With effective treatment options, #sexualhealth for female cancer survivors warrants inclusion in care.
Abstract
PURPOSE
The objectives of this narrative review are to describe (1) the evidence for interventions addressing four key issues affecting female sexual health in cancer populations (ie, low sexual desire, vulvovaginal symptoms, negative body image, and sexual partner relationships) that are ready or nearly ready for integration into practice and (2) the current state of patient-provider sexual health communication related to female sexual health as these findings could have implications for integrating sexual health into practice.
METHODS
A narrative review of recent intervention evidence for female cancer survivors' sexual health was conducted.
RESULTS
Strong evidence was found for behavioral interventions, such as psychosexual counseling and psychoeducation to treat concerns related to sexual health, including desire, body image, and sexual partner relationships. For partnered female survivors, couple-based psychosexual interventions have been found to be effective. There are no proven pharmacologic treatments for sexual-related concerns other than for vulvovaginal atrophy in female cancer survivors. Vaginal nonhormonal and low-dose hormonal agents are effective remedies for vulvovaginal symptoms. Laser treatment has not yet been fully evaluated. Sexual partners are a critical context for sexual health. Despite much need, discussions around this topic continue to be relatively infrequent. Recent technology-based interventions show promise in improving discussions around sexual health.
CONCLUSION
Effective interventions exist for many sexual health challenges for female survivors although more high-quality intervention research, particularly multimodal interventions, is needed. Many of the effective interventions are nonpharmacologic, and thus, evaluation of the use of digital delivery to improve access to these interventions is needed. Cancer care delivery research is urgently needed to translate existing effective interventions into practice, including strategies to improve patient-provider communication around this topic.
INTRODUCTION
Despite decades of research and numerous review articles, sexual health continues to be a substantially underappreciated and undertreated problem in female cancer survivors. 1 According to the WHO, sexual health is a fundamental part of overall health and well-being, with relevance not only for individuals and couples but also for the social and economic development of communities and countries. 2 Furthermore, sexual function is included in established guidelines for cancer care alongside other physical side effects of cancer treatment (eg, pain, fatigue). 3,4 Despite care guidelines for sexual health, effective interventions are not a regular part of standard care and efforts to routinely assess and treat sexual health concerns are needed. 1,5 In short, sexual health is a vital aspect of public health and care that warrants clinical attention.
CONTEXT
Key Objective
What is the evidence for interventions addressing key issues (low sexual desire, vulvovaginal atrophy [VVA], negative body image, and intimate partner relationships) of sexual health that are ready for integration into practice, and how well does patient-provider communication occur?
Knowledge Generated
Evidence supports primarily behavioral interventions for aspects of sexual health other than VVA, where pharmacologic treatments are effective. Discussions around sexual health continue to be infrequent, and technology-based interventions show promise in increasing these discussions.
Relevance (C. Zimmermann)
Discussions around sexual health may be facilitated by educational interventions directed at both patients and providers. High-quality research is needed to develop and test effective sexual health interventions that can be broadly disseminated.*
*Relevance section written by JCO Associate Editor Camilla Zimmermann, MD, PhD, FRCPC.
Sexual health concerns can be similar among female cancer survivors across different types of cancers although the prevalence of various components can vary by type of cancer 6-10 and specific needs vary by individual. Treatments to control cancer, especially hormone deprivation, can bring about changes in the vaginal tissue and women's skin more generally, as well as changes to bodily structures and function (including neurologic), and chronic morbidities such as fatigue, nerve damage, vulvovaginal atrophy (VVA), and lymphedema. 1 These effects can result in negative body image, loss of desire, loss of arousal, dyspareunia, and intimacy communication challenges. 1,6,7 According to Basson’s 11 biopsychosocial model of female sexual health, these effects are inter-related and affect overall female sexual health. In addition, findings from studies in cancer survivors support four main predictors of female sexual health: low sexual desire, vulvovaginal symptoms, negative body image, and sexual partner relationships. 12-16
A recent meta-analysis of observational studies that included at least 30 female cancer survivors and used the established cutoff score for dysfunction of 26.55 or less on the Female Sexual Function Index (FSFI) found over 60% of survivors across 15 different countries and at least four types of cancers had sexual function scores in the dysfunctional range. 7 Across studies, mean FSFI scores ranged from 14.6 to 23.39. The high prevalence of sexual dysfunction was supported by findings from a more recent review and meta-analysis, again with female cancer survivors with diverse cancer types, across at least 10 different countries. In over 5,400 women, the prevalence of female sexual dysfunction using the FSFI was 66%, with the highest prevalence in Africa and the lowest in Europe. 17 These studies indicate that sexual function is impaired in female cancer survivors throughout the world; however, rates of dysfunction must be interpreted cautiously because the FSFI can inflate rates of sexual dysfunction in those who are not sexually active, and these studies included both sexually active and inactive survivors. 18
Gaps remain in understanding sexual health needs of populations who are under-represented in the literature such as racial and ethnic minorities, sexual and gender minorities, and unpartnered women. There is some evidence that Black, Hispanic, and sexual minority women might have higher rates of sexual dysfunction compared with White women and heterosexual women. 19-21 Other research suggests that sexual minority women, when compared with heterosexual women, might have fewer issues with sexual identity and be less likely to have breast reconstruction surgery, which may correlate with less body image distress. 22 Unpartnered female cancer survivors have been found to have significant sexual problems that negatively affect their quality of life although sexual concerns of this population are often missed clinically. 23 Importantly, unpartnered women report interest in sexual health–related interventions. 5 Research is just starting to assess and identify gaps in knowledge related to the sexual health needs of these under-represented populations.
The greatest need in expanding the science of sexual health lies in the development and testing of effective interventions that can be broadly disseminated, and equally importantly, to explore how sexual health assessment and effective interventions can be integrated into cancer care delivery. The objectives of this narrative review are to describe (1) the evidence for interventions addressing four key issues affecting female sexual health in cancer populations (ie, low sexual desire, vulvovaginal symptoms, negative body image, and sexual partner relationships) that are ready or nearly ready for integration into practice and (2) the current state of patient-provider sexual health communication related to female sexual health since findings could have implications for integrating sexual health into practice. Although each component of sexual health is discussed separately, it is critical to understand that they affect each other, having both overlapping and unique etiologies. Therefore, female cancer survivors could be experiencing several issues, and a tiered or multicomponent, multimodal treatment approach may therefore be needed.
METHODS
The authors conducted a narrative review of the intervention literature for female cancer survivors' sexual health from diagnosis through cancer treatment and beyond for the four key issues described above. These topics were chosen because of their high prevalence and distressing nature. 1 The authors (N.A., D.L.B., J.B.R.) conducted searches in PubMed and Embase for the four sexual health concepts (sexual desire, vulvovaginal symptoms, negative body image, and intimate partner relationships) and sexual health communication related to female sexual health in cancer. In addition, the authors conducted hand searches of relevant articles’ reference lists and cited reference searches. Studies were limited to those in English, which were published since 2017, and clinical trials with preference given to randomized controlled trials.
RESULTS
Sexual Desire
Low sexual desire is defined as the loss of motivation for sexual activity. 24 It is one of the most commonly reported sexual issues among female cancer survivors. 25 As a multifactorial construct, it can have contributing factors including those that are biologic (eg, hormones, poor sleep), psychological (eg, depression, anxiety), interpersonal (eg, relationship issues), and cultural (eg, cultural and societal messages about sex for individuals with a medical condition). 26 Factors associated with low sexual desire in female cancer survivors include the type of cancer treatment, poor body image, general bodily pain, and adjuvant endocrine therapy. 25,27 Furthermore, according to Basson’s 11 intimacy-based cyclical model of female sexual response, when female cancer survivors experience pain and discomfort during sexual activity, this can significantly hamper motivation to engage in future activity, to avoid painful sex.
There are no proven efficacious pharmacologic agents for low sexual desire in female cancer survivors. Bupropion, a dopamine-norepinephrine reuptake inhibitor, was recently tested as an intervention to improve low sexual desire in female cancer survivors but did not report significant improvement when compared with placebo. 28 However, over 90% of women in that study reported vulvovaginal symptoms, which, if unaddressed, can negatively affect desire in that survivors are unlikely to desire sexual activity that is uncomfortable or painful. This might have limited the study's ability to fully assess bupropion's impact on sexual desire. There are two US Food and Drug Administration (FDA)–approved pharmacologic agents for hypoactive sexual desire disorder in premenopausal women: flibanserin and bremelanotide. 29,30 Neither flibanserin nor bremelanotide have been tested in female cancer survivors, and therefore, their risk/benefits are unknown for this population; however, flibanserin is currently being evaluated in an open-label observational study of females with a history of breast cancer who are taking tamoxifen or aromatase inhibitors with results expected later this year. 31 Cost is a potential barrier for both these pharmacologics as they may not be covered by insurance. 32
Recommendations for psychosexual counseling for female cancer survivors continue to be supported with four recent studies evaluating interventions such as psychological assessments to tailor their intervention, counseling, and sexual education demonstrating positive effects. 33-36 Two of these interventions used remote delivery formats of the intervention including WeChat, 34 a popular social media platform, and another delivering cognitive behavioral therapy (CBT) via the internet with e-mail support from a therapist. 33 One was a multidisciplinary study conducted in female cervical cancer survivors that was a 4-week nurse-led positive psychology intervention that included counseling on sexual psychological and physiological rehabilitation, couples’ sexual communication, and goal setting that was delivered by specialist nurses, gynecologists, psychological counselors, and physiotherapists. 34 While partners were not enrolled in the study, they were encouraged to participate in the couples’ sexual counseling. In addition, WeChat was used to communicate with participants and deliver education. Both overall sexual function and sexual desire significantly improved in the intervention group when compared with controls. 34 All four psychosexual counseling studies used either wait list or standard-of-care controls as the intervention comparator. Future trials of psychosexual counseling interventions could use stronger controls like an attention control which would enable the evaluation of nonspecific effects of general provider-centered interactions. However, there are potential ethical issues with attention controls, especially if they are designed to not address the expressed need of the participants. One solution to this is to provide the intervention to participants in the control arm once all data are collected although a delay in treatment could be problematic particularly in those with advanced disease. In addition, while psychosocial interventions are often considered minimal risk, none of the new studies reported whether they had adverse events, which makes it difficult to assess risk of harm. 33-36
Vulvovaginal Issues
Changes from chemotherapy and radiation therapy and/or estrogen deprivation in the vulvovaginal area can result in vaginal shortening and stenosis, tissue atrophy including thinning and loss of elasticity, fibrosis, and telangiectasias (small dilated blood vessels), all contributing to sexual problems such as a lack of lubrication, dryness, and pain with intercourse. 1,37,38 Arguably of all sexual health concerns, the largest amount of published intervention data is in treating vulvovaginal symptoms. 3,4,39
Nonhormonal topical agents include lubricants, moisturizers, and hyaluronic acid (HA). Polycarbophil-based vaginal moisturizers and HA-based moisturizers are often considered first-line nonhormonal treatments in reviews 1,39,40 and guidelines. 3,4 Efficacy of vaginal moisturizers comes from studies where they serve as a control for trials of other pharmaceutical interventions, and several of these studies have found no differences in patient-reported outcomes around symptoms of VVA when compared with HA or topical hormonal agents. 40-42 Important information around the use of vaginal moisturizers includes the use of hydrating products, frequent use (nightly), for a sufficient time (up to 12 weeks for initial results), inclusion of the vulvar vestibule and labia in moisturizing, and extended contact with the mucosa (use overnight after any sexual activity). 40 This area is ripe for well-designed comparative effectiveness trials with diverse female cancer survivors and trials designed for early intervention for prevention.
HA has a mechanism of action unique from polycarbophil-based moisturizers. 43 HA is normally present in the vaginal epithelium. The use of HA in the vagina directly results in water retention, and at least one study demonstrated maintenance of moisture and improved elasticity in vaginal epithelial tissue. 44 However, blinded, randomized controlled trials of sufficient sample sizes are lacking and there are no blinded randomized controlled trials in female cancer survivors, despite an open-label report showing benefit. 45
One study examined the use of 4% lidocaine applied to the vulvar vestibule compared with physiologic saline in breast cancer survivors who reported penetrative dyspareunia. 46 All participants also received a silicone lubricant. Participants in the lidocaine/silicone group reported significantly less pain and improved arousal at 4 weeks compared to the control group. Sexual distress scores improved significantly in both groups by 4 weeks. Adverse events were minimal and included a rash attributed to the silicone lubricant, and one participant experienced a tear at her introitus. Lidocaine could be an option for dyspareunia although it is not likely to directly improve tissue health. Therefore, if a woman has moderate to severe VVA, it may be that lidocaine would need to be accompanied by the use of a nonhormonal vaginal moisturizing agent.
Laser treatment has received significant attention for VVA recently. There are two types of lasers that have been studied to address VVA, erbium yttrium aluminum garnet and carbon dioxide (CO2), with the latter being the more widely studied of the two. 39,47 Most studies in female cancer survivors have been criticized as having a high risk of bias, 48 and in fact, several studies included nonblinded provider-graded outcomes. 39 There is only one randomized, sham controlled pilot trial of a CO2 laser in female cancer survivors, 49 and this trial closed to accrual early because of the issuance of a warning by the FDA on the use of laser treatment for genitourinary syndrome of menopause. 50 The warning was related to adverse effects such as vaginal burns, scarring, dyspareunia, and chronic pain. 50 There was no statistical difference in the primary end point, the Vaginal Assessment Scale, reported for the 10 women in the laser treatment versus eight in the sham arm 49 although the lack of a significant finding is equivocal in light of the small sample size. Further testing of laser treatment is questionable given a well-powered, well-designed sham controlled randomized trial with 85 women with menopause (not female cancer survivors) where laser treatment did not positively affect the coprimary outcomes of a visual analog scale for vulvovaginal symptoms and the Vulvovaginal Symptom Questionnaire. 51 In addition, there were no significant differences in the Vaginal Health Index, mean quality-of-life scores, or histologic characteristics. 51
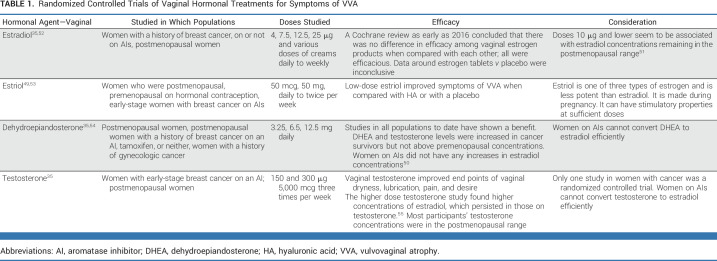
Hormonal options evaluated for the treatment of VVA include estradiol, estriol, testosterone, and dehydroepiandrosterone. The research in cancer survivors, particularly those with a history of breast cancer, has primarily focused on the lowest-dose or lower-potency estrogen. Table 1 describes recent key randomized controlled trials in this area. 40,52-58 Many of the studies that include sex steroid hormone concentration measures demonstrate a slight systemic increase initially, which then decreases. In studies with lower doses, concentrations often remain in the postmenopausal range. Unfortunately, there are no definitive data to inform women or clinicians on decision making about the amount of sex steroid hormone supplementation (oral or vaginal) that confers no risk to any given survivor. Hence, most guidelines written by oncology-related professionals recommend the use of low-dose hormonal agents only if other options are not helpful after a comprehensive discussion of risk versus benefits. 3,4
TABLE 1.
Randomized Controlled Trials of Vaginal Hormonal Treatments for Symptoms of VVA
Sexual rehabilitation interventions including psychoeducation, dilators, and pelvic floor muscle training with core strengthening and/or yoga have limited data in female cancer survivors but appear to be promising. 59-61 More research is needed to define frequency, duration, and practice integration.
Body Image
Body image is one's self-perception and beliefs about appearance, bodily sensations, and function. 62,63 Body image has been found to be predictors of anxiety, depression, sexual concerns, and shorter length of survival in female cancer survivors. 64 Factors associated with a negative body image in female cancer survivors include younger age, type of cancer treatment, being premenopausal, and bowel and bladder incontinence. 62,63,65
Recent data provide evidence for the effectiveness of psychosexual counseling for female cancer survivors. Six studies evaluating CBT, 33,64,66 couples-based sexual education and counseling, 67 self-healing training, 68 and group therapy with guided imagery found benefit. 69 Two of the psychosexual counseling interventions used remote delivery for their CBT intervention. One study used an internet-based CBT intervention that consisted of up to 24 weekly sessions with either a psychologist or a sexologist, internet-based CBT sexual health modules, and homework assignments designed to enhance acquisition of coping skills. 33 Partners were encouraged to participate but not required. The intervention improved both sexual desire and body image in female breast cancer survivors compared with controls. 33 A second study included 44 patients with head and neck cancer (61% female). The intervention consisted of five weekly CBT sessions with a psychologist via a telemedicine video platform (tele-CBT), workbook, and educational materials that included psychoeducation, self-monitoring, cognitive restructuring, coping strategies, and relapse prevention topics related to body image. 66 Compared with an attention control group, those participants in the tele-CBT group reported significantly less body image distress with a large effect size. 66 While these studies offer support for psychosexual counseling, none of the new psychosexual counseling studies reported adverse events. Access because of a lack of insurance coverage or knowledgeable professionals continues to be a challenge for counseling-based interventions.
Psychoeducation, group physical activity, and expressive writing are promising interventions for negative body image in female cancer survivors. One such intervention started normalizing and supporting body changes early in cancer care by using a psychoeducation self-care program that began in the inpatient setting during cancer treatment for patients with colorectal cancer. 70 In addition, group physical activities like belly dancing and mat Pilates have shown improvements in body image in female cancer survivors when compared with controls; however, similar to other physical activity interventions, they showed high attrition rates (30%). 71 An expressive writing intervention used a one-time, web-based, 30-minute structured writing exercise focused on self-compassionate attitudes demonstrating significant improvements in body image in female breast cancer survivors when compared with an attention control, which were sustained through the 3-month follow-up. 72 The group physical activity study reported no adverse events related to the interventions. 71 Neither the psychoeducational intervention nor expressive writing intervention reported on adverse events. Although promising, more evidence is needed before their adoption into clinical practice.
Relationship Context
For female cancer survivors in a partnered relationship, cancer-related sexual problems most often take place within the context of partnered sexual activity, making it critical to consider the relational context and the role of the partner when approaching such problems for these women. Recent frameworks of dyadic (couple-based) approaches to cancer outcomes recognize patient characteristics, relationship characteristics, and partner characteristics as both influencing and being influenced by sexuality and intimacy. 73 For example, in research involving breast cancer survivors and their sexual partners, couples reporting better relationship functioning (eg, emotional closeness, affectionate behaviors) have a greater likelihood of staying sexually active during the period after cancer treatment completion 74 or reporting greater sexual satisfaction. 75
Partners' experiences within the sexual relationship play an important role in women's experiences regarding their cancer-related sexual problems. For example, partner’s lack of interest in sex is a common reason for sexual inactivity among female cancer survivors, 76 and sexual problems in the survivors and their partners often co-occur. 27 In breast cancer survivors who had completed cancer treatment and met criteria for sexual dysfunction, two thirds of the male partners had erectile dysfunction, 27 a higher rate than the general male population. 77 In the same study, lower sexual satisfaction in partners was significantly associated with worse sexual function, arousal, and satisfaction in the survivors, whereas poorer sexual function in the partners was associated with worse sexual pain in the survivors. 27 As was recently shown in a qualitative study of patients with metastatic breast cancer and their partners, partners can face conflicting feelings about engaging in sex when their partners have sexual problems such as feeling guilty for initiating sex if their partners have pain during intercourse. 78
In light of the critical role of the relational context and partner's influence in women's sexual function after cancer treatment, partners should be considered for inclusion in approaches to management of these issues, 27 as excluding partners from clinical management could compromise efforts to address sexual health concerns. For instance, partners may harbor beliefs about the use of sexual aids or other strategies that could compromise the use of such aids in managing patients' sexual problems (eg, that using artificial lubricant feels unnatural). 78 By contrast, including the partner in coping efforts may bolster the success of such efforts, 79 which may be one reason why couple-based interventions are often effective in addressing sexual concerns for female cancer patients. 80-83 Including partners in such discussions is consistent with the preferences of female patients 84,85 with cancer and with guidelines from ASCO from 2017, which state that discussions about sexual function should be initiated by the health care team and could include the partner if the patient wishes. 3
Evidence from systematic reviews demonstrates that couple-based psychosexual interventions are efficacious in addressing women's sexual concerns after a cancer diagnosis. 81,82 The content of couple-based psychosexual interventions usually includes educational content (eg, on effects of the cancer on sexuality and sexual response) and training in skills for coping with sexual concerns such as communication and physical intimacy (eg, sensate focus or nonjudgmental sensual touching), 86 taught to both the survivor and the partner. 82 There are two rigorous ongoing trials of couple-based psychosexual interventions. 80,87 Previously tested in pilot trials, 83,88 these interventions are distinguished by a foundation within formative qualitative patient-centered work, 89,90 use of technology-based formats (ie, virtual teleconference or telephone), content designed to address a range of sexual function concerns, and inclusive eligibility criteria (eg, with respect to sexual minority couples). Furthermore, these trials have rigorous methodological design, including ample sample sizes and attention control conditions, elements that were often missing from previous trials of similar interventions. 82 If shown to be effective, these interventions have strong promise for widespread dissemination. Yet, couple-based interventions have limitations. For instance, unpartnered women cannot benefit and the couple-based nature makes it appropriate only for survivors whose partners are willing to participate. In addition, couple-based interventions require a trained counselor to administer them, which could limit broad dissemination. A self-management approach that is flexible to engage couples or singles would address these limitations.
Barriers to Addressing Sexual Concerns Clinically and Paths Forward
Despite the prevalence and severity of sexual concerns, inclusion of interventions for sexual function in clinical guidelines, and consistent data demonstrating that the majority of patients diagnosed with cancer want clinical discussions of sexual health to be included in their care, 91-93 such discussions in cancer care continue to be relatively infrequent. 94 Findings from a widely cited 2017 systematic review of studies examining the prevalence of sexual health discussions in cancer 95 revealed a striking disparity in discussions of sexual health by sex, whereas 60% of male cancer survivors reported receiving information about potential sexual side effects of their cancer treatments, fewer than half as many female survivors (28%) reported receiving the same information. In female cancer populations, fertility and reproductive concerns were more likely to be discussed than sexual function. 95
Rates of receiving information about sexual issues related to cancer treatment as low as 20% have been found for patients with breast cancer 96 ; data from other populations including gynecologic cancer, 97 head and neck cancer, 92 and hematopoietic cell transplantation 98 suggest similar challenges in these populations as well. The lack of communication has the potential to come at a significant detriment to patients' health-related quality of life; for instance, a recent study found that only 40% of women with vaginal atrophy related to breast cancer treatment recalled receiving a referral or intervention for this medical problem. 99
Substantial barriers constrain oncology clinicians' communication about sexual concerns, such that while >80% of clinicians believe that it is their professional obligation to discuss sexual concerns, fewer than 20% routinely hold such discussions in their practice. 100 Perhaps the single most significant barrier constraining communication about sexual concerns is the lack of sufficient training around how to discuss such concerns effectively. 98,101 However, certain beliefs commonly held by cancer clinicians may be exacerbating the hesitancy to raise such discussions (eg, conversations would take too long for routine clinic visits; patients will be embarrassed; older patients, unpartnered patients, or patients with advanced cancer do not have significant sexual health needs). 102-104 Furthermore, discomfort in discussing sexual health is often detectible by patients, 97 who face their own challenges in raising sexual health with their clinicians, including a lack of preparation or comfort in what to say or how to ask. 97,105
Efforts to increase clinical communication and care about sexual health concerns are taking shape. 101 In light of the significant barriers to patient-clinician communication about sexual health for women with cancer, some researchers have argued that a multipronged approach, consisting of clinician-focused and patient-focused interventions, may be needed to make substantive gains in this regard. 94,106
In the past 6 years, technology-supported interventions have shown promise in improving communication around sexual health. One study randomly assigned 144 breast cancer survivors to receive a list of resources on sexual and menopausal health (control arm) or a brief (20-minute) educational video and accompanying skills practice workbook (intervention arm). Participants in the intervention arm were significantly more likely to raise the topic of sexual health and to ask about sexual health at their next clinic encounters, which were audio recorded and coded for sexual health communication. 5 Women in the intervention arm were also more likely to be sexually active and had lower anxiety than those in the control arm at the 2-month follow-up, suggesting that educating female cancer survivors on how to raise sexual concerns effectively with their clinicians could lead to productive discussions and solutions for sexual problems. Furthermore, the same researchers adapted educational content from a workshop-based intervention for breast cancer clinicians to a technology-based (mLearning) intervention featuring an educational podcast series. 107 The intervention was well-received among clinicians and showed promise at bolstering clinicians' comfort in discussing sexual health concerns with their patients. 107 Findings from this pilot study suggested that a technology-based format, which allows clinicians to use educational materials in their own time, could be a fruitful means of disseminating sexual health information very widely to clinicians; efficacy testing is needed.
Overall, the growing efforts to integrate sexual health into the clinical care of women with cancer are encouraging although many questions remain of import, including how to achieve long-term openness in communication and optimal dissemination of effective interventions.
DISCUSSION
There are limitations associated with a narrative review, as opposed to a systematic review. Selection bias is possible since each author conducted a search on their topic areas with the intent to include recent randomized controlled trials ready for integration into practice. Early phase and smaller trials were not included unless there was a dearth of evidence in an area. In addition, we limited our review to publications written in English. This could pose a language bias; however, recent meta-analyses have demonstrated that limiting reviews to the English language did not affect review conclusions. 108,109
There is evidence that sexual health challenges exist for a wide variety of female cancer survivors and growing evidence that effective interventions exist for many of these challenges although more high-quality intervention research and multimodal intervention research are needed. As many of the effective interventions are nonpharmacologic in nature, the evaluation of the use of digital delivery to improve access to these types of interventions could potentially help to integrate these findings into practice.
Important knowledge gaps exist in better understanding the needs of populations from diverse backgrounds including unpartnered women. Cancer care delivery research is urgently needed to translate existing effective interventions into practice, including strategies to improve patient-provider communication around this topic.
Noël Arring
Employment: Beachbody
Debra L. Barton
Research Funding: Merck
No other potential conflicts of interest were reported.
SUPPORT
Supported by the Breast Cancer Research Foundation.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Clinical Practice Strategies to Address Sexual Health in Female Cancer Survivors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Noël Arring
Employment: Beachbody
Debra L. Barton
Research Funding: Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1. Smith T, Kingsberg SA, Faubion S. Sexual dysfunction in female cancer survivors: Addressing the problems and the remedies. Maturitas. 2022;165:52–57. doi: 10.1016/j.maturitas.2022.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Wordl Health Organization https://www.who.int/health-topics/sexual-health#tab=tab_1 Health topics: Sexual health. World Health Organization.
- 3. Carter J, Lacchetti C, Andersen BL, et al. Interventions to address sexual problems in people with cancer: American Society of Clinical Oncology Clinical Practice Guideline Adaptation of Cancer Care Ontario Guideline. J Clin Oncol. 2018;36:492–511. doi: 10.1200/JCO.2017.75.8995. [DOI] [PubMed] [Google Scholar]
- 4. Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: Consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596–608. doi: 10.1097/GME.0000000000001121. [DOI] [PubMed] [Google Scholar]
- 5. Reese JB, Sorice KA, Pollard W, et al. Efficacy of a multimedia intervention in facilitating breast cancer patients' clinical communication about sexual health: Results of a randomized controlled trial. Psychooncology. 2020;30:681–690. doi: 10.1002/pon.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wettergren L, Eriksson LE, Bergstrom C, et al. Prevalence and risk factors for sexual dysfunction in young women following a cancer diagnosis—A population-based study. Acta Oncol. 2022;61:1165–1172. doi: 10.1080/0284186X.2022.2112283. [DOI] [PubMed] [Google Scholar]
- 7. Maiorino MI, Chiodini P, Bellastella G, et al. Sexual dysfunction in women with cancer: A systematic review with meta-analysis of studies using the Female Sexual Function Index. Endocrine. 2016;54:329–341. doi: 10.1007/s12020-015-0812-6. [DOI] [PubMed] [Google Scholar]
- 8. Han CJ, Yang GS, Syrjala K. Symptom experiences in colorectal cancer survivors after cancer treatments: A systematic review and meta-analysis. Cancer Nurs. 2020;43:E132–E158. doi: 10.1097/NCC.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gjaerde LK, Eeltink C, Stringer J, et al. Sexual function of adult long-term survivors and their partners after allogeneic hematopoietic cell transplantation in Europe (S-FAST): A study from the Transplant Complications Working Party and Nurses Group of the EBMT. Bone Marrow Transpl. 2023;58:195–202. doi: 10.1038/s41409-022-01869-2. [DOI] [PubMed] [Google Scholar]
- 10. Bessa A, Martin R, Haggstrom C, et al. Unmet needs in sexual health in bladder cancer patients: A systematic review of the evidence. BMC Urol. 2020;20:64. doi: 10.1186/s12894-020-00634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Basson R. Female sexual response: The role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98:350–353. doi: 10.1016/s0029-7844(01)01452-1. [DOI] [PubMed] [Google Scholar]
- 12. Ganz PA, Desmond KA, Belin TR, et al. Predictors of sexual health in women after a breast cancer diagnosis. J Clin Oncol. 1999;17:2371–2380. doi: 10.1200/JCO.1999.17.8.2371. [DOI] [PubMed] [Google Scholar]
- 13. Gilbert E, Ussher JM, Perz J. Sexuality after breast cancer: A review. Maturitas. 2010;66:397–407. doi: 10.1016/j.maturitas.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 14. Gillen MM, Markey CH. A review of research linking body image and sexual well-being. Body Image. 2019;31:294–301. doi: 10.1016/j.bodyim.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 15. Lewis-Smith H, Diedrichs PC, Rumsey N, et al. Efficacy of psychosocial and physical activity-based interventions to improve body image among women treated for breast cancer: A systematic review. Psychooncology. 2018;27:2687–2699. doi: 10.1002/pon.4870. [DOI] [PubMed] [Google Scholar]
- 16. Woertman L, van den Brink F. Body image and female sexual functioning and behavior: A review. J Sex Res. 2012;49:184–211. doi: 10.1080/00224499.2012.658586. [DOI] [PubMed] [Google Scholar]
- 17. Esmat Hosseini S, Ilkhani M, Rohani C, et al. Prevalence of sexual dysfunction in women with cancer: A systematic review and meta-analysis. Int J Reprod Biomed. 2022;20:1–12. doi: 10.18502/ijrm.v20i1.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baser RE, Li Y, Carter J. Psychometric validation of the Female Sexual Function Index (FSFI) in cancer survivors. Cancer. 2012;118:4606–4618. doi: 10.1002/cncr.26739. [DOI] [PubMed] [Google Scholar]
- 19. Frimer M, Turker LB, Shankar V, et al. The association of sexual dysfunction with race in women with gynecologic malignancies. Gynecol Oncol Rep. 2019;30:100495. doi: 10.1016/j.gore.2019.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boehmer U, Ozonoff A, Timm A, et al. After breast cancer: Sexual functioning of sexual minority survivors. J Sex Res. 2014;51:681–689. doi: 10.1080/00224499.2013.772087. [DOI] [PubMed] [Google Scholar]
- 21. Boehmer U, Timm A, Ozonoff A, et al. Explanatory factors of sexual function in sexual minority women breast cancer survivors. Ann Oncol. 2012;23:2873–2878. doi: 10.1093/annonc/mds099. [DOI] [PubMed] [Google Scholar]
- 22. Kamen C. “Sex can be a great medicine”: Sexual health in oncology care for sexual and gender minority cancer patients. Curr Sex Health Rep. 2020;12:320–328. doi: 10.1007/s11930-020-00285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dorfman CS, Arthur SS, Kimmick GG, et al. Partner status moderates the relationships between sexual problems and self-efficacy for managing sexual problems and psychosocial quality-of-life for postmenopausal breast cancer survivors taking adjuvant endocrine therapy. Menopause. 2019;26:823–832. doi: 10.1097/GME.0000000000001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holloway V, Wylie K. Sex drive and sexual desire. Curr Opin Psychiatry. 2015;28:424–429. doi: 10.1097/YCO.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 25. Luo F, Link M, Grabenhorst C, et al. Low sexual desire in breast cancer survivors and patients: A review. Sex Med Rev. 2022;10:367–375. doi: 10.1016/j.sxmr.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 26. Kingsberg SA, Althof S, Simon JA, et al. Female sexual dysfunction—Medical and psychological treatments, committee 14. J Sex Med. 2017;14:1463–1491. doi: 10.1016/j.jsxm.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 27. Hummel SB, Hahn DEE, van Lankveld JJDM, et al. Factors associated with specific diagnostic and statistical manual of mental disorders, fourth edition sexual dysfunctions in breast cancer survivors: A study of patients and their partners. J Sex Med. 2017;14:1248–1259. doi: 10.1016/j.jsxm.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 28. Barton DL, Pugh SL, Ganz PA, et al. Randomized controlled phase II evaluation of two dose levels of bupropion versus placebo for sexual desire in female cancer survivors: NRG-CC004. J Clin Oncol. 2022;40:324–334. doi: 10.1200/JCO.21.01473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Food and Drug Adminstration https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-hypoactive-sexual-desire-disorder-premenopausal-women FDA approves new treatment for hypoactive sexual desire disorder in premenopausal women.
- 30. Edinoff AN, Sanders NM, Lewis KB, et al. Bremelanotide for treatment of female hypoactive sexual desire. Neurol Int. 2022;14:75–88. doi: 10.3390/neurolint14010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldfarb S, Carter J.https://clinicaltrials.gov/ct2/show/NCT03707340?term=sexual+desire&cond=Cancer&draw=1&rank=8 A study of flibanserin in breast cancer survivors on tamoxifen or aromatase inhibitors.
- 32.https://www.drugs.com/price-guide/vyleesi Vyleesi prices, coupons and patient assistance programs.
- 33. Hummel SB, van Lankveld J, Oldenburg HSA, et al. Efficacy of internet-based cognitive behavioral therapy in improving sexual functioning of breast cancer survivors: Results of a randomized controlled trial. J Clin Oncol. 2017;35:1328–1340. doi: 10.1200/JCO.2016.69.6021. [DOI] [PubMed] [Google Scholar]
- 34. Shi Y, Cai J, Wu Z, et al. Effects of a nurse-led positive psychology intervention on sexual function, depression and subjective well-being in postoperative patients with early-stage cervical cancer: A randomized controlled trial. Int J Nurs Stud. 2020;111:103768. doi: 10.1016/j.ijnurstu.2020.103768. [DOI] [PubMed] [Google Scholar]
- 35. Du J, Tian Y, Dan X, et al. The effect of empowerment education-based nursing intervention on the postoperative sexual function and depression state of cervical cancer patients of reproductive age. Int J Clin Exp Med. 2020;13:4052–4061. [Google Scholar]
- 36. Fatehi S, Maasoumi R, Atashsokhan G, et al. The effects of psychosexual counseling on sexual quality of life and function in Iranian breast cancer survivors: A randomized controlled trial. Breast Cancer Res Treat. 2019;175:171–179. doi: 10.1007/s10549-019-05140-z. [DOI] [PubMed] [Google Scholar]
- 37. Chan M, Olson R, Lapointe V, et al. Using a weekly patient-reported outcome questionnaire to track acute toxicity in patients undergoing pelvic radiotherapy for gynecologic cancers. Curr Oncol. 2022;29:3306–3317. doi: 10.3390/curroncol29050270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barcellini A, Dominoni M, Dal Mas F, et al. Sexual health dysfunction after radiotherapy for gynecological cancer: Role of physical rehabilitation including pelvic floor muscle training. Front Med (Lausanne) 2021;8:813352. doi: 10.3389/fmed.2021.813352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mension E, Alonso I, Castelo-Branco C. Genitourinary syndrome of menopause: Current treatment options in breast cancer survivors—Systematic review. Maturitas. 2021;143:47–58. doi: 10.1016/j.maturitas.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 40. Mendoza N, Carrion R, Mendoza-Huertas L, et al. Efficacy and safety of treatments to improve dyspareunia in breast cancer survivors: A systematic review. Breast Care (Basel) 2020;15:599–607. doi: 10.1159/000506148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barton DL, Sloan JA, Shuster LT, et al. Evaluating the efficacy of vaginal dehydroepiandosterone for vaginal symptoms in postmenopausal cancer survivors: NCCTG N10C1 (Alliance) Support Care Cancer. 2018;26:643–650. doi: 10.1007/s00520-017-3878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cagnacci A, Barattini DF, Casolati E, et al. Polycarbophil vaginal moisturizing gel versus hyaluronic acid gel in women affected by vaginal dryness in late menopausal transition: A prospective randomized trial. Eur J Obstet Gynecol Reprod Biol. 2022;270:239–245. doi: 10.1016/j.ejogrb.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 43. Nappi RE, Martella S, Albani F, et al. Hyaluronic acid: A valid therapeutic option for early management of genitourinary syndrome of menopause in cancer survivors? Healthcare (Basel) 2022;10:1528. doi: 10.3390/healthcare10081528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stabile G, Ricci G, Scalia MS, et al. Induced dryness stress on human vaginal epithelium: The efficacy of a new vaginal gel. Gels. 2021;7:157. doi: 10.3390/gels7040157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carter J, Baser RE, Goldfrank DJ, et al. A single-arm, prospective trial investigating the effectiveness of a non-hormonal vaginal moisturizer containing hyaluronic acid in postmenopausal cancer survivors. Support Care Cancer. 2020;29:311–322. doi: 10.1007/s00520-020-05472-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: A randomized controlled trial. J Clin Oncol. 2015;33:3394–3400. doi: 10.1200/JCO.2014.60.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jha S, Wyld L, Krishnaswamy PH. The impact of vaginal laser treatment for genitourinary syndrome of menopause in breast cancer survivors: A systematic review and meta-analysis. Clin Breast Cancer. 2019;19:e556–e562. doi: 10.1016/j.clbc.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 48. Febrina F, Triyoga IF, White M, et al. Efficacy of interventions to manage sexual dysfunction in women with cancer: A systematic review. Menopause. 2022;29:609–626. doi: 10.1097/GME.0000000000001953. [DOI] [PubMed] [Google Scholar]
- 49. Quick AM, Dockter T, Le-Rademacher J, et al. Pilot study of fractional CO(2) laser therapy for genitourinary syndrome of menopause in gynecologic cancer survivors. Maturitas. 2021;144:37–44. doi: 10.1016/j.maturitas.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.2018. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-efforts-safeguard-womens-health-deceptive-health-claims Statement from FDA Commissioner Scott Gottlieb, M.D., on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation”. U.S. Food & Drug Administration.
- 51. Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: A randomized clinical trial. JAMA. 2021;326:1381–1389. doi: 10.1001/jama.2021.14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hirschberg AL, Sanchez-Rovira P, Presa-Lorite J, et al. Efficacy and safety of ultra-low dose 0.005% estriol vaginal gel for the treatment of vulvovaginal atrophy in postmenopausal women with early breast cancer treated with nonsteroidal aromatase inhibitors: A phase II, randomized, double-blind, placebo-controlled trial. Menopause. 2020;27:526–534. doi: 10.1097/GME.0000000000001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barton DL, Shuster LT, Dockter T, et al. Systemic and local effects of vaginal dehydroepiandrosterone (DHEA): NCCTG N10C1 (Alliance) Support Care Cancer. 2018;26:1335–1343. doi: 10.1007/s00520-017-3960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Crean-Tate KK, Faubion SS, Pederson HJ, et al. Management of genitourinary syndrome of menopause in female cancer patients: A focus on vaginal hormonal therapy. Am J Obstet Gynecol. 2020;222:103–113. doi: 10.1016/j.ajog.2019.08.043. [DOI] [PubMed] [Google Scholar]
- 55. Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;31:CD001500. doi: 10.1002/14651858.CD001500.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Labrie F, Archer D, Bouchard C, et al. Intravaginal dehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause. 2009;16:907–922. doi: 10.1097/gme.0b013e31819e8e2d. [DOI] [PubMed] [Google Scholar]
- 57. Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: A multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575–1584. doi: 10.1111/jsm.12125. [DOI] [PubMed] [Google Scholar]
- 58. Melisko ME, Goldman ME, Hwang J, et al. Vaginal testosterone cream vs estradiol vaginal ring for vaginal dryness or decreased libido in women receiving aromatase inhibitors for early-stage breast cancer: A randomized clinical trial. JAMA Oncol. 2017;3:313–319. doi: 10.1001/jamaoncol.2016.3904. [DOI] [PubMed] [Google Scholar]
- 59. Brennen R, Lin K-Y, Denehy L, et al. The effect of pelvic floor muscle interventions on pelvic floor dysfunction after gynecological cancer treatment: A systematic review. Phys Ther. 2020;100:1357–1371. doi: 10.1093/ptj/pzaa081. [DOI] [PubMed] [Google Scholar]
- 60. Damast S, Jeffery DD, Son CH, et al. Literature review of vaginal stenosis and dilator use in radiation oncology. Pract Radiat Oncol. 2019;9:479–491. doi: 10.1016/j.prro.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kling JM, Saadedine M, Faubion SS, et al. Sexual health update in women. J Womens Health (Larchmt) 2023;32:10–14. doi: 10.1089/jwh.2022.0392. [DOI] [PubMed] [Google Scholar]
- 62. Morales-Sanchez L, Luque-Ribelles V, Gil-Olarte P, et al. Enhancing self-esteem and body image of breast cancer women through interventions: A systematic review. Int J Environ Res Public Health. 2021;18:1640. doi: 10.3390/ijerph18041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wilson CM, McGuire DB, Rodgers BL, et al. Body image, sexuality, and sexual functioning in women with gynecologic cancer: An integrative review of the literature and implications for research. Cancer Nurs. 2021;44:E252–E286. doi: 10.1097/NCC.0000000000000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lewis-Smith H, Diedrichs PC, Harcourt D. A pilot study of a body image intervention for breast cancer survivors. Body Image. 2018;27:21–31. doi: 10.1016/j.bodyim.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 65. Davis C, Tami P, Ramsay D, et al. Body image in older breast cancer survivors: A systematic review. Psychooncology. 2020;29:823–832. doi: 10.1002/pon.5359. [DOI] [PubMed] [Google Scholar]
- 66. Graboyes EM, Maurer S, Balliet W, et al. Efficacy of a brief tele-cognitive behavioral treatment vs attention control for head and neck cancer survivors with body image distress: A pilot randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2023;149:54–62. doi: 10.1001/jamaoto.2022.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Farnam F, Khakbazan Z, Nedjat S, et al. The effect of good enough sex (GES) model-based sexual counseling intervention on the body image in women surviving breast cancer: A randomized clinical trial. Asian Pac J Cancer Prev. 2021;22:2303–2310. doi: 10.31557/APJCP.2021.22.7.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Latifi Z, Soltani M, Mousavi S. Evaluation of the effectiveness of self-healing training on self-compassion, body image concern, and recovery process in patients with skin cancer. Complement Ther Clin Pract. 2020;40:101180. doi: 10.1016/j.ctcp.2020.101180. [DOI] [PubMed] [Google Scholar]
- 69. Esplen MJ, Wong J, Warner E, et al. Restoring body image after cancer (ReBIC): Results of a randomized controlled trial. J Clin Oncol. 2018;36:749–756. doi: 10.1200/JCO.2017.74.8244. [DOI] [PubMed] [Google Scholar]
- 70. Pakzad Khalilabad R, Aghebati N, Behnam Vashani HR. The effect of self-care program based on modeling and role modeling theory on body image nurturance in patients with colorectal cancer: A randomized clinical trial. Holist Nurs Pract. 2020;34:199–209. doi: 10.1097/HNP.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 71. Boing L, de Bem Fretta T, Stein F, et al. Can mat Pilates and belly dance be effective in improving body image, self-esteem, and sexual function in patients undergoing hormonal treatment for breast cancer? A randomized clinical trial. Arch Womens Ment Health. 2023;26:141–151. doi: 10.1007/s00737-023-01294-4. [DOI] [PubMed] [Google Scholar]
- 72. Sherman KA, Przezdziecki A, Alcorso J, et al. Reducing body image-related distress in women with breast cancer using a structured online writing exercise: Results from the my changed body randomized controlled trial. J Clin Oncol. 2018;36:1930–1940. doi: 10.1200/JCO.2017.76.3318. [DOI] [PubMed] [Google Scholar]
- 73. Thompson T, Ketcher D, Gray TF, et al. The dyadic cancer outcomes framework: A general framework of the effects of cancer on patients and informal caregivers. Soc Sci Med. 2021;287:114357. doi: 10.1016/j.socscimed.2021.114357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rottmann N, Larsen PV, Johansen C, et al. Sexual activity in couples dealing with breast cancer. A cohort study of associations with patient, partner and relationship-related factors. Front Psychol. 2022;13:828422. doi: 10.3389/fpsyg.2022.828422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rottmann N, Gilså Hansen D, dePont Christensen R, et al. Satisfaction with sex life in sexually active heterosexual couples dealing with breast cancer: A nationwide longitudinal study. Acta Oncol. 2017;56:212–219. doi: 10.1080/0284186X.2016.1266086. [DOI] [PubMed] [Google Scholar]
- 76. Marino JL, Saunders CM, Hickey M. Sexual inactivity in partnered female cancer survivors. Maturitas. 2017;105:89–94. doi: 10.1016/j.maturitas.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 77. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: Prevalence and predictors. JAMA. 1999;281:537–544. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 78. Reese JB, Zimmaro LA, McIlhenny S, et al. Coping with changes to sex and intimacy after a diagnosis of metastatic breast cancer: Results from a qualitative investigation with patients and partners. Front Psychol. 2022;13:864893. doi: 10.3389/fpsyg.2022.864893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Stulz A, Favez N, Flahault C. Emotional and sexual adaptation to colon cancer: Perceptual congruence of dyadic coping among couples. Front Psychol. 2022;13:802603. doi: 10.3389/fpsyg.2022.802603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Reese JB, Zimmaro LA, Lepore SJ, et al. Evaluating a couple-based intervention addressing sexual concerns for breast cancer survivors: Study protocol for a randomized controlled trial. Trials. 2020;21:173. doi: 10.1186/s13063-019-3975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Carroll AJ, Baron SR, Carroll RA. Couple-based treatment for sexual problems following breast cancer: A review and synthesis of the literature. Support Care Cancer. 2016;24:3651–3659. doi: 10.1007/s00520-016-3218-y. [DOI] [PubMed] [Google Scholar]
- 82. Candy B, Jones L, Vickerstaff V, et al. Interventions for sexual dysfunction following treatments for cancer in women. Cochrane Database Syst Rev. 2016;2:CD005540. doi: 10.1002/14651858.CD005540.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Reese JB, Smith KC, Handorf E, et al. A randomized pilot trial of a couple-based telephone intervention addressing sexual concerns for breast cancer survivors. J Psychosoc Oncol. 2019;37:242–263. doi: 10.1080/07347332.2018.1510869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Den Ouden MEM, Pelgrum-Keurhorst MN, Uitdehaag MJ, et al. Intimacy and sexuality in women with breast cancer: Professional guidance needed. Breast Cancer. 2019;26:326–332. doi: 10.1007/s12282-018-0927-8. [DOI] [PubMed] [Google Scholar]
- 85. Shaffer KM, Kennedy E, Glazer JV, et al. Including partners in discussions of sexual side effects from breast cancer: A qualitative study of survivors, partners, and providers. Support Care Cancer. 2022;30:4935–4944. doi: 10.1007/s00520-022-06917-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Masters WH, Johnson VE. Principles of the new sex therapy. Am J Psychiatry. 1976;133:548–554. doi: 10.1176/ajp.133.5.548. [DOI] [PubMed] [Google Scholar]
- 87. Gorman JR, Lyons KS, Harvey SM, et al. Opening the conversation: Study protocol for a Phase III trial to evaluate a couple-based intervention to reduce reproductive and sexual distress among young adult breast and gynecologic cancer survivor couples. Trials. 2022;23:730. doi: 10.1186/s13063-022-06665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gorman JR, Drizin JH, Smith E, et al. Feasibility of mindful after cancer: Pilot study of a virtual mindfulness-based intervention for sexual health in cancer survivorship. J Sex Med. 2022;19:1131–1146. doi: 10.1016/j.jsxm.2022.03.618. [DOI] [PubMed] [Google Scholar]
- 89. Reese JB, Porter LS, Casale KE, et al. Adapting a couple-based intimacy enhancement intervention to breast cancer: A developmental study. Health Psychol. 2016;35:1085–1096. doi: 10.1037/hea0000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gorman JR, Lyons KS, Reese JB, et al. Adapting a theory-informed intervention to help young adult couples cope with reproductive and sexual concerns after cancer. Front Psychol. 2022;13:813548. doi: 10.3389/fpsyg.2022.813548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Albers LF, van Belzen MA, van Batenburg C, et al. Discussing sexuality in cancer care: Towards personalized information for cancer patients and survivors. Support Care Cancer. 2020;28:4227–4233. doi: 10.1007/s00520-019-05257-3. [DOI] [PubMed] [Google Scholar]
- 92. Rhoten BA, Davis AJ, Baraff BN, et al. Priorities and preferences of patients with head and neck cancer for discussing and receiving information about sexuality and perception of self-report measures. J Sex Med. 2020;17:1529–1537. doi: 10.1016/j.jsxm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lehmann V, Laan ETM, den Oudsten BL. Sexual health-related care needs among young adult cancer patients and survivors: A systematic literature review. J Cancer Surviv. 2021;16:913–924. doi: 10.1007/s11764-021-01084-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Reese JB, Bober SL, Daly MB. Talking about women's sexual health after cancer: Why is it so hard to move the needle? Cancer. 2017;123:4757–4763. doi: 10.1002/cncr.31084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Reese JB, Sorice K, Beach MC, et al. Patient-provider communication about sexual concerns in cancer: A systematic review. J Cancer Surviv. 2017;11:175–188. doi: 10.1007/s11764-016-0577-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Albers LF, Van Ek GF, Krouwel EM, et al. Sexual health needs: How do breast cancer patients and their partners want information? J Sex Marital Ther. 2019;46:205–226. doi: 10.1080/0092623X.2019.1676853. [DOI] [PubMed] [Google Scholar]
- 97. Dai Y, Cook OY, Yeganeh L, et al. Patient-reported barriers and facilitators to seeking and accessing support in gynecologic and breast cancer survivors with sexual problems: A systematic review of qualitative and quantitative studies. J Sex Med. 2020;17:1326–1358. doi: 10.1016/j.jsxm.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 98. Eeltink CM, Witte BI, Stringer J, et al. Health-care professionals’ perspective on discussing sexual issues in adult patients after haematopoietic cell transplantation. Bone Marrow Transpl. 2018;53:235–245. doi: 10.1038/s41409-017-0027-y. [DOI] [PubMed] [Google Scholar]
- 99. Cook ED, Iglehart EI, Baum G, et al. Missing documentation in breast cancer survivors: Genitourinary syndrome of menopause. Menopause (New York, NY) 2017;24:1360–1364. doi: 10.1097/GME.0000000000000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dubin J, Patel S, Seldon CS, et al. Survey of oncology providers' attitudes and practices in evaluating sexual health in cancer care. Int J Radiat Oncol Biol Phys. 2021;111:e158–e159. [Google Scholar]
- 101. Albers LF, Palacios LAG, Pelger RCM, et al. Can the provision of sexual healthcare for oncology patients be improved? A literature review of educational interventions for healthcare professionals. J Cancer Surviv. 2020;14:858–866. doi: 10.1007/s11764-020-00898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Traa MJ, De Vries J, Roukema JA, et al. The sexual health care needs after colorectal cancer: The view of patients, partners, and health care professionals. Support Care Cancer. 2014;22:763–772. doi: 10.1007/s00520-013-2032-z. [DOI] [PubMed] [Google Scholar]
- 103. Arthur EK, Worly B, Carpenter KM, et al. Let's get it on: Addressing sex and intimacy in older cancer survivors. J Geriatr Oncol. 2021;12:312–315. doi: 10.1016/j.jgo.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 104. Hjalmarsson E, Lindroth M. “To live until you die could actually include being intimate and having sex”: A focus group study on nurses’ experiences of their work with sexuality in palliative care. J Clin Nurs. 2020;29:2979–2990. doi: 10.1111/jocn.15303. [DOI] [PubMed] [Google Scholar]
- 105. Hay CM, Donovan HS, Hartnett EG, et al. Sexual health as part of gynecologic cancer care: What do patients want? Int J Gynecol Cancer. 2018;28:1737–1742. doi: 10.1097/IGC.0000000000001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Reese JB, Beach MC, Smith KC, et al. Effective patient-provider communication about sexual concerns in breast cancer: A qualitative study. Support Care Cancer. 2017;25:3199–3207. doi: 10.1007/s00520-017-3729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Reese JB, Zimmaro LA, Bober SL, et al. Mobile technology-based (mLearning) intervention to enhance breast cancer clinicians' communication about sexual health: A pilot trial. J Natl Compr Canc Netw. 2021;19:1133–1140. doi: 10.6004/jnccn.2021.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dobrescu AI, Nussbaumer-Streit B, Klerings I, et al. Restricting evidence syntheses of interventions to English-language publications is a viable methodological shortcut for most medical topics: A systematic review. J Clin Epidemiol. 2021;137:209–217. doi: 10.1016/j.jclinepi.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 109. Morrison A, Polisena J, Husereau D, et al. The effect of English-language restriction on systematic review-based meta-analyses: A systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28:138–144. doi: 10.1017/S0266462312000086. [DOI] [PubMed] [Google Scholar]