeys injected with viral antigen found decreased brain size and cognitive alterations [118].
Lastly, while learning and cognition are difficult to separate, many animal studies have focused on learning deficits after in utero exposure to MIA. Learning deficits have been observed in mice and rats exposed to bacterial infections, LPS and viral mimics in-utero [94,125,144,145]. Some work has focused on the possibility of immunologic mediated risk of dyslexia. Vincent et al. injected sera from mothers with multiple children with dyslexia into pregnant mice and found resulting worse performance of mouse offspring on spatial coordination tasks and suggested that this was due to maternal antibodies [146]. In humans, studies into prenatal risk factors for dyslexia date back to the 1950s but have produced little suggestive published evidence [147]. Some epidemiologic evidence from China susceptible to a high degree of recall bias suggested that prenatal maternal infection may increase risk of dyslexia [148].
Minimal Evidence – Language and Sensory Disabilities
While there is an abundance of evidence on the increased risk of abnormal communication with maternal immune activation in animal models in line with work examining risk for autism spectrum disorder, there is little evidence that non-TORCH maternal infections increase the risk of speech or language disorders in humans [149-154]. For example, a study examining receptive language ability found no difference in scores between children exposed in utero to maternal upper respiratory tract infection compared to those who were not [157]. Conversely, another small retrospective cohort study of infants less than 30 weeks gestation found that histological chorioamnionitis was associated with language disability [158]. A cross-sectional study in France found no increased risk of language delay with prenatal inflammation among a group of preterm infants [99]. A large selection of studies have found that rats exposed to antenatal inflammation have alterations in communication compared to unexposed rats [127,155,156]. Although the complexity of human language is not perfectly recapitulated by studying communication and vocalization patterns in animal models, one can consider vocalization deficits in animals as an early sign of derailed neurodevelopment.
Finally, there is little evidence that exposure to non-TORCH infections during pregnancy increases the risk of sensory disabilities. The most common sensory disability implicated in maternal gestational infection is sensorineural hearing loss (SNHL) of which immune-mediated mechanisms secondary to viral infection as well as local cochlear inflammatory response are responsible for disease [159]. Two small retrospective studies present conflicting results for a link between chorioamnionitis and hearing loss [160,161]. One case-control study from Columbia suggested a possible increased risk of decreased visual or auditory acuity with maternal acute respiratory tract infection in pregnancy [162].
Understudied Impact of Malaria and Chikungunya Virus on Child Neurodevelopment
Malaria is one of several mosquito-borne infectious diseases that can increase risk of both maternal and fetal morbidity and mortality but has a poorly characterized impact on fetal neurodevelopment [163]. Nearly 125 million pregnant individuals reside in tropical and subtropical areas of the world and are susceptible to Malarial infection via the Anopheles mosquito. Despite this global infectious disease threat, malaria in pregnancy still lacks a reliable small animal model [164]. Two cohort studies in Malawi have found no association between maternal malaria and motor skills deficits [165,166], although language development may be impaired [166]. In a prospective study of 493 mother-offspring pairs in Benin, perinatal exposure to malaria, especially with a greater burden of parasites in maternal blood, was independently associated with impaired gross motor development in infants at 1 year of age, but not at 6 years of age, although the association was observed in the crude analysis [167]. In a recent randomized clinical trial conducted in Uganda examining the effect of malaria in pregnancy and chemoprevention regimens on child neurodevelopmental and behavioral outcomes, it was shown that children exposed to malaria in pregnancy had worse cognitive, behavioral, and executive function scores than unexposed controls [168]. Interestingly, it was demonstrated that more effective chemoprevention regimens did not result in better outcomes, possibly implying that more intensive prevention prior to and early in gestation may be more effective.
Over the last two decades, Chikungunya Virus (CHIKV) infection has become the most prevalent alphavirus disease in the world through the geographic expansion of the Aedes mosquito vectors (i.e., Aedes aegypti and Aedes albopictus) [169]. More recently, CHIKV was found to be the main neurotropic pathogen among children with brain infections for which cerebrospinal fluid was sampled in coastal Kenya, an area where cerebral malaria and bacterial meningitis had declined due to better vector control [170]. CHIKV can cause both neonatal encephalopathy [171] and encephalitis [172] when transmitted perinatally due to antepartum or intrapartum maternal viremia (absolute risk (AR) 22.5%, 95% CI 9.5 - 35.4% among the exposed; AR 47.6%, 95% CI 24.9 - 69.8% among the infected) [169,171-177]. When infection occurs perinatally, long-term neurological sequelae include cerebral palsy, blindness, and seizures [172,175,176].
On Reunion Island, where perinatal transmission of CHIKV was first documented [171], 51 percent of children exposed to perinatal mother-to-child CHIKV infection had global neurodevelopmental delay at two years of age, (aRR 2.79, 95%CI 1.45 - 3.02) compared to uninfected controls in a model adjusted for maternal social status, gestational age, small for gestational age and head circumference [176]. Specific cognitive domains affected included coordination, language, social, and to a lesser extent, gross motor, and postural abilities. Children with encephalopathy or encephalitis exhibited far lower scores across domains than children with milder neonatal disease. Postnatal microcephaly and cerebral palsy were also documented, suggesting that perinatal transmission of CHIKV shares features with classical TORCH pathogens [176]. Given the importance of preventing intrapartum infection with CHIKV, and likely malaria, there is a strong need for vaccines and pharmacotherapeutics which can block the perinatal transmission of these pathogens to the fetus and prevent severe lifelong brain damage.
Knowledge Gaps
The impact of non-TORCH infections on child development is greatly understudied and several systematic reviews are needed that focus on specific developmental domains that include evidence from both TORCH and non-TORCH infections. Although the impact of malaria and CHIKV exposure in utero on child neurodevelopment is a major knowledge gap in the field, there are many other pathogens that may adversely impact fetal neurodevelopment which are currently unknown. Studying the impact of a broad range of bacterial infections at different bodily sites and across a wide spectrum of disease severity is extremely challenging even in countries with national health databases containing decades of data. It also becomes difficult to study the impact of perinatal exposure to a viral or parasitic disease on child development when it becomes endemic in large parts of the world. Although hundreds of millions of pregnant individuals are at risk for acquiring the disease annually, many of these women were exposed once or repeatedly to the pathogen from a young age resulting in partial or protective immunity. Complex host responses to a pathogen can make the impact of the pathogen on the pregnant woman and her child more challenging to study, as it is unclear which individuals are truly susceptible and at greater risk for disease or transmission to the fetus. Further, public health investment in research typically decreases once a pathogen transitions from a pandemic/epidemic to endemic status and it becomes more difficult to set up expensive cohorts of exposed children that will require complex developmental domain testing over many years. Overall, determining the impact of a perinatal exposure to a pathogen on child development is expensive and requires investment in infrastructure to follow the children long-term.
Conclusions
TORCH infections are an important cause of disability worldwide across multiple developmental domains. However, there is strong suggestive evidence that non-TORCH infections during pregnancy may also increase the risk of motor delay and disability. We find moderate evidence that fetal exposure to maternal non-TORCH infections increases risk of cognitive delay and disability in the child. Except for perinatally acquired CHIKV encephalopathy/encephalitis, we find very little evidence for a causal relationship between non-TORCH maternal infections (including malaria) and speech and language and sensory disabilities. Larger, well-designed studies of whether and how malaria or CHIKV during pregnancy may affect neurodevelopment are needed. Further, research into the role of genitourinary tract infections in pregnancy would clarify the importance of these infections for fetal development. Information systems that align data from pregnancy care and pediatric developmental surveillance with new and reemerging pathogens would greatly facilitate long-term studies of neurodevelopmental effects of infection during pregnancy. Lastly, pathogen avoidance and vaccine administration continue to be cornerstones of infection prevention during pregnancy.
With the possible increased risk for global impairments in fetal and child neurodevelopment, prevention of infection during pregnancy continues to be crucial for pregnant women and their infants. While the role of malaria in gestation and subsequent neurodevelopment remains unclear, continued attention to improving coverage of prevention measures like intermittent prophylaxis, Anopheles mosquito reduction and bed nets [178] are essential. Although a malaria vaccine for use in pregnancy is not yet available, other infections and their sequelae can be prevented during pregnancy [179-181]. For CHIKV, the mechanism of mother-to-child perinatal transmission to the fetus remains elusive and a safe vaccine for pregnant women or immunotherapy protocols during labor or soon after birth are needed [182]. There is growing early evidence but still no consensus on whether infection with SARS-Cov2 during pregnancy increases risk of developmental delay in the child [183,184]. However, given the known increased risk of pregnancy complications for both influenza and SARS-COV2 during pregnancy and the availability of safe and effective vaccines, recommendations for continued vaccination and infection prevention measures for pregnant persons is warranted.
Highlights.
We reviewed the evidence for fetal exposure to non-TORCH maternal infections and inflammation on the motor, cognitive, language and sensory developmental domains.
We found strong evidence for increased risk of motor delay and disability after exposure to non- TORCH maternal infection or inflammation in utero.
We also found moderate evidence for increased risk of cognitive delay after non-TORCH maternal infection or inflammation in utero.
There was little evidence for increased risk of language or sensory disability.
Acknowledgments:
We thank and acknowledge Riley Raker for graphic design of the figures.
Funding:
This work was supported by funding from the National Institutes of Health grants AI164588, AI143625, AI133976, R01HD098713, R01AI145890, and OD010425 to K.A.W. BJS al-Haddad has previously been supported by T32 GM008244 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- CHIKV
chikungunya virus
- CMV
cytomegalovirus
- CP
cerebral palsy
- HIV
human immunodeficiency virus
- HR
Hazard Ratio
- HSV
herpes simplex virus
- LPS
lipopolysaccharide
- OR
Odds Ratio
- RR
Risk Ratio
- TORCH
Toxoplasma, “Other” (Zika, Varicella, Parvovirus B19), Rubella, Cytomegalovirus, Herpes simplex virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures:
Dr Patrick Gérardin reported having received consulting fees from Takeda and Valneva. Dr. Kristina Adams Waldorf reported receiving consultant fees from GlaxoSmithKline. Ms. Devaraju, Ms. A. Li, Ms. Ha, Ms. M. Li, Ms. Shivakumar, Ms. H. Li, Dr. Phelps Nishiguchi, and Dr. al-Haddad reported no biomedical financial interests or potential conflicts of interests.
References:
- 1.Bale JF Fetal Infections and Brain Development. Clin. Perinatol 2009, 36, 639–653, doi: 10.1016/j.clp.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Adams Waldorf KM; McAdams RM Influence of Infection during Pregnancy on Fetal Development. Reprod. Camb. Engl 2013, 146, R151–162, doi: 10.1530/REP-13-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams Waldorf KM; Stencel-Baerenwald JE; Kapur RP; Studholme C; Boldenow E; Vornhagen J; Baldessari A; Dighe MK; Thiel J; Merillat S; et al. Fetal Brain Lesions after Subcutaneous Inoculation of Zika Virus in a Pregnant Nonhuman Primate. Nat. Med 2016, 22, 1256–1259, doi: 10.1038/nm.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams Waldorf KM; Nelson BR; Stencel-Baerenwald JE; Studholme C; Kapur RP; Armistead B; Walker CL; Merillat S; Vornhagen J; Tisoncik-Go J; et al. Congenital Zika Virus Infection as a Silent Pathology with Loss of Neurogenic Output in the Fetal Brain. Nat. Med 2018, 24, 368–374, doi: 10.1038/nm.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams Waldorf KM; Olson EM; Nelson BR; Little M-TE; Rajagopal L The Aftermath of Zika: Need for Long-Term Monitoring of Exposed Children. Trends Microbiol. 2018, 26, 729–732, doi: 10.1016/j.tim.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen-Saines K; Brasil P; Kerin T; Vasconcelos Z; Gabaglia CR; Damasceno L; Pone M; Abreu de Carvalho LM; Pone SM; Zin AA; et al. Delayed Childhood Neurodevelopment and Neurosensory Alterations in the Second Year of Life in a Prospective Cohort of ZIKV-Exposed Children. Nat. Med 2019, 25, 1213–1217, doi: 10.1038/s41591-019-0496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobral da Silva PF; Eickmann SH; Arraes de Alencar Ximenes R; Ramos Montarroyos U; de Carvalho Lima M; Turchi Martelli CM; Velho Barreto de Araújo T; Brickley EB; Cunha Rodrigues L; Lima da Silva Pastich Gonçalves FC; et al. Pediatric Neurodevelopment by Prenatal Zika Virus Exposure: A Cross-Sectional Study of the Microcephaly Epidemic Research Group Cohort. BMC Pediatr. 2020, 20, 472, doi: 10.1186/s12887-020-02331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown AS; Derkits EJ Prenatal Infection and Schizophrenia: A Review of Epidemiologic and Translational Studies. Am. J. Psychiatry 2010, 167, 261–280, doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tioleco N; Silberman AE; Stratigos K; Banerjee-Basu S; Spann MN; Whitaker AH; Turner JB Prenatal Maternal Infection and Risk for Autism in Offspring: A Meta-Analysis. Autism Res. Off. J. Int. Soc. Autism Res. 2021, 14, 1296–1316, doi: 10.1002/aur.2499. [DOI] [PubMed] [Google Scholar]
- 10.Cheslack-Postava K; Brown AS Prenatal Infection and Schizophrenia: A Decade of Further Progress. Schizophr. Res 2022, 247, 7–15, doi: 10.1016/j.schres.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nahmias AJ; Walls KW; Stewart; Herrmann KL; Flynt WJ The ToRCH Complex-Perinatal Infections Associated with Toxoplasma and Rubella, Cytomegol- and Herpes Simplex Viruses. Pediatr. Res 1971, 5, 405–406, doi: 10.1203/00006450-197108000-00144. [DOI] [Google Scholar]
- 12.Neu N; Duchon J; Zachariah P TORCH Infections. Clin. Perinatol 2015, 42, 77–103, viii, doi: 10.1016/j.clp.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Mehrjardi MZ Is Zika Virus an Emerging TORCH Agent? An Invited Commentary. Virol. Res. Treat 2017, 8, 1178122X17708993, doi: 10.1177/1178122X17708993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coyne CB; Lazear HM Zika Virus - Reigniting the TORCH. Nat. Rev. Microbiol 2016, 14, 707–715, doi: 10.1038/nrmicro.2016.125. [DOI] [PubMed] [Google Scholar]
- 15.Bryson Y. Zika Virus Congenital Syndrome, the New Z in TORCHZ? Prospects for Diagnosis Prevention and Treatment. Curr. Opin. Pediatr 2017, 29, 94–96, doi: 10.1097/MOP.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 16.Morand A; Zandotti C; Charrel R; Minodier P; Fabre A; Chabrol B; De Lamballerie X [From TORCH to TORCHZ: Zika virus infection highlights infectious fetopathies]. Arch. Pediatr. Organe Off. Soc. Francaise Pediatr 2017, 24, 911–913, doi: 10.1016/j.arcped.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Tahotná A; Brucknerová J; Brucknerová I Zika Virus Infection from a Newborn Point of View. TORCH or TORZiCH? Interdiscip. Toxicol 2018, 11, 241–246, doi: 10.2478/intox-2018-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Melo ASO; Aguiar RS; Amorim MMR; Arruda MB; de Melo FO; Ribeiro STC; Batista AGM; Ferreira T; Dos Santos MP; Sampaio VV; et al. Congenital Zika Virus Infection: Beyond Neonatal Microcephaly. JAMA Neurol. 2016, 73, 1407–1416, doi: 10.1001/jamaneurol.2016.3720. [DOI] [PubMed] [Google Scholar]
- 19.Mulkey SB; Arroyave-Wessel M; Peyton C; Bulas DI; Fourzali Y; Jiang J; Russo S; McCarter R; Msall ME; du Plessis AJ; et al. Neurodevelopmental Abnormalities in Children With In Utero Zika Virus Exposure Without Congenital Zika Syndrome. JAMA Pediatr. 2020, 174, 269–276, doi: 10.1001/jamapediatrics.2019.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auriti C; Bucci S; De Rose DU; Coltella L; Santisi A; Martini L; Maddaloni C; Bersani I; Lozzi S; Campi F; et al. Maternal-Fetal Infections (Cytomegalovirus, Toxoplasma, Syphilis): Short-Term and Long-Term Neurodevelopmental Outcomes in Children Infected and Uninfected at Birth. Pathogens 2022, 11, 1278, doi: 10.3390/pathogens11111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chase C; Vibbert M; Pelton SI; Coulter DL; Cabral H Early Neurodevelopmental Growth in Children with Vertically Transmitted Human Immunodeficiency Virus Infection. Arch. Pediatr. Adolesc. Med 1995, 149, 850–855, doi: 10.1001/archpedi.1995.02170210024004. [DOI] [PubMed] [Google Scholar]
- 22.Wheeler AC; Toth D; Ridenour T; Lima Nóbrega L; Borba Firmino R; Marques da Silva C; Carvalho P; Marques D; Okoniewski K; Ventura LO; et al. Developmental Outcomes Among Young Children With Congenital Zika Syndrome in Brazil. JAMA Netw. Open 2020, 3, e204096, doi: 10.1001/jamanetworkopen.2020.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desmond MM; Fisher ES; Vorderman AL; Schaffer HG; Andrew LP; Zion TE; Catlin FI The Longitudinal Course of Congenital Rubellaencephalitis in Nonretarded Children. J. Pediatr 1978, 93, 584–591, doi: 10.1016/S0022-3476(78)80892-0. [DOI] [PubMed] [Google Scholar]
- 24.Corey L; Stone EF; Whitley RichardJ.; Mohan K DIFFERENCE BETWEEN HERPES SIMPLEX VIRUS TYPE I AND TYPE 2 NEONATAL ENCEPHALITIS IN NEUROLOGICAL OUTCOME. The Lancet 1988, 331, 1–4, doi: 10.1016/S0140-6736(88)90997-X. [DOI] [PubMed] [Google Scholar]
- 25.Whitley R; Arvin A; Prober C; Corey L; Burchett S; Plotkin S; Starr S; Jacobs R; Powell D; Nahmias A; et al. Predictors of Morbidity and Mortality in Neonates with Herpes Simplex Virus Infections. N. Engl. J. Med 1991, 324, 450–454, doi: 10.1056/NEJM199102143240704. [DOI] [PubMed] [Google Scholar]
- 26.Brasil P; Pereira JP; Moreira ME; Ribeiro Nogueira RM; Damasceno L; Wakimoto M; Rabello RS; Valderramos SG; Halai U-A; Salles TS; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med 2016, 375, 2321–2334, doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Einspieler C; Utsch F; Brasil P; Panvequio Aizawa CY; Peyton C; Hydee Hasue R; Françoso Genovesi F; Damasceno L; Moreira ME; Adachi K; et al. Association of Infants Exposed to Prenatal Zika Virus Infection With Their Clinical, Neurologic, and Developmental Status Evaluated via the General Movement Assessment Tool. JAMA Netw. Open 2019, 2, e187235, doi: 10.1001/jamanetworkopen.2018.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazarte-Rantes C; Rodríguez-Anccasi R; Rivas-Campos C; Silva E Congenital Toxoplasmosis: Findings in Fetal MRI. Cureus 2021, 13, e16894, doi: 10.7759/cureus.16894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel P. Congenital Infections of the Nervous System. Contin. Minneap. Minn 2021, 27, 1105–1126, doi: 10.1212/CON.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 30.Rudnick CM; Hoekzema GS Neonatal Herpes Simplex Virus Infections. Am. Fam. Physician 2002, 65, 1138–1142. [PubMed] [Google Scholar]
- 31.Jacobs RF Neonatal Herpes Simplex Virus Infections. Semin. Perinatol 1998, 22, 64–71, doi: 10.1016/s0146-0005(98)80008-6. [DOI] [PubMed] [Google Scholar]
- 32.Papola P; Alvarez M; Cohen HJ Developmental and Service Needs of School-Age Children with Human Immunodeficiency Virus Infection: A Descriptive Study. Pediatrics 1994, 94, 914–918. [PubMed] [Google Scholar]
- 33.Malm G; Engman M-L Congenital Cytomegalovirus Infections. Semin. Fetal. Neonatal Med 2007, 12, 154–159, doi: 10.1016/j.siny.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 34.A Search for Congenital Rubella in Psychiatric Day Treatment, Language and Learning Centres - Feldman Ronald B., Mendelson Jack, Golick Margie, Rothman Stanley J., Portnoy Joseph, Eaton William W., 1983. Available online: https://journals-sagepub-com.ezp1.lib.umn.edu/doi/10.1177/070674378302800110?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 18 November 2022). [DOI] [PubMed] [Google Scholar]
- 35.Brackis-Cott E; Kang E; Dolezal C; Abrams EJ; Mellins CA The Impact of Perinatal HIV Infection on Older School-Aged Children’s and Adolescents’ Receptive Language and Word Recognition Skills. AIDS Patient Care STDs 2009, 23, 415–421, doi: 10.1089/apc.2008.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caiaffa WT; Chiari CA; Figueiredo AR; Orefice F; Antunes CM Toxoplasmosis and Mental Retardation--Report of a Case-Control Study. Mem. Inst. Oswaldo Cruz 1993, 88, 253–261, doi: 10.1590/s0074-02761993000200013. [DOI] [PubMed] [Google Scholar]
- 37.Lim J; Yoon SJ; Shin JE; Han JH; Lee SM; Eun HS; Park MS; Park KI l. Outcomes of Infants Born to Pregnant Women with Syphilis: A Nationwide Study in Korea. BMC Pediatr. 2021, 21, 47, doi: 10.1186/s12887-021-02502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Jong EP; Lindenburg IT; van Klink JM; Oepkes D; van Kamp IL; Walther FJ; Lopriore E Intrauterine Transfusion for Parvovirus B19 Infection: Long-Term Neurodevelopmental Outcome. Am. J. Obstet. Gynecol 2012, 206, 204.e1–5, doi: 10.1016/j.ajog.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 39.Fairlie L; Chernoff M; Cotton MF; Bwakura-Dangarembizi M; Violari A; Familiar-Lopez I; Barlow-Mosha L; Kamthunzi P; McCarthy K; Jean-Philippe P; et al. Antiretroviral Choice and Severe Disease Predict Poorer Neuropsychological Outcomes in HIV+ Children from Africa. Front. Pediatr 2022, 10, 899002, doi: 10.3389/fped.2022.899002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boivin MJ; Green SD; Davies AG; Giordani B; Mokili JK; Cutting WA A Preliminary Evaluation of the Cognitive and Motor Effects of Pediatric HIV Infection in Zairian Children. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc 1995, 14, 13–21, doi: 10.1037//0278-6133.14.1.13. [DOI] [PubMed] [Google Scholar]
- 41.Diamond GW; Gurdin P; Wiznia AA; Belman AL; Rubinstein A; Cohen HJ Effects of Congenital HIV Infection on Neurodevelopmental Status of Babies in Foster Care. Dev. Med. Child Neurol 1990, 32, 999–1004, doi: 10.1111/j.1469-8749.1990.tb08123.x. [DOI] [PubMed] [Google Scholar]
- 42.Gordon-Lipkin E; Peacock G The Spectrum of Developmental Disability with Zika Exposure: What Is Known, What Is Unknown, and Implications for Clinicians. J. Dev. Behav. Pediatr. JDBP 2019, 40, 387–395, doi: 10.1097/DBP.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson KM; Simons EA; Badizadegan K; Reef SE; Cooper LZ Characterization of the Risks of Adverse Outcomes Following Rubella Infection in Pregnancy. Risk Anal. Off. Publ. Soc. Risk Anal 2016, 36, 1315–1331, doi: 10.1111/risa.12264. [DOI] [PubMed] [Google Scholar]
- 44.Lanzieri TM; Leung J; Caviness AC; Chung W; Flores M; Blum P; Bialek SR; Miller JA; Vinson SS; Turcich MR; et al. Long-Term Outcomes of Children with Symptomatic Congenital Cytomegalovirus Disease. J. Perinatol. Off. J. Calif. Perinat. Assoc 2017, 37, 875–880, doi: 10.1038/jp.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engman M-L; Adolfsson I; Lewensohn-Fuchs I; Forsgren M; Mosskin M; Malm G Neuropsychologic Outcomes in Children with Neonatal Herpes Encephalitis. Pediatr. Neurol 2008, 38, 398–405, doi: 10.1016/j.pediatrneurol.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Mattson SN; Jones KL; Gramling LJ; Schonfeld AM; Riley EP; Harris JA; Chambers CD Neurodevelopmental Follow-up of Children of Women Infected with Varicella during Pregnancy: A Prospective Study. Pediatr. Infect. Dis. J 2003, 22, 819–823, doi: 10.1097/01.inf.0000086402.18710.54. [DOI] [PubMed] [Google Scholar]
- 47.da Silva TC; do Santos LJPN; Arrais NMR; Balen SA Development of Infants Presented with Congenital Syphilis in Their First Months of Life. Rev. CEFAC Atualizacao Cient. Em Fonoaudiol. E Educ 2021, 23, NA–NA, doi: 10.1590/1982-0216/20212369321. [DOI] [Google Scholar]
- 48.Mwaniki MK; Atieno M; Lawn JE; Newton CRJC Long-Term Neurodevelopmental Outcomes after Intrauterine and Neonatal Insults: A Systematic Review. Lancet Lond. Engl 2012, 379, 445–452, doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magai DN; Karyotaki E; Mutua AM; Chongwo E; Nasambu C; Ssewanyana D; Newton CR; Koot HM; Abubakar A Long-Term Outcomes of Survivors of Neonatal Insults: A Systematic Review and Meta-Analysis. PLOS ONE 2020, 15, e0231947, doi: 10.1371/journal.pone.0231947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walsh H; Zuwala J; Hunter J; Oh Y Congenital Cytomegalovirus and Human Immunodeficiency Virus: Effects on Hearing, Speech and Language Development, and Clinical Outcomes in Children. Front. Pediatr 2021, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malm G; Forsgren M; el Azazi M; Persson A A Follow-up Study of Children with Neonatal Herpes Simplex Virus Infections with Particular Regard to Late Nervous Disturbances. Acta Paediatr. Scand 1991. 80, 226–234, doi: 10.1111/j.1651-2227.1991,tb11838.x. [DOI] [PubMed] [Google Scholar]
- 52.Melamed J; Eckert GU; Spadoni VS; Lago EG; Uberti F Ocular Manifestations of Congenital Toxoplasmosis. Eye 2010, 24, 528–534, doi: 10.1038/eye.2009.140. [DOI] [PubMed] [Google Scholar]
- 53.KLAUDER JV; MEYER GP CHORIORETINITIS OF CONGENITAL SYPHILIS. AMA Arch. Ophthalmol 1953, 49, 139–157, doi: 10.1001/archopht.1953.00920020144002. [DOI] [PubMed] [Google Scholar]
- 54.Lambert SR; Taylor D; Kriss A; Holzel H; Heard S Ocular Manifestations of the Congenital Varicella Syndrome. Arch. Ophthalmol. Chic. Ill 1960 1989, 107, 52–56, doi: 10.1001/archopht.1989.01070010054026. [DOI] [PubMed] [Google Scholar]
- 55.Armstrong NT The Ocular Manifestations of Congenital Rubella Syndrome. Insight Am. Soc. Ophthalmic Regist. Nurses 1992, 17, 14–16. [PubMed] [Google Scholar]
- 56.O’Neill JF The Ocular Manifestations of Congenital Infection: A Study of the Early Effect and Long-Term Outcome of Maternally Transmitted Rubella and Toxoplasmosis. Trans. Am. Ophthalmol. Soc 1998, 96, 813–879. [PMC free article] [PubMed] [Google Scholar]
- 57.Coats DK; Demmler GJ; Paysse EA; Du LT; Libby C Ophthalmologic Findings in Children with Congenital Cytomegalovirus Infection. J. AAPOS Off. Publ. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2000, 4, 110–116, doi: 10.1067/mpa.2000.103870. [DOI] [PubMed] [Google Scholar]
- 58.Nahmias AJ; Visintine AM; Caldwell DR; Wilson LA Eye Infections with Herpes Simplex Viruses in Neonates. Surv. Ophthalmol 1976, 21, 100–105, doi: 10.1016/0039-6257(76)90086-2. [DOI] [PubMed] [Google Scholar]
- 59.Angueyra C; Abou Hatab H; Pathak A Congenital Cytomegalovirus and Zika Infections. Indian J. Pediatr 2020, 87, 840–845, doi: 10.1007/s12098-020-03260-9. [DOI] [PubMed] [Google Scholar]
- 60.de Barbosa MHM; de Magalhães-Barbosa MC; Robaina JR; Prata-Barbosa A; Lima M.A. de M.T. de; da Cunha AJLA Auditory Findings Associated with Zika Virus Infection: An Integrative Review. Braz. J. Otorhinolaryngol 2019, 85, 642–663, doi: 10.1016/j.bjorl.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fandiño-Cárdenas M; Idrovo AJ; Velandia R; Molina-Franky J; Alvarado-Socarras JL Zika Virus Infection during Pregnancy and Sensorineural Hearing Loss among Children at 3 and 24 Months Post-Partum. J. Trop. Pediatr 2019, 65, 328–335, doi: 10.1093/tropej/fmy055. [DOI] [PubMed] [Google Scholar]
- 62.Leal MC; Muniz LF; Ferreira TSA; Santos CM; Almeida LC; Van Der Linden V; Ramos RCF; Rodrigues LC; Neto SSC Hearing Loss in Infants with Microcephaly and Evidence of Congenital Zika Virus Infection - Brazil, November 2015-May 2016. MMWR Morb. Mortal. Wkly. Rep 2016, 65, 917–919, doi: 10.15585/mmwr.mm6534e3. [DOI] [PubMed] [Google Scholar]
- 63.da Hora LCD; Muniz LF; Griz SMS; da Silva JD; de Britto DBLA; Venâncio LGA; de Filho DBM; de Leal MC Frequency-Following Response and Auditory Behavior in Children with Prenatal Exposure to the Zika Virus. Int. Arch. Otorhinolaryngol 2022, 26, e380–e389, doi: 10.1055/s-0041-1726048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Corrêa CC; Maximino LP; Weber SAT Hearing Disorders in Congenital Toxoplasmosis: A Literature Review. Int. Arch. Otorhinolaryngol 2018, 22, 330–333, doi: 10.1055/S-0037-1605377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chau J; Atashband S; Chang E; Westerberg BD; Kozak FK A Systematic Review of Pediatric Sensorineural Hearing Loss in Congenital Syphilis. Int. J. Pediatr. Otorhinolaryngol 2009, 73, 787–792, doi: 10.1016/j.ijporl.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 66.Brookhouser PE; Bordley JE Congenital Rubella Deafness. Pathology and Pathogenesis. Arch. Otolaryngol. Chic. Ill 1960 1973, 98, 252–257, doi: 10.1001/archotol.1973.00780020262008. [DOI] [PubMed] [Google Scholar]
- 67.Fowler KB Congenital Cytomegalovirus Infection: Audiologic Outcome. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am 2013, 57, S182–S184, doi: 10.1093/cid/cit609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohen BE; Durstenfeld A; Roehm PC Viral Causes of Hearing Loss: A Review for Hearing Health Professionals. Trends Hear. 2014, 18, 2331216514541361, doi: 10.1177/2331216514541361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mitsikas D; Gabrani C; Giannakou K; Lamnisos D Intrauterine Exposure to Zika Virus and Hearing Loss within the First Few Years of Life: A Systematic Literature Review. Int. J. Pediatr. Otorhinolaryngol 2021, 147, 110801, doi: 10.1016/j.ijporl.2021.110801. [DOI] [PubMed] [Google Scholar]
- 70.Atladóttir HÓ; Henriksen TB; Schendel DE; Parner ET Autism after Infection, Febrile Episodes, and Antibiotic Use during Pregnancy: An Exploratory Study. Pediatrics 2012, 130, e1447–1454, doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Croen LA; Qian Y; Ashwood P; Zerbo O; Schendel D; Pinto-Martin J; Daniele Fallin M; Levy S; Schieve LA; Yeargin-Allsopp M; et al. Infection and Fever in Pregnancy and Autism Spectrum Disorders: Findings from the Study to Explore Early Development. Autism Res. Off. J. Int. Soc. Autism Res 2019, 12, 1551–1561, doi: 10.1002/aur.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gustavson K; Ask H; Ystrom E; Stoltenberg C; Lipkin WI; Surén P; Håberg SE; Magnus P; Knudsen GP; Eilertsen E; et al. Maternal Fever during Pregnancy and Offspring Attention Deficit Hyperactivity Disorder. Sci. Rep 2019, 9, 9519, doi: 10.1038/s41598-019-45920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bergdolt L; Dunaevsky A Brain Changes in a Maternal Immune Activation Model of Neurodevelopmental Brain Disorders. Prog. Neurobiol 2019, 175, 1–19, doi: 10.1016/j.pneurobio.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Connor TG; Ciesla AA Maternal Immune Activation Hypotheses for Human Neurodevelopment: Some Outstanding Questions. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 471–479, doi: 10.1016/j.bpsc.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santoni M; Frau R; Pistis M Transgenerational Sex-Dependent Disruption of Dopamine Function Induced by Maternal Immune Activation. Front. Pharmacol 2022, 13, 821498, doi: 10.3389/fphar.2022.821498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luchicchi A; Lecca S; Melis M; De Felice M; Cadeddu F; Frau R; Muntoni AL; Fadda P; Devoto P; Pistis M Maternal Immune Activation Disrupts Dopamine System in the Offspring. Int. J. Neuropsychopharmacol 2016, 19, pyw007, doi: 10.1093/ijnp/pyw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corradini I; Focchi E; Rasile M; Morini R; Desiato G; Tomasoni R; Lizier M; Ghirardini E; Fesce R; Morone D; et al. Maternal Immune Activation Delays Excitatory-to-Inhibitory Gamma-Aminobutyric Acid Switch in Offspring. Biol. Psychiatry 2018, 83, 680–691, doi: 10.1016/j.biopsych.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 78.Fernandez A; Dumon C; Guimond D; Tyzio R; Bonifazi P; Lozovaya N; Burnashev N; Ferrari DC; Ben-Ari Y The GABA Developmental Shift Is Abolished by Maternal Immune Activation Already at Birth. Cereb. Cortex N. Y. N 1991 2019, 29, 3982–3992, doi: 10.1093/cercor/bhy279. [DOI] [PubMed] [Google Scholar]
- 79.Mirabella F; Desiato G; Mancinelli S; Fossati G; Rasile M; Morini R; Markicevic M; Grimm C; Amegandjin C; Termanini A; et al. Prenatal Interleukin 6 Elevation Increases Glutamatergic Synapse Density and Disrupts Hippocampal Connectivity in Offspring. Immunity 2021, 54, 2611–2631.e8, doi: 10.1016/j.immuni.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amodeo DA; Lai C-Y; Hassan O; Mukamel EA; Behrens MM; Powell SB Maternal Immune Activation Impairs Cognitive Flexibility and Alters Transcription in Frontal Cortex. Neurobiol. Dis 2019, 125, 211–218, doi: 10.1016/j.nbd.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reisinger SN; Kong E; Khan D; Schulz S; Ronovsky M; Berger S; Horvath O; Cabatic M; Berger A; Pollak DD Maternal Immune Activation Epigenetically Regulates Hippocampal Serotonin Transporter Levels. Neurobiol. Stress 2016, 4, 34–43, doi: 10.1016/j.ynstr.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Csatlosova K; Bogi E; Durisova B; Grinchii D; Paliokha R; Moravcikova L; Lacinova L; Jezova D; Dremencov E Maternal Immune Activation in Rats Attenuates the Excitability of Monoamine-Secreting Neurons in Adult Offspring in a Sex-Specific Way. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol 2021, 43, 82–91, doi: 10.1016/j.euroneuro.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 83.Goeden N; Velasquez J; Arnold KA; Chan Y; Lund BT; Anderson GM; Bonnin A Maternal Inflammation Disrupts Fetal Neurodevelopment via Increased Placental Output of Serotonin to the Fetal Brain. J. Neurosci. Off. J. Soc. Neurosci 2016, 36, 6041–6049, doi: 10.1523/JNEUROSCI.2534-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Canetta S; Brown A Prenatal Infection, Maternal Immune Activation, and Risk for Schizophrenia. Transl. Neurosci 2012, 3, 320–327, doi: 10.2478/s13380-012-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Speers LJ; Cheyne KR; Cavani E; Hayward T; Schmidt R; Bilkey DK Hippocampal Sequencing Mechanisms Are Disrupted in a Maternal Immune Activation Model of Schizophrenia Risk. J. Neurosci 2021, 41, 6954–6965, doi: 10.1523/JNEUROSCI.0730-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Makinodan M; Tatsumi K; Manabe T; Yamauchi T; Makinodan E; Matsuyoshi H; Shimoda S; Noriyama Y; Kishimoto T; Wanaka A Maternal Immune Activation in Mice Delays Myelination and Axonal Development in the Hippocampus of the Offspring. J. Neurosci. Res 2008, 86, 2190–2200, doi: 10.1002/jnr.21673. [DOI] [PubMed] [Google Scholar]
- 87.Boccazzi M; Van Steenwinckel J; Schang A-L; Faivre V; Le Charpentier T; Bokobza C; Csaba Z; Verderio C; Fumagalli M; Mani S; et al. The Immune-Inflammatory Response of Oligodendrocytes in a Murine Model of Preterm White Matter Injury: The Role of TLR3 Activation. Cell Death Dis 2021, 12, 166, doi: 10.1038/s41419-021-03446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abraham M; Peterburs J; Mundorf A Oligodendrocytes Matter: A Review of Animal Studies on Early Adversity. J. Neural Transm. Vienna Austria 1996 2023, doi: 10.1007/s00702-023-02643-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mouihate A; Al-Hashash H; Rakhshani-Moghadam S; Kalakh S Impact of Prenatal Immune Challenge on the Demyelination Injury during Adulthood. CNS Neurosci. Ther 2017, 23, 724–735, doi: 10.1111/cns.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnson T; Saatci D; Handunnetthi L Maternal Immune Activation Induces Methylation Changes in Schizophrenia Genes. PLOS ONE 2022, 17, e0278155, doi: 10.1371/journal.pone.0278155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Straley ME; Van Oeffelen W; Theze S; Sullivan AM; O’Mahony SM; Cryan JF; O’Keeffe GW Distinct Alterations in Motor & Reward Seeking Behavior Are Dependent on the Gestational Age of Exposure to LPS-Induced Maternal Immune Activation. Brain. Behav. Immun 2017, 63, 21–34, doi: 10.1016/j.bbi.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 92.Al-Haddad BJS; Oler E; Armistead B; Elsayed NA; Weinberger DR; Bernier R; Burd I; Kapur R; Jacobsson B; Wang C; et al. The Fetal Origins of Mental Illness. Am. J. Obstet. Gynecol 2019, 221, 549–562, doi: 10.1016/j.ajog.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Al-Haddad BJS; Jacobsson B; Chabra S; Modzelewska D; Olson EM; Bernier R; Enquobahrie DA; Hagberg H; Östling S; Rajagopal L; et al. Long-Term Risk of Neuropsychiatric Disease After Exposure to Infection In Utero. JAMA Psychiatry 2019, 76, 594–602, doi: 10.1001/jamapsychiatry.2019.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Granja MG; Alves LP; Leardini-Tristão M; Saul ME; Bortoni LC; de Moraes FM; Ferreira EC; de Moraes BPT; da Silva VZ; dos Santos AFR; et al. Inflammatory, Synaptic, Motor, and Behavioral Alterations Induced by Gestational Sepsis on the Offspring at Different Stages of Life. J. Neuroinflammation 2021, 18, 60, doi: 10.1186/s12974-021-02106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haida O; Al Sagheer T; Balbous A; Francheteau M; Matas E; Soria F; Fernagut PO; Jaber M Sex-Dependent Behavioral Deficits and Neuropathology in a Maternal Immune Activation Model of Autism. Transl. Psychiatry 2019, 9, 1–12, doi : 10.1038/s41398-019-0457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cavarsan CF; Gorassini MA; Quinlan KA Animal Models of Developmental Motor Disorders: Parallels to Human Motor Dysfunction in Cerebral Palsy. J. Neurophysiol 2019, 122, 1238–1253, doi: 10.1152/jn.00233.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saadani-Makki F; Kannan S; Lu X; Janisse J; Dawe E; Edwin S; Romero R; Chugani D Intrauterine Administration of Endotoxin Leads to Motor Deficits in a Rabbit Model: A Link between Prenatal Infection and Cerebral Palsy. Am. J. Obstet. Gynecol 2008, 199, 651.e1–7, doi: 10.1016/j.ajog.2008.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen C; Lu D; Xue L; Ren P; Zhang H; Zhang J Association between Placental Inflammatory Pathology and Offspring Neurodevelopment at 8 Months and 4 and 7 Years of Age. J. Pediatr 2020, 225, 132–137.e2, doi: 10.1016/j.jpeds.2020.05.049. [DOI] [PubMed] [Google Scholar]
- 99.Giraud A; Chaux R; Allard M-J; Celle M; Teyssier G; Roche F; Chapelle C; Chabrier S; Sébire G; Patural H Perinatal Inflammation Is Associated with Social and Motor Impairments in Preterm Children without Severe Neonatal Brain Injury. Eur. J. Paediatr. Neurol 2020, 28, 126–132, doi: 10.1016/j.ejpn.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 100.Mann JR; Mcdermott S; Bao H; Bersabe A Maternal Genitourinary Infection and Risk of Cerebral Palsy. Dev. Med. Child Neurol 2009, 51, 282–288, doi: 10.1111/j.1469-8749.2008.03226.x. [DOI] [PubMed] [Google Scholar]
- 101.Miller JE; Pedersen LH; Streja E; Bech BH; Yeargin-Allsopp M; Van Naarden Braun K; Schendel DE; Christensen D; Uldall P; Olsen J Maternal Infections during Pregnancy and Cerebral Palsy: A Population-Based Cohort Study. Paediatr. Perinat. Epidemiol 2013, 27, 542–552, doi: 10.1111/ppe.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Streja E; Miller JE; Bech BH; Greene N; Pedersen LH; Yeargin-Allsopp M; Van Naarden Braun K; Schendel DE; Christensen D; Uldall P; et al. Congenital Cerebral Palsy and Prenatal Exposure to Self-Reported Maternal Infections, Fever, or Smoking. Am. J. Obstet. Gynecol 2013, 209, 332.e1–332.e10, doi: 10.1016/j.ajog.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu CS; Pedersen LH; Miller JE; Sun Y; Streja E; Uldall P; Olsen J Risk of Cerebral Palsy and Childhood Epilepsy Related to Infections before or during Pregnancy. PloS One 2013, 8, e57552, doi: 10.1371/journal.pone.0057552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ahlin K; Himmelmann K; Hagberg G; Kacerovsky M; Cobo T; Wennerholm U-B; Jacobsson B Cerebral Palsy and Perinatal Infection in Children Born at Term. Obstet. Gynecol 2013, 122, 41–49, doi: 10.1097/AOG.0b013e318297f37f. [DOI] [PubMed] [Google Scholar]
- 105.Leviton A; Allred EN; Kuban KCK; O’Shea TM; Paneth N; Onderdonk AB; Fichorova RN; Dammann O; ELGAN Study Investigators The Development of Extremely Preterm Infants Born to Women Who Had Genitourinary Infections During Pregnancy. Am. J. Epidemiol 2016, 183, 28–35, doi: 10.1093/aje/kwv129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tita ATN; Andrews WW Diagnosis and Management of Clinical Chorioamnionitis. Clin. Perinatol 2010, 37, 339–354, doi: 10.1016/j.clp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cappelletti M; Presicce P; Kallapur SG Immunobiology of Acute Chorioamnionitis. Front. Immunol 2020, 11, 649, doi: 10.3389/fimmu.2020.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McCartney SA; Kapur R; Liggitt HD; Baldessari A; Coleman M; Orvis A; Ogle J; Katz R; Rajagopal L; Adams Waldorf KM Amniotic Fluid Interleukin 6 and Interleukin 8 Are Superior Predictors of Fetal Lung Injury Compared with Maternal or Fetal Plasma Cytokines or Placental Histopathology in a Nonhuman Primate Model. Am. J. Obstet. Gynecol 2021, 225, 89.e1–89.e16, doi: 10.1016/j.ajog.2020.12.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beck C; Gallagher K; Taylor LA; Goldstein JA; Mithal LB; Gernand AD Chorioamnionitis and Risk for Maternal and Neonatal Sepsis: A Systematic Review and Meta-Analysis. Obstet. Gynecol 2021, 137, 1007–1022, doi: 10.1097/AOG.0000000000004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jain VG; Willis KA; Jobe A; Ambalavanan N Chorioamnionitis and Neonatal Outcomes. Pediatr. Res 2022, 91, 289–296, doi: 10.1038/s41390-021-01633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bear JJ; Wu YW Maternal Infections During Pregnancy and Cerebral Palsy in the Child. Pediatr. Neurol 2016, 57, 74–79, doi: 10.1016/j.pediatrneurol.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu YW; Colford JM Chorioamnionitis as a Risk Factor for Cerebral Palsy: A Meta-Analysis. JAMA 2000, 284, 1417–1424, doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 113.Jones HF; Han VX; Patel S; Gloss BS; Soler N; Ho A; Sharma S; Kothur K; Nosadini M; Wienholt L; et al. Maternal Autoimmunity and Inflammation Are Associated with Childhood Tics and Obsessive-Compulsive Disorder: Transcriptomic Data Show Common Enriched Innate Immune Pathways. Brain. Behav. Immun 2021, 94, 308–317, doi: 10.1016/j.bbi.2020.12.035. [DOI] [PubMed] [Google Scholar]
- 114.Mathews CA; Scharf JM; Miller LL; Macdonald-Wallis C; Lawlor DA; Ben-Shlomo Y Association between Pre- and Perinatal Exposures and Tourette Syndrome or Chronic Tic Disorder in the ALSPAC Cohort. Br. J. Psychiatry 2014, 204, 40–45, doi: 10.1192/bjp.bp.112.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cubo E; Hortigüela M; Jorge-Roldan S; Ciciliani SE; Lopez P; Velasco L; Sastre E; Ausin V; Delgado V; Saez S; et al. Prenatal and Perinatal Morbidity in Children with Tic Disorders: A Mainstream School-Based Population Study in Central Spain. Tremor Hyperkinetic Mov. 2014, 4, 272, doi: 10.5334/tohm.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abdulkadir M; Tischfield JA; King RA; Fernandez TV; Brown LW; Cheon K-A; Coffey BJ; de Bruijn SFTM; Elzerman L; Garcia-Delgar B; et al. Pre- and Perinatal Complications in Relation to Tourette Syndrome and Co-Occurring Obsessive-Compulsive Disorder and Attention-Deficit/Hyperactivity Disorder. J. Psychiatr. Res 2016, 82, 126–135, doi: 10.1016/j.jpsychires.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang T; Brander G; Isung J; Isomura K; Sidorchuk A; Larsson H; Chang Z; Mataix-Cols D; Fernández de la Cruz, L. Prenatal and Early Childhood Infections and Subsequent Risk of Obsessive-Compulsive Disorder and Tic Disorders: A Nationwide, Sibling-Controlled Study. Biol. Psychiatry 2022, doi: 10.1016/j.biopsych.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 118.Vlasova RM; Iosif A-M; Ryan AM; Funk LH; Murai T; Chen S; Lesh TA; Rowland DJ; Bennett J; Hogrefe CE; et al. Maternal Immune Activation during Pregnancy Alters Postnatal Brain Growth and Cognitive Development in Nonhuman Primate Offspring. J. Neurosci. Off. J. Soc. Neurosci 2021, 41, 9971–9987, doi: 10.1523/JNEUROSCI.0378-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ryan AM; Bauman MD Primate Models as a Translational Tool for Understanding Prenatal Origins of Neurodevelopmental Disorders Associated With Maternal Infection. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 510–523, doi: 10.1016/j.bpsc.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Perez-Palomar B; Erdozain AM; Erkizia-Santamaría I; Ortega JE; Meana JJ Maternal Immune Activation Induces Cortical Catecholaminergic Hypofunction and Cognitive Impairments in Offspring. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol 2023, doi: 10.1007/s11481-023-10070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Labouesse MA; Dong E; Grayson DR; Guidotti A; Meyer U Maternal Immune Activation Induces GAD1 and GAD2 Promoter Remodeling in the Offspring Prefrontal Cortex. Epigenetics 2015, 10, 1143–1155, doi: 10.1080/15592294.2015.1114202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Richetto J; Calabrese F; Meyer U; Riva MA Prenatal versus Postnatal Maternal Factors in the Development of Infection-Induced Working Memory Impairments in Mice. Brain. Behav. Immun 2013, 33, 190–200, doi: 10.1016/j.bbi.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 123.Ozawa K; Hashimoto K; Kishimoto T; Shimizu E; Ishikura H; Iyo M Immune Activation during Pregnancy in Mice Leads to Dopaminergic Hyperfunction and Cognitive Impairment in the Offspring: A Neurodevelopmental Animal Model of Schizophrenia. Biol. Psychiatry 2006, 59, 546–554, doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 124.Giovanoli S; Notter T; Richetto J; Labouesse MA; Vuillermot S; Riva MA; Meyer U Late Prenatal Immune Activation Causes Hippocampal Deficits in the Absence of Persistent Inflammation across Aging. J. Neuroinflammation 2015, 12, 221, doi: 10.1186/s12974-015-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murray BG; Davies DA; Molder JJ; Howland JG Maternal Immune Activation during Pregnancy in Rats Impairs Working Memory Capacity of the Offspring. Neurobiol. Learn. Mem 2017, 141, 150–156, doi: 10.1016/j.nlm.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 126.Connor CM; Dincer A; Straubhaar J; Galler JR; Houston IB; Akbarian S Maternal Immune Activation Alters Behavior in Adult Offspring, with Subtle Changes in the Cortical Transcriptome and Epigenome. Schizophr. Res 2012, 140, 175–184, doi: 10.1016/j.schres.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carbone E; Buzzelli V; Manduca A; Leone S; Rava A; Trezza V Maternal Immune Activation Induced by Prenatal Lipopolysaccharide Exposure Leads to Long-Lasting Autistic-like Social, Cognitive and Immune Alterations in Male Wistar Rats. Int. J. Mol. Sci 2023, 24, 3920, doi: 10.3390/ijms24043920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gogos A; Sbisa A; Witkamp D; van den Buuse M Sex Differences in the Effect of Maternal Immune Activation on Cognitive and Psychosis-like Behaviour in Long Evans Rats. Eur. J. Neurosci 2020, 52, 2614–2626, doi: 10.1111/ejn.14671. [DOI] [PubMed] [Google Scholar]
- 129.Su Y; Lian J; Hodgson J; Zhang W; Deng C Prenatal Poly I:C Challenge Affects Behaviors and Neurotransmission via Elevated Neuroinflammation Responses in Female Juvenile Rats. Int. J. Neuropsychopharmacol 2022, 25, 160–171, doi: 10.1093/ijnp/pyab087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Takei N; Murray G; O’Callaghan E; Sham PC; Glover G; Murray RM Prenatal Exposure to Influenza Epidemics and Risk of Mental Retardation. Eur. Arch. Psychiatry Clin. Neurosci 1995, 245, 255–259, doi: 10.1007/BF02191805. [DOI] [PubMed] [Google Scholar]
- 131.Eriksen W; Sundet JM; Tambs K Register Data Suggest Lower Intelligence in Men Born the Year after Flu Pandemic. Ann. Neurol 2009, 66, 284–289, doi: 10.1002/ana.21702. [DOI] [PubMed] [Google Scholar]
- 132.Haber H; Xing G; Walker C 21: Prenatal Infections and Risk of Autism, Intellectual Disability and/or Epilepsy. Am. J. Obstet. Gynecol 2016, 215, S824, doi: 10.1016/j.ajog.2016.09.022. [DOI] [Google Scholar]
- 133.Lee YH; Papandonatos GD; Savitz DA; Heindel WC; Buka SL Effects of Prenatal Bacterial Infection on Cognitive Performance in Early Childhood. Paediatr. Perinat. Epidemiol 2020, 34, 70–79, doi: 10.1111/ppe.12603. [DOI] [PubMed] [Google Scholar]
- 134.Kwok J; Hall HA; Murray AL; Lombardo MV; Auyeung B Maternal Infections during Pregnancy and Child Cognitive Outcomes. BMC Pregnancy Childbirth 2022, 22, 848, doi: 10.1186/s12884-022-05188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brynge M; Sjöqvist H; Gardner RM; Lee BK; Dalman C; Karlsson H Maternal Infection during Pregnancy and Likelihood of Autism and Intellectual Disability in Children in Sweden: A Negative Control and Sibling Comparison Cohort Study. Lancet Psychiatry 2022, 9, 782–791, doi: 10.1016/S2215-0366(22)00264-4. [DOI] [PubMed] [Google Scholar]
- 136.Md R; Am G; E F; O M-D; Jm R; R N; S E; Pd W; C B; Da F Maternal IL-6 during Pregnancy Can Be Estimated from Newborn Brain Connectivity and Predicts Future Working Memory in Offspring. Nat. Neurosci 2018, 21, doi: 10.1038/s41593-018-0128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rasmussen JM; Graham AM; Entringer S; Gilmore JH; Styner M; Fair DA; Wadhwa PD; Buss C Maternal Interleukin-6 Concentration during Pregnancy Is Associated with Variation in Frontolimbic White Matter and Cognitive Development in Early Life. Neuroimage 2019, 185, 825–835, doi: 10.1016/j.neuroimage.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Spann MN; Monk C; Scheinost D; Peterson BS Maternal Immune Activation During the Third Trimester Is Associated with Neonatal Functional Connectivity of the Salience Network and Fetal to Toddler Behavior. J. Neurosci. Off. J. Soc. Neurosci 2018, 38, 2877–2886, doi: 10.1523/JNEUROSCI.2272-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu C; Chen Y; Zhao D; Zhang J; Zhang Y Association Between Funisitis and Childhood Intellectual Development: A Prospective Cohort Study. Front. Neurol 2019, 10, 612, doi: 10.3389/fneur.2019.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Camp BW; Broman SH; Nichols PL; Leff M Maternal and Neonatal Risk Factors for Mental Retardation: Defining the ‘at-Risk’ Child. Early Hum. Dev 1998, 50, 159–173, doi: 10.1016/S0378-3732(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 141.Langridge AT; Glasson EJ; Nassar N; Jacoby P; Pennell C; Hagan R; Bourke J; Leonard H; Stanley FJ Maternal Conditions and Perinatal Characteristics Associated with Autism Spectrum Disorder and Intellectual Disability. PLOS ONE 2013, 8, e50963, doi: 10.1371/journal.pone.0050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McDermott S; Callaghan W; Szwejbka L; Mann H; Daguise V Urinary Tract Infections during Pregnancy and Mental Retardation and Developmental Delay. Obstet. Gynecol 2000, 96, 113–119, doi: 10.1016/s0029-7844(00)00823-1. [DOI] [PubMed] [Google Scholar]
- 143.McDermott S; Szwejbka L; Mann H; Durkin M; Callaghan W Urinary Tract Infections during Pregnancy in South Carolina. J. S. C. Med. Assoc. 1975 2001, 97, 195–200. [PubMed] [Google Scholar]
- 144.Schaafsma W; Basterra LB; Jacobs S; Brouwer N; Meerlo P; Schaafsma A; Boddeke EWGM; Eggen BJL Maternal Inflammation Induces Immune Activation of Fetal Microglia and Leads to Disrupted Microglia Immune Responses, Behavior, and Learning Performance in Adulthood. Neurobiol. Dis 2017, 106, 291–300, doi: 10.1016/j.nbd.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 145.Thangarajan R; Tantradi RR; Rai KS; Gopalakrishnan S; Perumal V Inflammation During Gestation Induced Spatial Memory and Learning Deficits: Attenuated by Physical Exercise in Juvenile Rats. J. Clin. Diagn. Res. JCDR 2015, 9, CF01–04, doi: 10.7860/JCDR/2015/12565.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vincent A; Deacon R; Dalton P; Salmond C; Blamire AM; Pendlebury S; Johansen-Berg H; Rajogopalan B; Styles P; Stein J Maternal Antibody-Mediated Dyslexia? Evidence for a Pathogenic Serum Factor in a Mother of Two Dyslexic Children Shown by Transfer to Mice Using Behavioural Studies and Magnetic Resonance Spectroscopy. J. Neuroimmunol 2002, 130, 243–247, doi: 10.1016/S0165-5728(02)00226-6. [DOI] [PubMed] [Google Scholar]
- 147.Kawi AA; Pasamanick B ASSOCIATION OF FACTORS OF PREGNANCY WITH READING DISORDERS IN CHILDHOOD. J. Am. Med. Assoc 1958, 166, 1420–1423, doi: 10.1001/jama.1958.02990120012003. [DOI] [PubMed] [Google Scholar]
- 148.Liu L; Wang J; Shao S; Luo X; Kong R; Zhang X; Song R Descriptive Epidemiology of Prenatal and Perinatal Risk Factors in a Chinese Population with Reading Disorder. Sci. Rep 2016, 6, 36697, doi: 10.1038/srep36697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Malkova NV; Yu CZ; Hsiao EY; Moore MJ; Patterson PH Maternal Immune Activation Yields Offspring Displaying Mouse Versions of the Three Core Symptoms of Autism. Brain. Behav. Immun 2012, 26, 607–616, doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yee N; Schwarting RKW; Fuchs E; Wöhr M Increased Affective Ultrasonic Communication during Fear Learning in Adult Male Rats Exposed to Maternal Immune Activation. J. Psychiatr. Res 2012, 46, 1199–1205, doi: 10.1016/j.jpsychires.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 151.Machado CJ; Whitaker AM; Smith SEP; Patterson PH; Bauman MD Maternal Immune Activation in Nonhuman Primates Alters Social Attention in Juvenile Offspring. Biol. Psychiatry 2015, 77, 823–832, doi: 10.1016/j.biopsych.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gzielo K; Potasiewicz A; Litwa E; Piotrowska D; Popik P; Nikiforuk A The Effect of Maternal Immune Activation on Social Play-Induced Ultrasonic Vocalization in Rats. Brain Sci. 2021, 11, 344, doi: 10.3390/brainsci11030344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gzieło K; Piotrowska D; Litwa E; Popik P; Nikiforuk A Maternal Immune Activation Affects Socio-Communicative Behavior in Adult Rats. Sci. Rep 2023, 13, 1918, doi: 10.1038/s41598-023-28919-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Usui N; Matsumoto-Miyai K; Koyama Y; Kobayashi Y; Nakamura Y; Kobayashi H; Shimada S Social Communication of Maternal Immune Activation-Affected Offspring Is Improved by Si-Based Hydrogen-Producing Agent. Front. Psychiatry 2022, 13, 872302, doi: 10.3389/fpsyt.2022.872302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Scott KJ; Tashakori-Sabzevar F; Bilkey DK Maternal Immune Activation Alters the Sequential Structure of Ultrasonic Communications in Male Rats. Brain Behav. Immun. - Health 2021, 16, 100304, doi: 10.1016/j.bbih.2021.100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Baharnoori M; Bhardwaj SK; Srivastava LK Neonatal Behavioral Changes in Rats with Gestational Exposure to Lipopolysaccharide: A Prenatal Infection Model for Developmental Neuropsychiatric Disorders. Schizophr. Bull 2012, 38, 444–456, doi: 10.1093/schbul/sbq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Parker SE; Lijewski VA; Janulewicz PA; Collett BR; Speltz ML; Werler MM Upper Respiratory Infection during Pregnancy and Neurodevelopmental Outcomes among Offspring. Neurotoxicol. Teratol 2016, 57, 54–59, doi: 10.1016/j.ntt.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee I; Neil JJ; Huettner PC; Smyser CD; Rogers CE; Shimony JS; Kidokoro H; Mysorekar IU; Inder TE The Impact of Prenatal and Neonatal Infection on Neurodevelopmental Outcomes in Very Preterm Infants. J. Perinatol. Off. J. Calif. Perinat. Assoc 2014, 34, 741–747, doi: 10.1038/jp.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen X; Fu Y-Y; Zhang T-Y Role of Viral Infection in Sudden Hearing Loss. J. Int. Med. Res 2019, 47, 2865–2872, doi: 10.1177/0300060519847860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vedovato S; Lo Iacono A; Morando C; Suppiej A; Orzan E; Trevisanuto D; Visentin S; Cavallin F; Chiarelli S; Zanardo V Sensorineural Hearing Loss in Very Low Birth Weight Infants with Histological Chorioamnionitis. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet 2015, 28, 895–899, doi: 10.3109/14767058.2014.936375. [DOI] [PubMed] [Google Scholar]
- 161.Suppiej A; Franzoi M; Vedovato S; Marucco A; Chiarelli S; Zanardo V Neurodevelopmental Outcome in Preterm Histological Chorioamnionitis. Early Hum. Dev 2009, 85, 187–189, doi: 10.1016/j.earlhumdev.2008.09.410. [DOI] [PubMed] [Google Scholar]
- 162.Manotas M; Sarmiento K; Ibañez-Morantes A; Suárez-Obando F; Gelvez N; López G; Ayala-Ramírez P; Angel J; Prieto J; Tamayo N; et al. Risk Factors Associated with Congenital Defects That Alter Hearing or Vision in Children Born in the City of Bogotá between 2002 and 2016. Int. J. Pediatr. Otorhinolaryngol 2019, 126, 109594, doi: 10.1016/j.ijporl.2019.109594. [DOI] [PubMed] [Google Scholar]
- 163.Rijken MJ; McGready R; Boel ME; Poespoprodjo R; Singh N; Syafruddin D; Rogerson S; Nosten F Malaria in Pregnancy in the Asia-Pacific Region. Lancet Infect. Dis 2012, 12, 75–88, doi: 10.1016/S1473-3099(11)70315-2. [DOI] [PubMed] [Google Scholar]
- 164.Fischer PR Wistar Rat-Plasmodium Berghei Model Does Not Approximate Human Congenital Malaria. J. Parasitol 1996, 82, 635–637. [PubMed] [Google Scholar]
- 165.Buchwald AG; Boudova S; Peterson I; Divala T; Mungwira R; Mawindo P; Gladstone M; Cairo C; Laufer MK The Association among Malaria in Pregnancy, Neonatal Inflammation, and Neurocognitive Development in a Cohort of Malawian Infants. Am. J. Trop. Med Hyg 2022, 107, 1036–1040, doi: 10.4269/ajtmh.22-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Weckman AM; Conroy AL; Madanitsa M; Gnaneswaran B; McDonald CR; Kalilani-Phiri L; Chandna J; Ali D; Mwapasa V; Khairallah C; et al. Neurocognitive Outcomes in Malawian Children Exposed to Malaria during Pregnancy: An Observational Birth Cohort Study. PLoS Med. 2021, 18, e1003701, doi: 10.1371/journal.pmed.1003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Garrison A; Boivin MJ; Fiévet N; Zoumenou R; Alao JM; Massougbodji A; Cot M; Bodeau-Livinec F The Effects of Malaria in Pregnancy on Neurocognitive Development in Children at 1 and 6 Years of Age in Benin: A Prospective Mother-Child Cohort. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am 2021, 74, 766–775, doi: 10.1093/cid/ciab569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bangirana P; Conroy AL; Opoka RO; Semrud-Clikeman M; Jang JH; Apayi C; Kakuru A; Muhindo MK; Georgieff MK; Dorsey GM; et al. Effect of Malaria and Malaria Chemoprevention Regimens in Pregnancy and Childhood on Neurodevelopmental and Behavioral Outcomes in Children at 12, 24, and 36 Months: A Randomized Clinical Trial. Clin. Infect. Dis 2023, 76, 600–608, doi: 10.1093/cid/ciac815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bartholomeeusen K; Daniel M; LaBeaud DA; Gasque P; Peeling RW; Stephenson KE; Ng LFP; Ariën KK Chikungunya Fever. Nat. Rev. Dis. Primer 2023, 9, 1–21, doi: 10.1038/S41572-023-00429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nyamwaya DK; Otiende M; Mwango L; Kariuki SM; Otieno B; Omuoyo DO; Githinji G; Kitsao BS; Karanja HK; Gitonga JN; et al. Incidence of Chikungunya Virus Infections among Kenyan Children with Neurological Disease, 2014–2018: A Cohort Study. PLoS Med. 2022, 19, e1003994, doi: 10.1371/journal.pmed.1003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gérardin P; Barau G; Michault A; Bintner M; Randrianaivo H; Choker G; Lenglet Y; Touret Y; Bouveret A; Grivard P; et al. Multidisciplinary Prospective Study of Mother-to-Child Chikungunya Virus Infections on the Island of La Réunion. PLOS Med. 2008,5, e60, doi: 10.1371/journal.pmed.0050060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gérardin P; Couderc T; Bintner M; Tournebize P; Renouil M; Lémant J; Boisson V; Borgherini G; Staikowsky F; Schramm F; et al. Chikungunya Virus-Associated Encephalitis: A Cohort Study on La Réunion Island, 2005–2009. Neurology 2016, 86, 94–102, doi: 10.1212/WNL.0000000000002234. [DOI] [PubMed] [Google Scholar]
- 173.Fritel X; Rollot O; Gérardin P; Gaüzère B-A; Bideault J; Lagarde L; Dhuime B; Orvain E; Cuillier F; Ramful D; et al. Chikungunya Virus Infection during Pregnancy, Réunion, France, 2006. Emerg. Infect. Dis 2010, 16, 418–425, doi: 10.3201/eid1604.091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Oliveira JRM; Gérardin P; Couderc T; Randrianaivo H; Fritel X; Lecuit M Chikungunya Virus-Associated Encephalitis: A Cohort Study on La Réunion Island, 2005-2009. Neurology 2016, 86, 2025–2026, doi: 10.1212/WNL.0000000000002732. [DOI] [PubMed] [Google Scholar]
- 175.Ramos R; Viana R; Brainer-Lima A; FloreÂncio T; Carvalho MD; van der Linden V; Amorim A; Rocha M.Aâ.; Medeiros F Perinatal Chikungunya Virus-Associated Encephalitis Leading to Postnatal-Onset Microcephaly and Optic Atrophy. Pediatr. Infect. Dis. J 2018, 37, 94–95, doi: 10.1097/INF.0000000000001690. [DOI] [PubMed] [Google Scholar]
- 176.Gérardin P; Sampériz S; Ramful D; Boumahni B; Bintner M; Alessandri J-L; Carbonnier M; Tiran-Rajaoefera I; Beullier G; Boya I; et al. Neurocognitive Outcome of Children Exposed to Perinatal Mother-to-Child Chikungunya Virus Infection: The CHIMERE Cohort Study on Reunion Island. PLoS Negl. Trop. Dis 2014, 8, e2996, doi: 10.1371/journal.pntd.0002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Waechter R; Ingraham E; Evans R; Cudjoe N; Krystosik A; Isaac R; Watts A; Noël T; Landon B; Fernandes M; et al. Pre and Postnatal Exposure to Chikungunya Virus Does Not Affect Child Neurodevelopmental Outcomes at Two Years of Age. PLoS Negl. Trop. Dis 2020, 14, e0008546, doi: 10.1371/journal.pntd.0008546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Okello G; Gerrets R; Zakayo S; Molyneux S; Jones C “Every Day They Keep Adding New Tools but They Don’t Take Any Away”: Producing Indicators for Intermittent Preventive Treatment for Malaria in Pregnancy (IPTp) from Routine Data in Kenya. PLOS ONE 2018, 13, e0189699, doi: 10.1371/journal.pone.0189699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Healy SA; Fried M; Richie T; Bok K; Little M; August A; Riley L; Swamy GK; Wylie BJ; Menendez C; et al. Malaria Vaccine Trials in Pregnant Women: An Imperative without Precedent. Vaccine 2019, 37, 763–770, doi: 10.1016/j.vaccine.2018.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Duffy PE; Healy S; Gorres JP; Fried M Chapter 14 - Malaria. In Maternal Immunization; Leuridan EE, Nunes MC, Jones CE, Eds.; Academic Press, 2020; pp. 321–337 ISBN 978-0-12-814582-1. [Google Scholar]
- 181.Gamain B; Chêne A; Viebig NK; Tuikue Ndam N; Nielsen MA Progress and Insights Toward an Effective Placental Malaria Vaccine. Front. Immunol 2021, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Couderc T; Khandoudi N; Grandadam M; Visse C; Gangneux N; Bagot S; Prost J-F; Lecuit M Prophylaxis and Therapy for Chikungunya Virus Infection. J. Infect. Dis 2009, 200, 516–523, doi: 10.1086/600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Edlow AG; Castro VM; Shook LL; Kaimal AJ; Perlis RH Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 During Pregnancy. JAMA Netw. Open 2022, 5, e2215787, doi: 10.1001/jamanetworkopen.2022.15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shuffrey LC; Firestein MR; Kyle MH; Fields A; Alcantara C; Amso D; Austin J; Bain JM; Barbosa J; Bence M; et al. Association of Birth During the COVID-19 Pandemic With Neurodevelopmental Status at 6 Months in Infants With and Without In Utero Exposure to Maternal SARS-CoV-2 Infection. JAMA Pediatr. 2022, 176, e215563, doi: 10.1001/jamapediatrics.2021.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Scharf RJ; Scharf GJ; Stroustrup A Developmental Milestones. Pediatr. Rev 2016, 37, 25–38, doi: 10.1542/pir.2014-0103. [DOI] [PubMed] [Google Scholar]