Abstract
The effects of limiting concentrations of ammonium on the metabolic activity of Nitrosomonas europaea, an obligate ammonia-oxidizing soil bacterium, were investigated. Cells were harvested during late logarithmic growth and were incubated for 24 h in growth medium containing 0, 15, or 50 mM ammonium. The changes in nitrite production and the rates of ammonia- and hydroxylamine-dependent oxygen consumption were monitored. In incubations without ammonium, there was little change in the ammonia oxidation activity after 24 h. With 15 mM ammonium, an amount that was completely consumed, there was an 85% loss of the ammonia oxidation activity after 24 h. In contrast, there was only a 35% loss of the ammonia oxidation activity after 24 h in the presence of 50 mM ammonium, an amount that was not consumed to completion. There was little effect on the hydroxylamine oxidation activity in any of the incubations. The loss of ammonia oxidation activity was not due to differences in steady-state levels of ammonia monooxygenase (AMO) mRNA (amoA) or to degradation of the active site-containing subunit of AMO protein. The incubations were also conducted at a range of pH values to determine whether the loss of ammonia oxidation activity was correlated to the residual ammonium concentration. The loss of ammonia oxidation activity after 24 h was less at lower pH values (where the unoxidized ammonium concentration was higher). When added in conjunction with limiting ammonium, short-chain alkanes, which are alternative substrates for AMO, prevented the loss of ammonia oxidation activity at levels corresponding to their binding affinity for AMO. These results suggest that substrates of AMO can preserve the ammonia-oxidizing activity of N. europaea in batch incubations by protecting either AMO itself or other molecules associated with ammonia oxidation.
Autotrophic ammonia oxidation is an environmentally and economically significant process that has been largely characterized at the molecular level in a single bacterial isolate, Nitrosomonas europaea (17). Ammonia-oxidizing bacteria are thought to live primarily in oligotrophic environments, such as in soils or the open ocean, where ammonium is often present in extremely low concentrations (17). Thus, ammonium limitation (when ammonium is present in amounts that can be metabolized to completion) and starvation (survival in the absence of ammonium) are important environmental stresses to which these bacteria must adapt. However, although much is known about the general metabolism of ammonia-oxidizing bacteria (27), little is known about their physiological responses to limiting ammonium concentrations.
Previous studies of ammonium limitation and starvation in N. europaea have focused on cells either attached to a solid matrix or in suspension culture. When provided with limiting ammonium, N. europaea cells attached to a solid matrix grew slowly and exhibited problems with adhesion (18). When N. europaea cells attached to a sand matrix were starved of ammonium for 7.7 to 43.2 days, they were immediately capable of producing nitrite upon reintroduction of ammonium (1). However, in suspension cultures, N. europaea cells starved for up to 42 days showed an increasing lag period in nitrite production after ammonium was reintroduced. This study indicated that N. europaea cells were more capable of tolerating ammonium starvation in biofilms than in suspension cultures, perhaps due to the production of and response to the “quorum-sensing molecule,” N-acyl homoserine lactone (1, 24). Ammonium starvation studies in liquid cultures have also been conducted with the marine isolate Nitrosomonas cryotolerans (11, 13), a close relative of N. europaea. When incubated in ammonium-free medium for up to 25 weeks, N. cryotolerans cells did not die but their endogenous respiration rate and ability to oxidize ammonia decreased significantly within the first week (13). Physiological studies of N. cryotolerans cells starved for 10 weeks showed no changes in protein, DNA, or RNA levels; the cells did not miniaturize; an active electron transport system was maintained; and the intracellular ATP levels were stable (11). These responses were markedly different from those observed in energy-starved heterotrophic bacteria, in which the listed attributes changed markedly (2, 15, 20).
In contrast to the above studies, the present study has focused on the responses of N. europaea to ammonium limitation and starvation in batch incubations over a shorter timescale (24 h). Because ammonia oxidation is the central metabolism of N. europaea, this study has focused primarily on the changes in ammonia- and hydroxylamine-oxidizing activities. The metabolic activities of ammonia monooxygenase (AMO) and the subsequent flow of reductant through the electron transport chain to the terminal oxidase can be measured by the rate of NH4+-dependent O2 uptake (6). The rate of hydroxylamine (NH2OH)-dependent O2 uptake measures the metabolic activity from hydroxylamine oxidoreductase (HAO) through to the terminal oxidase. By measuring these activities independently, we were able to determine whether the observed changes in this study were specific to the ammonia oxidation activity or to an activity from hydroxylamine oxidoreductase to the terminal oxidase.
The activity of AMO is regulated by the presence of ammonia at the transcriptional (21), translational (7, 23), and posttranslational (23) levels. Therefore, we wanted to determine if there was also an effect on the ammonia oxidation activity when cells were exposed to limiting concentrations of ammonium. We found that ammonium limitation leads to dramatic and specific changes in only the ammonia oxidation activity within 24 h. These changes occur strictly at the posttranslational level and can be ameliorated by the presence of ammonium or the addition of alternative substrates for AMO. However, these effects were observed only when the incubation mixtures contained ammonium, not when ammonium was absent from the beginning. Thus, these results represent effects from limiting ammonium concentrations rather than starvation of ammonium.
MATERIALS AND METHODS
Materials.
Reagents for electrophoresis were obtained from ICN Biochemicals (Costa Mesa, Calif.). Na214CO3 (6.2 mCi/mmol) was supplied by Sigma. [α-32P]dCTP (3,000 Ci/mmol) was supplied by DuPont-NEN Products (Wilmington, Del.). All other chemicals were of reagent grade.
Growth of N. europaea.
Batch cultures (1.5 liters) of N. europaea (ATCC 19178) were grown in Erlenmeyer flasks on a rotary shaker (200 rpm) at 30°C in the dark. The defined growth medium was as previously described (6). Cells were harvested during late logarithmic growth by centrifugation (10,000 × g for 10 min) 3 days after inoculation. The cell pellet was resuspended and sedimented three times in 1.5 ml of sodium phosphate buffer (50 mM NaH2PO4, 2 mM MgCl2 [pH 8.0]). The cells were resuspended in sodium phosphate buffer (1.5 ml) and incubated at 4°C for 12 to 18 h to allow degradation of intracellular amoA mRNA (21). No changes in the NH4+- or NH2OH-dependent O2 uptake activities occurred during this period (data not shown).
Batch incubations.
In the experiments in this study, we monitored the changes in NH4+- and NH2OH-dependent O2 uptake activities and nitrite production from cells originally harvested during late logarithmic growth. The batch incubations were conducted in Erlenmeyer flasks (125 ml) containing 25 ml of ammonium-free medium (pH 8.1) which contained Na2CO3 (4 mM). Ammonium sulfate was added to achieve a final concentration of 0, 15, or 50 mM ammonium. The reactions were initiated by the addition of washed cells (250 μl; ca. 109 cells · ml−1), and the reaction mixtures were incubated at 30°C on a rotary shaker (200 rpm) in the dark. The pH of the incubations was not controlled by adding additional buffer because of the negative effects of high ionic strength on the cells. At the indicated times, samples (1 ml) of the incubation medium were removed and the cells in these samples were sedimented (14,000 × g for 2 min). The resulting supernatant was collected and used to determine the accumulation of nitrite (5) and the pH of the incubation medium. The sedimented cells were washed, resuspended in sodium phosphate buffer (50 μl), and used to measure the NH4+- and NH2OH-dependent O2 uptake rates (hereafter referred to as ammonia and hydroxylamine oxidation activities, respectively) (6) and protein concentrations (4). The rate of NH4+-dependent O2 uptake referred to as 100% activity was approximately 120 nmol of O2 min−1 ml−1. The rate of NH2OH-dependent O2 uptake referred to as 100% activity was approximately 35 nmol of O2 min−1 ml−1. The total remaining ammonium in the medium after the 24-h incubation was determined by microdiffusion followed by Nesslerization as previously described (3). In incubations initiated at different pH values, the pH was adjusted with 10 N HCl. The incubations including short-chain alkanes were conducted in sealed glass vials (160 ml), and the gas was added as an overpressure to the headspace in the vials. The concentration of each alkane in the liquid phase of the incubation was calculated from the coefficients for Henry’s law constants for gases in water (22).
Analysis of amoA gene expression.
Batch incubations of N. europaea cells were conducted in NH4+-free medium (150 ml) containing 0, 15, or 50 mM ammonium in Erlenmeyer flasks (500 ml). The incubations were initiated by the addition of washed cells (1.5 ml), and the flasks were incubated on a rotary shaker at 30°C in the dark. Total RNA was isolated from aliquots (24 ml) from each incubation at the indicated time points in a CsCl step gradient (19). Northern hybridizations were performed on Nytran membranes (Schleicher & Schuell, Keene, N.H.). The same amount of RNA was loaded onto each lane (2 μg). Hybridization standardization was assessed by hybridization with a 23S rRNA probe, obtained by PCR with genomic N. europaea DNA as a template, and the oligonucleotide primers S2301 and S23R01 (25). A probe for amoA mRNA (21) was used for specific transcript hybridization. Hybridization was quantified by exposure on a storage phosphor screen with Imagequant software (Molecular Dynamics, Sunnyvale, Calif.). The efficiency of hybridization was determined by loading an equal amount of mRNA from ammonia-induced cells and normalizing the amoA signal from this control for each blot.
Na214CO3 labeling reactions.
Batch incubations were conducted in glass serum vials (160 ml) sealed with butyl rubber and aluminum crimp seals. Ammonium-free medium (30 ml) containing 4 mM Na2CO3, 0, 7.5, or 25 mM (NH4)2SO4, and 5 μCi of Na214CO3 (2 to 10 mCi · mmol−1) was added to the vials. The incubations were initiated by the addition of washed cells (300 μl), and the vials were shaken at 30°C in the dark. At the indicated time points, aliquots were sampled (1 ml), the cells were sedimented (14,000 × g for 2 min), and the supernatant was discarded. The cell pellets were solubilized in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (100 μl) and frozen at −80°C. The apparent molecular masses of the polypeptides visualized on SDS-PAGE gels (12% polyacrylamide) were determined by comparison with Rf values for molecular mass markers as described previously (6). Polypeptides that had accumulated 14C from the fixation of Na214CO3 were visualized by exposure on storage phosphor screens (Molecular Dynamics). Densitometry was conducted with Imagequant software.
RESULTS
Time-dependent changes in ammonia oxidation activity from cells incubated in batch cultures containing different amounts of ammonium.
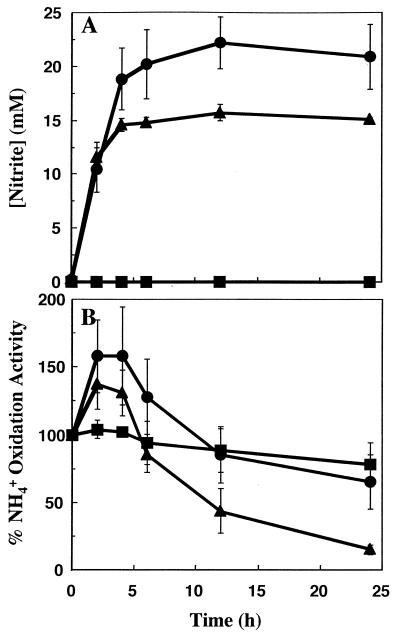
Changes in nitrite production and ammonia oxidation activity were monitored over a 24-h time course for cells incubated with 0, 15, or 50 mM ammonium. Within 6 h, all of the ammonium was consumed in the incubation containing 15 mM ammonium, as shown by the accumulation of 15 mM nitrite in the medium (Fig. 1A). The complete conversion of the 15 mM ammonium to nitrite was confirmed by the direct measurement of less than 0.1 mM total ammonium (NH3 and NH4+) after 6 h (data not shown). In contrast, the incubation containing 50 mM ammonium retained up to 27 mM ammonium and produced up to 23 mM nitrite after 6 h. The average initial and final pH values were, respectively, 8.4 and 8.6 in the incubations without ammonium, 8.2 and 6.7 in the incubations with 15 mM ammonium, and 8.1 and 5.6 in the incubations with 50 mM ammonium (data not shown).
FIG. 1.
Time course of changes in ammonia oxidation activity and nitrite production for N. europaea cells incubated with different concentrations of ammonium. N. europaea cells were incubated in growth medium containing 0, 15, or 50 mM ammonium. Washed cells were assayed for ammonia oxidation activity, and the supernatant was assayed for nitrite at the indicated time points as described in Materials and Methods. Error bars represent the standard deviation for the average of five replicate experiments. (A) Nitrite accumulation over a 24-h time course for cells incubated with 50 (•), 15 (▴), or 0 (▪) mM ammonium. (B) Percent change in the NH4+-dependent O2 uptake rate for cells incubated with 50 (•), 15 (▴), or 0 (▪) mM ammonium. The rate of oxygen consumption at 100% activity was approximately 120 nmol of O2 consumed min−1 ml−1.
In the incubations containing either 15 or 50 mM ammonium, there was a stimulation in the ammonia oxidation activity within the first 2 h (Fig. 1B). The extent of the increase in ammonia oxidation activity was smaller for the cells incubated in 15 M ammonium than for those incubated in 50 mM ammonium, which was similar to the previously characterized stimulatory effect of ammonia on AMO activity (23). By 12 and 24 h, the ammonia oxidation activity in the incubation with 15 mM ammonium had declined to approximately 40 and 15%, respectively, of its preincubation level (Fig. 1B). However, the activity of ammonia oxidation in the incubation with 50 mM ammonium was near 80% of its initial level after 12 h and at approximately 65% after 24 h. In the incubation without ammonium, there was no detectable stimulation of ammonia oxidation activity and no dramatic loss of ammonia oxidation activity after 12 h, and the activity had declined to only about 78% of its initial level after 24 h. In contrast to the ammonia oxidation activity, the hydroxylamine oxidation activity remained relatively stable, ranging between 78 and 100% of the initial level in all of the incubations, and did not correlate with changes in the ammonium concentration (data not shown).
Expression of the amoA gene in response to changing levels of ammonium.
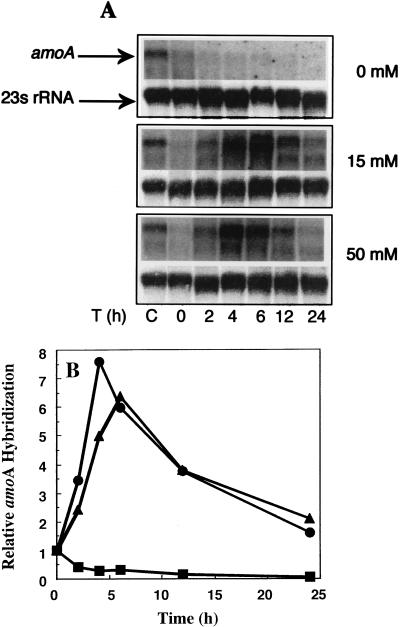
The results from Fig. 1 suggested that the levels of ammonia oxidation activity were responsive to both the initial amount of ammonium in the incubation medium and the changes occurring in the incubation medium over time. Because there was such a large difference after 24 h between the amount of ammonia oxidation activity in the incubations with 15 or 50 mM ammonium, we wished to investigate the nature of the activity loss by analyzing the effects on AMO levels. To this end, the steady-state levels of amoA mRNA transcript were examined by Northern hybridization of RNA from cells incubated in medium containing 0, 15, or 50 mM ammonium. A small amount of amoA transcript was detectable in all of the samples at the beginning of each incubation (Fig. 2A). Hybridization to the 23S rRNA molecules was performed to show that equivalent amounts of RNA were loaded in all of the lanes (Fig. 2A). Between 4 and 6 h, there was a large amount of specific hybridization to the amoA transcript in the incubations containing 15 or 50 mM ammonium (Fig. 2B). However, both the increases and decreases in the amoA mRNA pools occurred at approximately the same rate in the incubations with 15 or 50 mM ammonium, indicating that the differential losses of ammonia oxidation activity seen in Fig. 1 were not correlated to differences in amoA gene expression. In the incubation without ammonium, the small amount of initial transcript continued to decay until it was undetectable by Northern hybridization (Fig. 2B).
FIG. 2.
Time-dependent changes in the steady-state levels of amoA mRNA in N. europaea cells incubated with different concentrations of ammonium. Total RNA was extracted at the indicated times from N. europaea cells incubated in medium containing 0, 15, or 50 mM ammonium as described in Materials and Methods. (A) A PhosphorImager composite of RNA blotted on a Nytran membrane and hybridized with a single-stranded DNA probe specific for amoA mRNA or 23S rRNA, as indicated by the arrows. The blots are a single representative of three replicate experiments. A control mRNA (lane C), as described in Materials and Methods, is shown for each blot. (B) Relative densitometric values of the mRNA band hybridized to the amoA probe from the above experiment plotted against time for cells incubated with 50 (•), 15 (▴), or 0 (▪) mM ammonium. Densitometric values from each blot were normalized to the level of hybridization to the amoA mRNA in the control lane, as described in Materials and Methods.
Stability of AMOa protein.
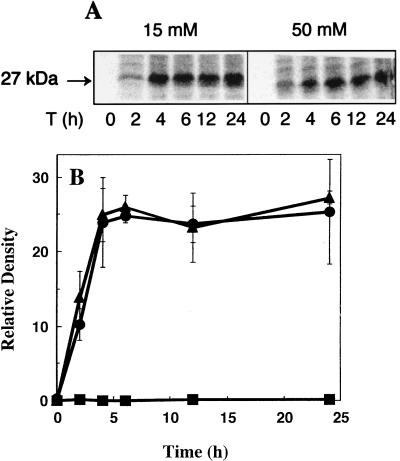
We examined the changes in the levels of both newly synthesized and preexisting AMOa, a 27-kDa polypeptide containing the enzyme active site (8), to determine if the loss of activity observed in the incubation with 15 mM ammonium was due to the degradation of AMO protein during the 24 h incubation. To visualize the de novo-synthesized polypeptides, N. europaea cells were incubated in medium containing 0, 15, or 50 mM ammonium in the presence of Na214CO3. The incorporation of 14C into AMOa continued for up to 4 h and then stabilized after approximately 6 h (Fig. 3A). The total amount of labeled AMOa polypeptide was similar in cells incubated in medium with either 15 or 50 mM initial ammonium (Fig. 3B). Furthermore, the amount of radiolabeled polypeptide remained stable for the rest of the 24-h incubation with no indication of degradation. In the incubation without ammonium, there was no detectable protein synthesis, confirming the results from a previous study (7). The total radiolabeled protein profiles from cells incubated in 15 or 50 mM ammonium were generally equivalent in banding pattern and label intensity, indicating that the changes in the ammonia oxidation activity were not due to visible differences in general protein synthesis (data not shown).
FIG. 3.
Time-dependent changes in the de novo-synthesized AMOa polypeptide in N. europaea cells incubated with different amounts of ammonium. N. europaea cells were incubated in growth medium containing 0, 15, or 50 mM ammonium plus Na214CO3. Incorporation of 14C into de novo-synthesized polypeptides was analyzed by SDS-PAGE and a PhosphorImager as described in Materials and Methods. (A) PhosphorImager image of the 14C-labeled 27-kDa polypeptide in cells incubated with 0, 15, or 50 mM ammonium at the indicated time points. The image is a single representative of three replicate experiments. (B) Radiolabel incorporation into the 27-kDa band for cells incubated with 50 (•), 15 (▴), or 0 (▪) mM ammonium from three replicate experiments. Error bars represent the standard deviation for the average of three replicate experiments.
A culture of N. europaea was also grown in the presence of 15 μCi of Na214CO3 to incorporate 14C into all proteins throughout the growth of the culture. The cells were then subjected to the same incubations as above to determine whether the preexisting pool of AMOa was subject to degradation and could account for the loss of ammonia oxidation activity. Measurable loss of the radiolabeled AMOa polypeptide was not observed for 24 h, regardless of the ammonium concentration, further indicating that protein degradation did not account for the loss of ammonia oxidation activity during the incubations (data not shown).
Influence of the remaining ammonium on ammonia oxidation activity.
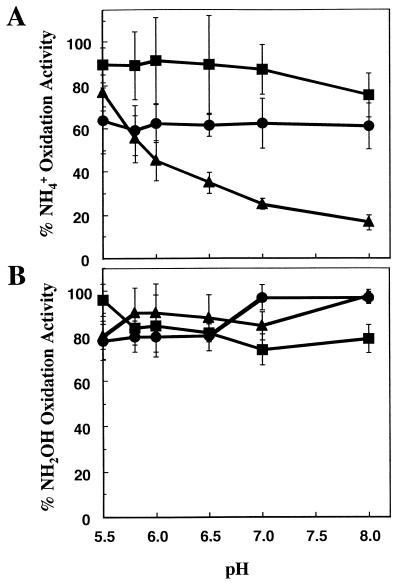
The above experiments suggested that the loss of ammonia oxidation activity was probably due to either the posttranslational loss of AMO activity or the loss of a factor required for ammonia oxidation but not for hydroxylamine oxidation. To directly investigate the role of ammonium and its involvement in the loss of ammonia oxidation activity, incubations with 0, 15, or 50 mM ammonium were conducted with the medium at a variety of starting pH values from 5.5 to 8.0. Ammonia oxidation is an acidogenic reaction. In the presence of excess ammonium, the pH of the medium decreases during ammonia oxidation until it is sufficiently low that further ammonia oxidation is prevented. If the starting pH is lowered, the amount of ammonia which must be oxidized to reach this limiting pH is decreased. Thus, at lower pH values, more ammonium remained in the medium of incubations containing 15 or 50 mM initial ammonium (as measured by a less nitrite production, data not shown).
In an incubation with 15 mM ammonium at pH 5.5, about 77% of the initial ammonia oxidation activity remained after 24 h (Fig. 4A). At more basic pH values, less ammonia oxidation activity remained after 24 h. For example, at pH 6, 45% of the activity remained, whereas at pH 8, 17% of the activity remained. The level of remaining activity correlated with the amount of ammonium remaining in the medium. For example, at pH 5.5, all of the ammonium remained in the incubation medium, as indicated by the lack of nitrite accumulation (data not shown). At pH 6.0, approximately 2 mM ammonium remained.
FIG. 4.
Effect of pH on the ammonia oxidation activity in cells incubated for 24 h with different amounts of ammonium. N. europaea cells were incubated in growth medium at a range of pH values from 5.5 to 8.0 and containing 0, 15, or 50 mM ammonium. Error bars represent the standard deviation for the average of three replicate experiments. (A) Percentage of ammonia oxidation activity remaining after 24 h relative to the level at the start of the incubation, for cells incubated with 50 (•), 15 (▴), or 0 (▪) mM ammonium at the indicated pHs. (B) Percentage of hydroxylamine oxidation activity remaining after 24 h for the same incubations as in panel A.
In contrast to the incubations containing 15 mM ammonium, all of the incubations containing 50 mM ammonium maintained about 62% of the original ammonia oxidation activity regardless of the initial pH or the remaining ammonium in the medium (Fig. 4A). At least 23 mM ammonium remained in the incubations initially containing 50 mM ammonium (data not shown). In incubations without ammonium, the ammonia oxidation activity varied considerably, between 65 and 112% of the original level, but the variation did not correlate with the pH of the incubation medium (Fig. 4A). Changes in the hydroxylamine oxidation activity did not correlate with differences in the medium pH in any of the incubations, although it did range between 78 and 98% of the original level (Fig. 4B).
Effect of short-chain alkanes on ammonia oxidation activity.
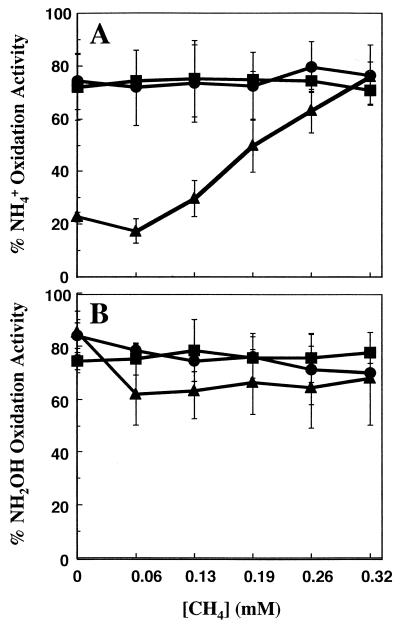
Because residual ammonium appeared to prevent some of the loss of ammonia oxidation activity, we wished to determine whether this phenomenon was limited to ammonium or if other AMO substrates could provide a similar protection of ammonia oxidation activity. Therefore, cells were incubated with 0, 15, or 50 mM ammonium and with a range of methane concentrations from 0 to 0.32 mM in the liquid phase of the incubation. These concentrations corresponded to additions of 0 to 25% (vol/vol) of the gas headspace of the vials. Methane is a competitive inhibitor of ammonia oxidation by AMO (10, 12) and was thus considered an appropriate substrate for comparison to ammonium. In incubations with limiting ammonium, 15 mM, higher methane concentrations resulted in more protection of ammonia oxidation activity during the 24-h incubation (Fig. 5A). For example, with 0.06 mM methane in the liquid phase of the incubation (5% [vol/vol] of the gas headspace), approximately 17% of the original ammonia oxidation activity was maintained. With 0.19 mM methane (15% [vol/vol] of the gas headspace), 50% of the ammonia oxidation activity remained, and with 0.32 mM methane (25% [vol/vol] of the gas headspace), 76% of the ammonia oxidation activity was preserved.
FIG. 5.
Effect of methane on ammonia oxidation activity for cells incubated for 24 h with different amounts of ammonium. N. europaea cells were incubated in growth medium containing 0, 15, or 50 mM ammonium in sealed vials and with different concentrations of methane in the liquid phase. Error bars represent standard deviation for the average of four replicate experiments. (A) Percentage of ammonia oxidation activity remaining after 24 h relative to the initial level at the beginning of the incubation for cells incubated with 50 (•), 15 (▴), or 0 (▪) mM ammonium and the indicated concentrations of methane in the liquid phase. (B) Percentage of hydroxylamine oxidation activity remaining after 24 h in the same incubations as in panel A.
In the incubations with 15 mM ammonium, virtually all of the ammonium was consumed and the pH declined to 6.7 (data not shown). However, about 1 mM ammonium could not be accounted for as nitrite produced. The discrepancy in nitrite production and ammonia consumption was presumably due to the conversion of nitrite to nitrous oxide when the atmosphere in the sealed vials became O2 limited several hours after the ammonium was oxidized to completion (data not shown) (16). With 0 or 50 mM ammonium in the incubations, the ammonia oxidation activity remained relatively constant (between 70 and 80% of the original level after 24 h) and was thus not affected by the presence of methane (Fig. 5A). The hydroxylamine oxidation activities fluctuated between 62 and 85% of the original level but did not follow a noticeable trend with methane concentration (Fig. 5B).
Other alkanes—ethane, propane, and butane—were also tested for their ability to confer protection on ammonia oxidation activity in the presence of limiting ammonium concentrations (Table 1). All three of these alternative substrates are classified as noncompetitive inhibitors of ammonia oxidation by AMO and have different affinities for the enzyme (9, 14). In the incubations containing 15 mM ammonium, all of the alkanes (including methane) protected the ammonia oxidation activity to an extent that correlated with their binding affinity for AMO. The dissociation constant (KiE) is 3.24 mM for methane, 0.22 mM for ethane, 1.4 mM for propane, and 0.92 mM for butane (14). Thus, in incubations with 15 mM ammonium, ethane protected the ammonia oxidation activity to the greatest extent, followed by butane, propane, and then methane (Table 1; Fig. 5). The different solubilities of the alkanes in the liquid phase of the incubations did not alter the correlation between protection of activity and binding affinity for AMO. For example, butane had the lowest solubility in water of any of the alkanes tested but it protected more ammonia oxidation activity than did propane or methane (Table 1; Fig. 5).
TABLE 1.
Effect of short-chain alkanes on ammonia and hydroxylamine oxidation activities and nitrite production in cells incubated with varied amounts of ammonium
| Alkane concn (mM) in liquid | % of gas in headspace (vol/vol) | Ammonium concn (mM) | % NH4+ oxidation activitya | % NH2OH oxidation activitya | Nitrite concn (mM)a |
|---|---|---|---|---|---|
| Control incubationb | 0 | 76.1 ± 11 | 72.7 ± 12 | 0 | |
| 15 | 18.7 ± 3 | 87.2 ± 13 | 12.7 ± 0.6 | ||
| 50 | 72.2 ± 12 | 89.7 ± 10 | 19.2 ± 1.1 | ||
| Ethane | |||||
| 0.019 | 1 | 0 | 73.2 ± 14 | 71.6 ± 2 | 0 |
| 0.095 | 5 | 0 | 73.3 ± 11 | 69.4 ± 2 | 0 |
| 0.19 | 10 | 0 | 72.7 ± 13 | 66.0 ± 9 | 0 |
| 0.019 | 1 | 15 | 23.9 ± 4 | 88.7 ± 3 | 14.3 ± 1.0 |
| 0.095 | 5 | 15 | 59.9 ± 5 | 54.4 ± 15 | 13.2 ± 0.4 |
| 0.19 | 10 | 15 | 70.4 ± 7 | 49.0 ± 9 | 12.4 ± 0.4 |
| 0.019 | 1 | 50 | 75.2 ± 12 | 86.3 ± 1 | 19.3 ± 0.4 |
| 0.095 | 5 | 50 | 87.7 ± 15 | 80.5 ± 17 | 18.8 ± 0.2 |
| 0.19 | 10 | 50 | 98.9 ± 8 | 82.5 ± 14 | 18.6 ± 0.4 |
| Propane | |||||
| 0.011 | 1 | 0 | 62.9 ± 4 | 75.9 ± 2 | 0 |
| 0.055 | 5 | 0 | 66.9 ± 7 | 72.2 ± 5 | 0 |
| 0.11 | 10 | 0 | 63.4 ± 4 | 72.1 ± 7 | 0 |
| 0.011 | 1 | 15 | 14.8 ± 0.9 | 78.9 ± 11 | 13.6 ± 0.5 |
| 0.055 | 5 | 15 | 18.6 ± 3 | 66.9 ± 10 | 13.7 ± 0.8 |
| 0.11 | 10 | 15 | 24.5 ± 2 | 61.6 ± 9 | 13.6 ± 0.5 |
| 0.011 | 1 | 50 | 78.9 ± 10 | 81.8 ± 5 | 18.7 ± 1.7 |
| 0.055 | 5 | 50 | 66.9 ± 10 | 71.5 ± 14 | 18.0 ± 1.4 |
| 0.11 | 10 | 50 | 61.6 ± 9 | 65.9 ± 15 | 18.3 ± 1.7 |
| Butane | |||||
| 0.008 | 1 | 0 | 71.7 ± 16 | 64.1 ± 10 | 0 |
| 0.04 | 5 | 0 | 66.6 ± 12 | 63.2 ± 11 | 0 |
| 0.08 | 10 | 0 | 55.1 ± 11 | 57.4 ± 9 | 0 |
| 0.008 | 1 | 15 | 20.6 ± 7 | 55.1 ± 13 | 13.5 ± 0.6 |
| 0.04 | 5 | 15 | 26.3 ± 6 | 52.8 ± 2 | 14.0 ± 0.8 |
| 0.08 | 10 | 15 | 40.3 ± 15 | 47.3 ± 15 | 13.5 ± 0.5 |
| 0.008 | 1 | 50 | 90.3 ± 17 | 78.9 ± 8 | 20.3 ± 1.1 |
| 0.04 | 5 | 50 | 87.3 ± 17 | 61.3 ± 5 | 18.8 ± 0.9 |
| 0.08 | 10 | 50 | 58.6 ± 15 | 50.0 ± 7 | 17.3 ± 0.3 |
All measurements were made following a 24-h incubation. The standard deviation in all of the experimental treatments is representative of three replicate experiments.
Control incubations had no alkane addition. The standard deviation in all of the control incubations is representative of eight replicate experiments.
The presence of the alkanes led to more complex actions on the hydroxylamine oxidation activity than on the ammonia oxidation activity. For example, the presence of ethane and butane resulted in a dramatic loss of hydroxylamine oxidation activity in incubations containing 15 mM ammonium whereas the presence of propane and methane resulted in a smaller degree of activity loss. The interactions of the alkanes leading to the loss of hydroxylamine oxidation activity are unclear but may be the result of feedback inhibition by hydroxylamine, the diversion of an inactivating agent from its preferred target to HAO, or the inactivation of HAO by the products of alkane oxidation, mainly alcohols and aldehydes (26).
DISCUSSION
The purposes of this study were to determine whether limiting amounts of ammonium have a unique regulatory effect on the ammonia-oxidizing activity of N. europaea and to expand the knowledge of the physiological responses of ammonia-oxidizing bacteria to limiting substrate concentrations. The concentrations of ammonium chosen for this study fit the criteria for comparing the effects of ammonium starvation (0 mM; ammonium is immediately removed), ammonium limitation (15 mM; all of the ammonium is consumed during the incubation), and nonlimiting ammonium (50 mM; excess ammonium remains at the end of the incubation) on the metabolic activity of these bacteria. Our results suggest that in batch incubations of N. europaea, the limiting ammonium concentration, 15 mM, resulted in a large loss of ammonia-oxidizing activity—specifically via AMO, or molecules associated with ammonia oxidation, but not HAO—over a 24-h time course (Fig. 1). In contrast, little change in the ammonia oxidation activity was observed in the absence of ammonium and only a small loss occurred when ammonium was abundant.
Because only the ammonia oxidation activity was affected in the batch incubations, we examined the regulation of AMO at the transcriptional and translational levels. The same steady-state levels of amoA mRNA were synthesized and degraded in the incubations with limiting or nonlimiting concentrations of ammonium, indicating a lack of differential regulation at the transcriptional level (Fig. 2). Furthermore, the subunit of AMO which contains the active site was not degraded over the 24-h time period in any of the incubations, with or without ammonium (Fig. 3). Therefore, the loss of ammonia oxidation activity was probably due to a posttranslational modification of AMO, the inactivation of an electron carrier such as a cytochrome or quinone that delivers reductant to AMO for further ammonia oxidation, or the loss of another molecule associated with ammonia oxidation. Perhaps the loss of ammonia oxidation activity is similar to the activity loss characterized in a previous study of the regulatory effects of NH3 and pH on AMO activity (23). In the previous study, cells were incubated with 50 mM ammonium and the ammonia oxidation activity increased and then returned to a level similar to that measured at the beginning of the incubation over an 8-h period. This response is similar to the present result with abundant ammonium (50 mM) in the incubation, although some additional activity loss was observed during the more extended, 24-h incubation. In contrast, the dramatic loss of ammonia oxidation activity in the ammonium-limited incubations suggests that an additional mechanism is at work in this case. It is possible that the dynamic pH changes which occur during ammonia oxidation contributed to the losses of activity in the incubation with 15 mM ammonium. However, pH changes alone cannot explain the greater amount of remaining activity and the lower pH in the incubation with 50 mM ammonium than in the incubation with 15 mM ammonium. Furthermore, there was no difference in the amount of remaining activity after 24 h with 50 mM ammonium at any pH between 5.5 and 8 (Fig. 4). This lack of a specific response to pH is interesting because dynamic pH changes most probably occur in natural environments, suggesting that the mechanism protecting ammonia oxidation activity, perhaps involving the presence of an AMO substrate, may play an important role outside of batch cultures.
The results of the present study suggest that the presence of an AMO substrate can ameliorate most of the inactivating effects on ammonia oxidation activity when ammonium is limiting in the incubation. First, only a modest loss of ammonia oxidation activity was observed in the presence of nonlimiting ammonium concentrations (Fig. 1 and 4). The preservation of ammonia oxidation activity in incubations with abundant ammonium was correlated with the presence of a large amount of unoxidized ammonium remaining in the incubation medium after 24 h. Second, this apparent protective effect of ammonium was further defined and characterized by the results in Fig. 4, in which the incubation medium was made more acidic to prevent the complete oxidation of ammonium. Third, the alternative AMO substrates, methane, ethane, propane, and butane, could also protect the ammonia oxidation activity once the ammonium was completely consumed in the incubations with limiting ammonium concentrations (Fig. 5; Table 1). Because the KiE for ethane is the lowest of the alkanes tested, more of the ammonia oxidation activity was preserved with 15 mM ammonium and ethane than with the same concentration of methane, propane, or butane, regardless of the concentration of the alkanes in the liquid phase of the incubations. Furthermore, the extent of protection decreased as the KiE for the substrate increased. These results suggest that AMO must interact directly with a substrate molecule to avoid the deleterious effects of the inactivating agent.
The experiments conducted in the absence of ammonium corroborated observations made in previous studies of ammonium starvation in obligate ammonia-oxidizing bacteria (1, 11, 13). The ammonia and hydroxylamine oxidation activities did not change considerably during the 24-h incubations under any of the conditions tested. These results suggest that in the absence of ammonium, cells remain physiologically stable and do not react unless the surrounding environment is altered.
This study is different from the previous studies of ammonium limitation or starvation in obligate ammonia oxidizers (1, 11, 13, 18), primarily because both ammonium and the end products of ammonia oxidation, nitrite and an acidic environment, accumulated and remained in the medium throughout the experiment. Thus, the effect of the incubations on ammonia oxidation activity was not necessarily due to the effect of ammonium limitation only but, rather, was due to the effect of the changing culture conditions over time. For example, the stimulation in ammonia oxidation activity during the first few hours of the incubations with 15 or 50 mM initial ammonium (Fig. 1) was most probably due to regulated events influencing AMO activity in response to changes in the proportion of NH3 to NH4+, as described previously (23). However, the further loss of ammonia oxidation activity in the incubation with 15 mM ammonium was most probably due to both the loss of the protective effects of ammonium and to the toxic effects of other environmental components, mainly nitrite and the acidic environment, since these are the only other aspects that changed during the incubation. This model of changing culture conditions and their effects on ammonia oxidation activity would explain why none of the effects were observed when ammonium was absent from the incubations.
Our results demonstrate that the presence of AMO substrates, especially substrates that have a high binding affinity for AMO, is capable of protecting the ammonia-oxidizing activity in incubations with limiting ammonium concentrations. This phenomenon has broad implications for both the physiology of ammonia-oxidizing bacteria and our understanding of the interactions between AMO and its substrates. Most importantly, because ammonium and other substrates can protect the energy-generating activity of ammonia-oxidizing bacteria from the potentially toxic by-products of their metabolism, the interactions between ammonia oxidizers, their metabolic substrates, and other microbes in the environment may be defined by avoidance of this toxicity.
ACKNOWLEDGMENTS
This work was supported by EPA grant R821405 to D.J.A. and P. J. Bottomley.
We thank Luis Sayavedra-Soto and Norman Hommes for technical support.
REFERENCES
- 1.Batchelor S E, Cooper M, Chhabra S R, Glover L A, Stewart G S A B, Williams P, Prosser J I. Cell density-regulated recovery of starved biofilm populations of ammonia-oxidizing bacteria. Appl Environ Microbiol. 1997;63:2281–2286. doi: 10.1128/aem.63.6.2281-2286.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawes E A. Starvation, survival and energy reserves. In: Fletcher M, Floodgate G D, editors. Bacteria in their natural environments. London, United Kingdom: Academic Press, Ltd.; 1985. pp. 43–80. [Google Scholar]
- 3.Dilworth M J, Subramanian D, Munson T O, Burris R H. The adenosine triphosphate requirement for nitrogen fixation in cell-free extracts of Clostridium pasteurianum. Biochim Biophys Acta. 1965;99:486–503. doi: 10.1016/s0926-6593(65)80202-8. [DOI] [PubMed] [Google Scholar]
- 4.Gornall A G, Bardawill C J, David M M. Determination of serum proteins by means of the Biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 5.Hageman R H, Hucklesby D P. Nitrate reductase in higher plants. Methods Enzymol. 1971;23:491–503. [Google Scholar]
- 6.Hyman M R, Arp D J. 14C2H2- and 14CO2-labeling studies of the de novo synthesis of polypeptides by Nitrosomonas europaea during recovery from acetylene and light inactivation of ammonia monooxygenase. J Biol Chem. 1992;267:1534–1545. [PubMed] [Google Scholar]
- 7.Hyman M R, Arp D J. Effects of ammonia on the de novo synthesis of polypeptides in cells of Nitrosomonas europaea denied ammonia as an energy source. J Bacteriol. 1995;177:4974–4979. doi: 10.1128/jb.177.17.4974-4979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyman M R, Arp D J. The small-scale production of [U-14C]acetylene from Ba14CO3: application to labeling of ammonia monooxygenase in autotrophic nitrifying bacteria. Anal Biochem. 1990;190:348–353. doi: 10.1016/0003-2697(90)90206-o. [DOI] [PubMed] [Google Scholar]
- 9.Hyman M R, Murton I B, Arp D J. Interaction of ammonia monooxygenase from Nitrosomonas europaea with alkanes, alkenes, and alkynes. Appl Environ Microbiol. 1988;54:3187–3190. doi: 10.1128/aem.54.12.3187-3190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyman M R, Wood P M. Methane oxidation by Nitrosomonas europaea. Biochem J. 1983;212:31–37. doi: 10.1042/bj2120031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnstone B H, Jones R D. Physiological effects of long-term energy-source deprivation on the survival of a marine chemolithotrophic ammonium-oxidizing bacterium. Mar Ecol Prog Ser. 1988;49:295–303. [Google Scholar]
- 12.Jones R D, Morita R Y. Methane oxidation by Nitrosococcus oceanus and Nitrosomonas europaea. Appl Environ Microbiol. 1983;45:401–410. doi: 10.1128/aem.45.2.401-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones R D, Morita R Y. Survival of a marine ammonium oxidizer under energy-source deprivation. Mar Ecol Prog Ser. 1985;26:175–179. [Google Scholar]
- 14.Keener W K, Arp D J. Kinetic studies of ammonia monooxygenase inhibition in Nitrosomonas europaea by hydrocarbons and halogenated hydrocarbons in an optimized whole-cell assay. Appl Environ Microbiol. 1993;59:2501–2510. doi: 10.1128/aem.59.8.2501-2510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolter R, Siegele D A, Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 16.Lipschultz F, Zafiriou O C, Wofsy S C, McElroy M B, Valois F W, Watson S W. Production of NO and N2O in soil nitrifying bacteria. Nature (London) 1981;294:641–643. [Google Scholar]
- 17.Prosser J I. Autotrophic nitrification in bacteria. Adv Microb Physiol. 1989;30:125–177. doi: 10.1016/s0065-2911(08)60112-5. [DOI] [PubMed] [Google Scholar]
- 18.Prosser J I, Gray T R G. Use of finite difference method to study a model system of nitrification at low substrate concentrations. J Gen Microbiol. 1977;102:119–128. [Google Scholar]
- 19.Reddy K J, Gilman M. Preparation of bacterial RNA. In: Ausubel F M, et al., editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1993. pp. 4.4.1–4.4.7. [DOI] [PubMed] [Google Scholar]
- 20.Roszak D B, Colwell R R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987;51:365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayavedra-Soto L A, Hommes N G, Russell S A, Arp D J. Induction of ammonia monooxygenase and hydroxylamine oxidoreductase mRNAs by ammonium in Nitrosomonas europaea. Mol Microbiol. 1996;20:541–548. doi: 10.1046/j.1365-2958.1996.5391062.x. [DOI] [PubMed] [Google Scholar]
- 22.Smith M R, Baresi L. Methane estimation for methanogenic and methanotrophic bacteria. In: Linskens H F, Jackson J F, editors. Gases in plant and microbial cells. Berlin, Germany: Springer-Verlag; 1989. pp. 275–308. [Google Scholar]
- 23.Stein L Y, Arp D J, Hyman M R. Regulation of the synthesis and activity of ammonia monooxygenase in Nitrosomonas europaea by altering pH to affect NH3 availability. Appl Environ Microbiol. 1997;63:4588–4592. doi: 10.1128/aem.63.11.4588-4592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swift S, Throup J P, Salmond G P C, Stewart G S A B. Quorum sensing: a population density component in the determination of bacterial phenotype. Trends Biochem Sci. 1997;21:214–219. [PubMed] [Google Scholar]
- 25.Thiem S M, Krumme M L, Smith R L, Tiedje J M. Use of molecular techniques to evaluate the survival of a microorganism injected into an aquifer. Appl Environ Microbiol. 1994;60:1059–1067. doi: 10.1128/aem.60.4.1059-1067.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voysey P A, Wood P M. Methanol and formaldehyde oxidation by an autotrophic nitrifying bacterium. J Gen Microbiol. 1987;33:283–290. [Google Scholar]
- 27.Wood P M. Nitrification as a bacterial energy source. In: Prosser J I, editor. Nitrification. Oxford, United Kingdom: Society for General Microbiology and IRL Press; 1986. pp. 39–62. [Google Scholar]