Abstract
PURPOSE
Magrolimab is a first-in-class humanized monoclonal antibody against cluster of differentiation 47, an antiphagocytic signal used by cancer cells to evade phagocytosis. Azacitidine upregulates prophagocytic signals on AML cells, further increasing phagocytosis when combined with magrolimab. We report final phase Ib data for magrolimab with azacitidine in patients with untreated AML ineligible for intensive chemotherapy (ClinicalTrials.gov identifier: NCT03248479).
PATIENTS AND METHODS
Patients with previously untreated AML, including TP53-mutant AML, received magrolimab intravenously as an initial dose (1 mg/kg, days 1 and 4), followed by 15 mg/kg once on day 8 and 30 mg/kg once weekly or every 2 weeks as maintenance. Azacitidine 75 mg/m2 was administered intravenously/subcutaneously once daily on days 1-7 of each 28-day cycle. Primary end points were safety/tolerability and proportion with complete remission (CR).
RESULTS
Eighty-seven patients were enrolled and treated; 72 (82.8%) had TP53 mutations with a median variant allele frequency of 61% (range, 9.8-98.7). Fifty-seven (79.2%) of TP53-mutant patients had European LeukemiaNet 2017 adverse-risk cytogenetics. Patients received a median of 4 (range, 1-39) cycles of treatment. The most common treatment-emergent adverse events included constipation (49.4%), nausea (49.4%), and diarrhea (48.3%). Thirty (34.5%) experienced anemia, and the median hemoglobin change from baseline to first postdose assessment was –0.9 g/dL (range, –3.6 to 2.5 g/dL). Twenty-eight (32.2%) patients achieved CR, including 23 (31.9%) patients with TP53 mutations. The median overall survival in TP53-mutant and wild-type patients were 9.8 months and 18.9 months, respectively.
CONCLUSION
Magrolimab with azacitidine was relatively well tolerated with promising efficacy in patients with AML ineligible for intensive induction chemotherapy, including those with TP53 mutations, warranting further evaluation of magrolimab with azacitidine in AML. The phase III randomized ENHANCE-2 (ClinicalTrials.gov identifier: NCT04778397) and ENHANCE-3 (ClinicalTrials.gov identifier: NCT05079230) studies are recruiting frontline patients with AML.
INTRODUCTION
Older age, performance status, and genetic risk group are independent predictors of poor outcomes in AML.1 Patients 65 years and older with AML have a 5-year overall survival (OS) of only 11% in recent SEER data,2 because of intolerance to intensive chemotherapy (IC),1,3 and a higher burden of poor-risk genetic abnormalities and genetic secondary AML.4,5 Newly diagnosed patients with TP53-mutant AML treated with IC have a median OS of only 4-7 months and high induction mortality.6-8 Lower-intensity treatments, including standard-of-care venetoclax-azacitidine for older/unfit TP53-mutant AML, have a similarly poor median OS of 5.5-7.2 months.8-10 Evidence suggests that outcomes may be even poorer in patients with TP53-mutant AML with concomitant adverse-risk cytogenetics, a TP53 variant allele frequency (VAF) of >40%, or multihit TP53-mutant disease.7,11-13 However, a recent study reported no association between any TP53 molecular characteristics and survival (2-year OS, 12.8%) and suggested that TP53 mutation represents a distinct disease entity.14 These findings highlight a major unmet need for patients with TP53-mutant AML.
CONTEXT
Key Objective
Is the first-in-class anticluster of differentiation 47 monoclonal antibody magrolimab safe, well-tolerated, and efficacious when combined with azacitidine in patients with untreated AML unfit for intensive induction chemotherapy, particularly those with TP53 mutations, a population with a very high unmet need?
Knowledge Generated
Efficacy data support the magrolimab-azacitidine synergy observed in preclinical studies, with encouraging complete remission (CR) rates, durations of CR, and overall survival in patients with AML regardless of TP53 mutation status including a subset that bridged to allogeneic hematopoietic stem-cell transplantation. Magrolimab + azacitidine was generally well-tolerated, with manageable expected early anemia and hemoglobin improvement over time on treatment.
Relevance (C.F. Craddock)
It will be important to confirm this promising early-phase data relating to tolerability and efficacy of the novel magrolimab-azacitidine combination in adults with de novo AML deemed ineligible for intensive chemotherapy, and the results of ongoing randomized trials are awaited with interest.*
*Relevance section written by JCO Associate Editor Charles F. Craddock, MD.
The cluster of differentiation 47 (CD47) is a widely expressed cell surface protein that binds signal-regulatory protein alpha (SIRPα) on phagocytic cells providing an antiphagocytic don't eat me signal. Its overexpression on cancer cells is postulated to overcome prophagocytic eat me signals to evade phagocytosis.15,16 Magrolimab is a first-in-class humanized immunoglobulin G4 anti-CD47 monoclonal antibody that blocks CD47-SIRPα interaction, promoting cancer cell phagocytosis (Data Supplement, Fig S1 [online only]). Azacitidine upregulates eat me signals, such as calreticulin, on AML cell surfaces.17,18 In preclinical studies, magrolimab promoted AML cell phagocytosis19 and was synergistic with azacitidine in vitro and in vivo.17,18
Magrolimab monotherapy was well tolerated in two phase I clinical studies in solid tumors and lymphoma (ClinicalTrials.gov identifier: NCT02216409)20 and in relapsed/refractory AML (ClinicalTrials.gov identifier: NCT02678338, CAMELLIA study).21 We report results from a phase Ib trial (5F9005, ClinicalTrials.gov identifier: NCT03248479) of magrolimab + azacitidine in untreated patients with AML ineligible for IC.
PATIENTS AND METHODS
Study Design and Participants
This open-label, multicenter, multicohort, phase Ib trial had a screening period of ≤30 days, a 3 + 3 dose-evaluation phase, and an expansion phase (Data Supplement, Fig S2). Eligible patients were previously untreated adults (18 years and older) with histologically confirmed AML by WHO 2008 classification, who could not receive standard IC because of age, comorbidities, treating physicians' discretion, or who refused IC. After 25 patients were enrolled in the expansion, the study accrued only patients with TP53 mutations on the basis of poor outcomes in this molecular subgroup with hypomethylating agent (HMA)-venetoclax combinations.22,23 Patients had an Eastern Cooperative Oncology Group performance score of 0-2 and a WBC count of ≤20× 103/μL before the first and each magrolimab dose in cycle 1. Hydroxyurea or oral etoposide was permitted for WBC count control. No minimum hemoglobin (Hb) value at baseline was required. Enrolled patients were required to have AST and ALT ≤5× upper limit of normal (ULN); bilirubin ≤1.5× or ≤3.0× ULN and primarily unconjugated if documented history of Gilbert's syndrome or genetic equivalent; and serum creatinine ≤1.5× ULN or calculated glomerular filtration rate ≥40 mL/min/1.73 m2. Patients with acute promyelocytic leukemia or clinical suspicion of active CNS involvement by leukemia were excluded. Each patient was tested for TP53 and prespecified somatic mutations using local next-generation sequencing (NGS) per each institutional standard. TP53 VAF was available at screening in a subset of patients; another subset underwent baseline and longitudinal central whole-exome sequencing (WES) using customized processing pipelines combining published algorithms with novel filtering, curation, and quality control steps that were developed using a VAF cutoff of 0.07. Full inclusion/exclusion criteria are given in the Protocol (online only) and sample size description is given in the Data Supplement (Methods).
All patients provided written informed consent before study participation. The study was conducted according to International Conference on Harmonisation Good Clinical Practice guidelines, Declaration of Helsinki, and local Institutional Review Board requirements.
Procedures
RBC phenotyping or genotyping, type and screen (ABO/Rh), and direct antiglobulin test were performed at screening to select appropriate RBC products for transfusion because magrolimab can interfere with pretransfusion test results and crossmatching (Data Supplement, Methods). The initial cohort evaluated dose-limiting toxicities in eight patients (seven AML and one myelodysplastic syndrome [MDS]) during the first 28 days before expansion (Protocol). In expansion, magrolimab was administered intravenously at 1 mg/kg once each on days 1 and 4; 15 mg/kg once on day 8; and 30 mg/kg once each on days 11, 15, and 22. Beginning in cycle 2, 30 mg/kg was administered once weekly, followed by 30-mg/kg maintenance once weekly or biweekly from cycle 3. Azacitidine 75 mg/m2 was administered subcutaneously/intravenously once daily on days 1-7 of each 28-day cycle. Treatment was continued until unacceptable drug-related toxicity, disease progression, or death. Dose modifications and delays were allowed per protocol. Magrolimab and azacitidine were delayed together until a protocol amendment (August 2021, implemented after enrollment completion) decoupled treatments and recommended magrolimab to be continued per schedule if azacitidine was delayed because of adverse events (AEs) not considered related to magrolimab. AEs and serious AEs occurring after the first dose through 30 days after the last dose of study drugs were assessed according to Common Terminology Criteria for Adverse Events version 4.03 or the customized protocol-defined AE severity grading system for RBC agglutination. Bone marrow (BM) response assessments were conducted at screening and on day 1 of cycles 3, 5, 7, and every three cycles thereafter. Measurable residual disease (MRD) was assessed on BM aspirates obtained at BM biopsy time points by a central laboratory (Hematologics, Inc, Seattle, WA) using multiparameter flow cytometry for AML with a sensitivity of 0.02%. RBC and platelet transfusion independence were defined as ≥8 consecutive weeks without transfusion for patients who were transfusion-dependent at baseline (ie, requiring transfusion[s] within 4 weeks before first study treatment). Additional details are provided in the Protocol.
Outcomes
Primary end points were safety and tolerability measured by AEs and efficacy measured by investigator-assessed European LeukemiaNet (ELN) 20173 complete remission (CR) rate. Secondary and exploratory end points are listed in the Data Supplement (Methods).
Statistical Analysis
Intent-to-treat (ITT) analysis was conducted on patients who received ≥1 dose of magrolimab. Objective response rate (ORR) was defined as CR, CR with incomplete blood count recovery (CRi), CR with partial hematologic recovery, partial response, and morphologic leukemia-free state. Event-free survival (EFS) was defined as the time from the date of study treatment initiation until the date of documented disease progression, death from any cause, or failure to achieve CR/CRi by cycle 5 day 1.24 OS was measured from the date of study treatment initiation until the date of death from any cause. Patients who did not die during the trial active period were censored at their last known alive date from survival follow-up. Durations of responses and survival outcomes were not censored for allogeneic hematopoietic stem-cell transplant (allo-HSCT).
RESULTS
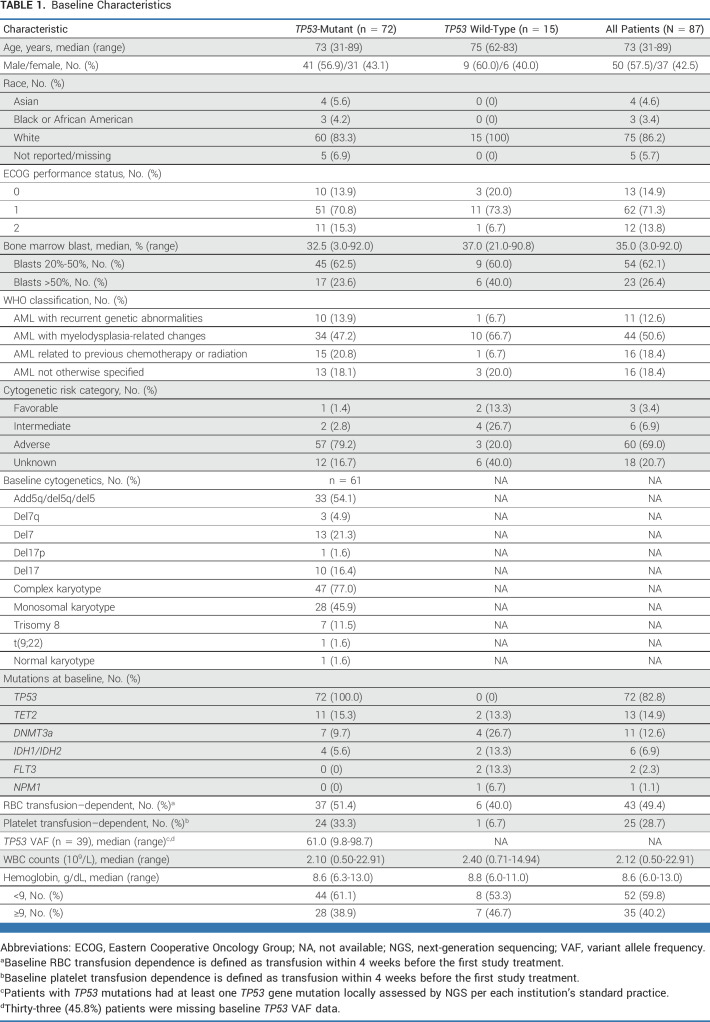
Eighty-seven patients were enrolled between February 13, 2018, and May 21, 2021; 72 had TP53 mutations (Table 1; Data Supplement, Fig S3). The median age of patients with TP53 mutations was 73 years (range, 31-89); 57 (79.2%) were 65 years and older, and 27 (37.5%) were 75 years and older. The median percentage of BM blasts was 35.0% (range, 3.0%-92.0%). Fifty-seven (79.2%) patients with TP53 mutation had adverse cytogenetic risk per ELN 2017 criteria (specific abnormalities in Table 1). Sixteen (18.4%) patients had therapy-related AML, and 44 (50.6%) had AML with myelodysplasia-related changes. One patient received previous HMA for MDS and was technically ineligible per protocol but was enrolled and included in the analysis. TP53 was the most common mutation detected at screening with central WES (Data Supplement, Table S1). TP53 mutations identified by local NGS had 91% sequence concordance with central WES in the WES subset, and all were documented as pathogenic with low transcriptional activity.
TABLE 1.
Baseline Characteristics
The median duration of treatment for all patients was 3.48 (range, 0.03-38.21) months for magrolimab and 3.02 (range, 0.03-37.85) months for azacitidine. Patients received a median of 4 (range, 1-39) cycles of treatment (10 [3-39] cycles in patients who achieved CR but did not receive allo-HSCT). Eighty-six (98.9%) patients discontinued treatment as of data cutoff; primary reasons were progressive disease (PD; 32.2%), AE (17.2%), lack of efficacy (11.5%), stem-cell transplant (10.3%), death (9.2%), patient decision (8.0%), and physician decision (8.0%).
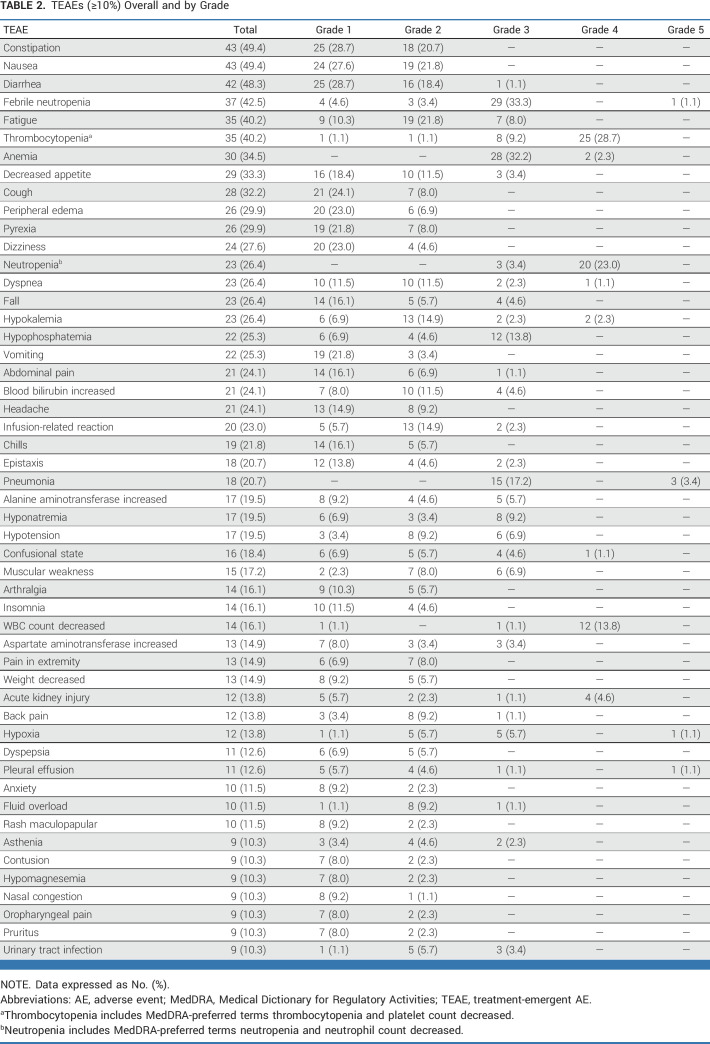
Nineteen (21.8%) patients had grade 3 anemia at baseline, and 60 (69.0%) had grade 3/4 neutropenia. The most common treatment-emergent AEs (TEAEs) regardless of causality were constipation (49.4%), nausea (49.4%), diarrhea (48.3%), febrile neutropenia (42.5%), and fatigue (40.2%; Table 2; Data Supplement, Table S2). The most common grade ≥3 TEAEs were thrombocytopenia (37.9%), anemia (34.5%), febrile neutropenia (34.5%), neutropenia (26.4%), and pneumonia (20.7%; Data Supplement, Table S3); serious AEs occurring in >1 patient included febrile neutropenia (24.1%), pneumonia (12.6%), and infusion-related reactions (IRRs; 6.9%; Data Supplement, Table S4).
TABLE 2.
TEAEs (≥10%) Overall and by Grade
The most common TEAEs considered related to magrolimab included anemia (28.7%; including one grade 4 occurring on cycle 1 day 1), IRRs (23.0%; most grade 1/2, only 2.3% grade 3), and fatigue (21.8%; Data Supplement, Table S5); possible immune-related reactions related to magrolimab were rare (one grade 2 enterocolitis infection, one grade 3 ulcerative colitis). The most common TEAEs considered related to azacitidine included thrombocytopenia (33.3%), nausea (28.7%), and anemia (27.6%; all grade ≤3; Data Supplement, Table S5).
Dose reductions were uncommon; few TEAEs led to dose reductions (1 [1.1%] for magrolimab, 9 [10.3%] for azacitidine). Dose delays were common (40 [46.0%] for magrolimab, 32 [36.8%] for azacitidine; Data Supplement, Table S6). TEAEs (regardless of attribution) led to discontinuation of magrolimab in 26 (29.9%) and azacitidine in 25 (28.7%) patients; the most common TEAEs for both were pneumonia (4.6%), acute respiratory failure, sepsis, and septic shock (2.3% each). One patient discontinued treatment because of a grade 2 IRR. Hemolysis occurred in two patients: one grade 1 and one grade 3 refractory to RBC transfusion and eventually resolved with Hb improvement after 2 weeks in whom treatment was discontinued per patient/investigator decision.
Fifty-two (59.8%) patients had a Hb level of <9 g/dL at baseline. The median Hb change from baseline to first assessment postmagrolimab dose in cycle 1 was –0.9 g/dL (range, –3.6 to 2.5 g/dL). The median maximum Hb decrease between first and third doses (ie, over the first 7 days of therapy) among 82 patients with data was –1.3 g/dL (range, –6.2 to 1.4 g/dL). Only one patient had a 6.2 g/dL Hb decrease. Importantly, 24 (29.3%) patients had a ≥2 g/dL decrease and 5 (6.1%) had a ≥3 g/dL decrease in Hb between magrolimab doses 1 and 3. Decreases in Hb in the first 24 hours after magrolimab dose 1 among 72 patients with data were <1 g/dL in 27 (37.5%), 1-2 g/dL in 27 (37.5%), and >2 g/dL in 8 (11.1%); 10 (13.9%) had no Hb decrease in the first 24 hours. Despite initial decreases, Hb levels improved over time (Fig 1A) and the median number of packed RBC/whole blood units transfused decreased over time on treatment (Fig 1B). Forty-three patients (49.4%) were RBC transfusion–dependent at baseline, 14 (32.6%) of whom became transfusion-independent. Twenty-five (28.7%) were platelet transfusion–dependent at baseline, and 12 (48.0%) became transfusion-independent.
FIG 1.
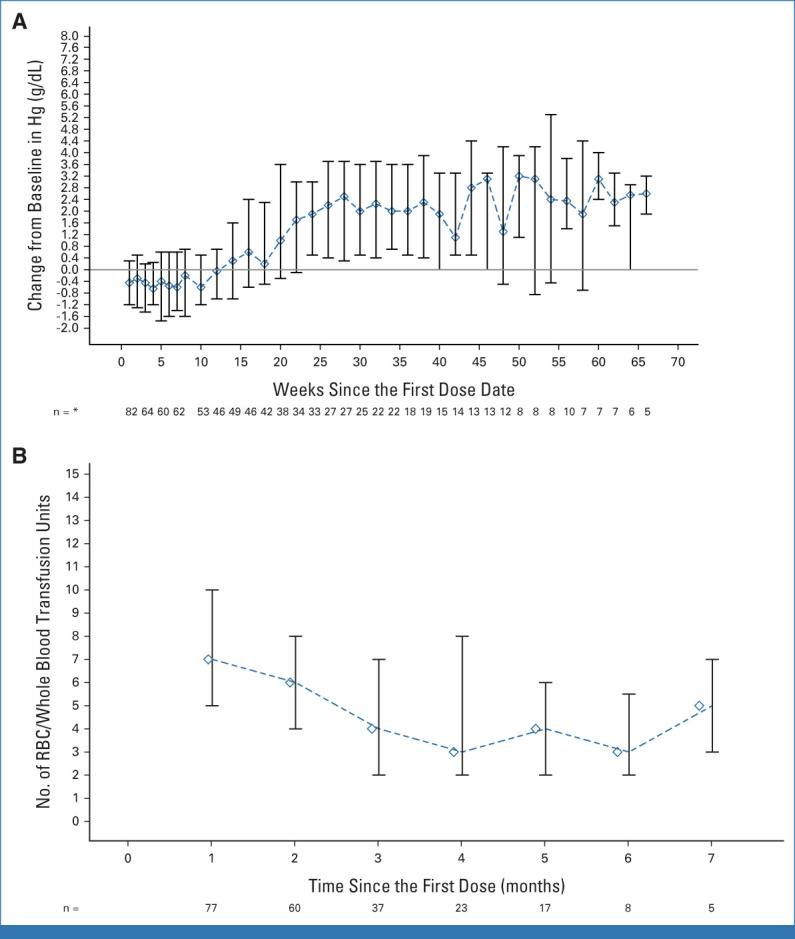
Hb change from baseline and RBC/whole blood transfusion over time. (A) Hb (g/dL) change from baseline over time. Median (IQR) shown for all patients (n = 87). Numbers below the graph indicate the number of patients with Hb assessment at that time point. (B) RBC/whole blood units transfused over time in patients remaining on treatment from the overall study population (n = 87); median (IQR). Numbers below the graph indicate the number of patients who received a transfusion at that time point. *Time points with at least five patients. Hb, hemoglobin.
The 30-day mortality was 6.9%, and the 60-day mortality was 16.1% (14 patients total); all patients with early deaths had TP53 mutations (12 because of AEs unrelated to treatment before first response assessment, one because of PD, and one because of other cause; details are provided in the Data Supplement, Table S7). Overall, 66 patients (75.9%) died during the study, 39 (44.8%) from PD and 18 (20.7%) from AEs (none treatment-related; Data Supplement, Table S7).
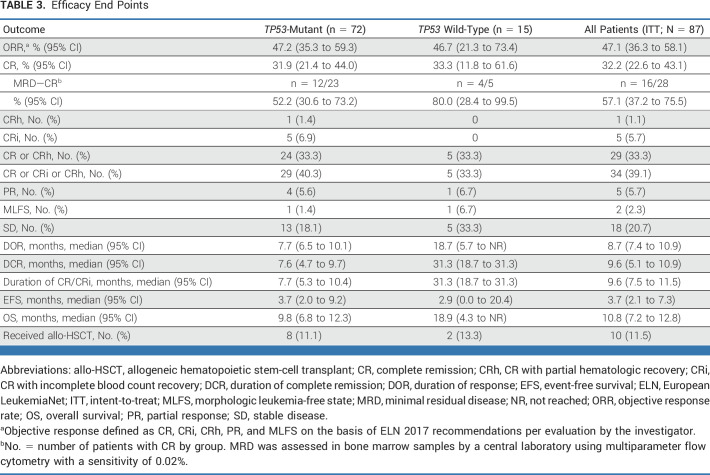
Efficacy data for all 87 patients are shown in Table 3. Twenty-eight patients achieved CR (32.2% [95% CI, 22.6 to 43.1]). Among 63 response-evaluable patients, 58 (92%) had a reduction in BM blasts from baseline (Fig 2A). Among TP53-mutant patients, responses were as follows: CR, 31.9% (95% CI, 21.4 to 44.0); CR/CRi, 40.3% (28.9 to 52.5); partial remission (PR), 5.6%; and ORR, 47.2% (35.3 to 59.3). The median time to first objective response was 2.0 (range, 1.0-5.6) months, and to CR was 3.7 (1.8-9.6) months. In patients with TP53 mutations, the median duration of CR was 7.6 (95% CI, 4.7 to 9.7) months (Fig 2B) and the median duration of response (DOR) was 7.7 (95% CI, 6.5 to 10.1) months (Data Supplement, Fig S4A). In patients with TP53 mutations who achieved CR (n = 23), 26% occurred after 4 months on therapy (range, 1.8-9.6 months; Data Supplement, Fig S5); and 52.2% (12 of 23) demonstrated MRD negativity (Table 3).
TABLE 3.
Efficacy End Points
FIG 2.
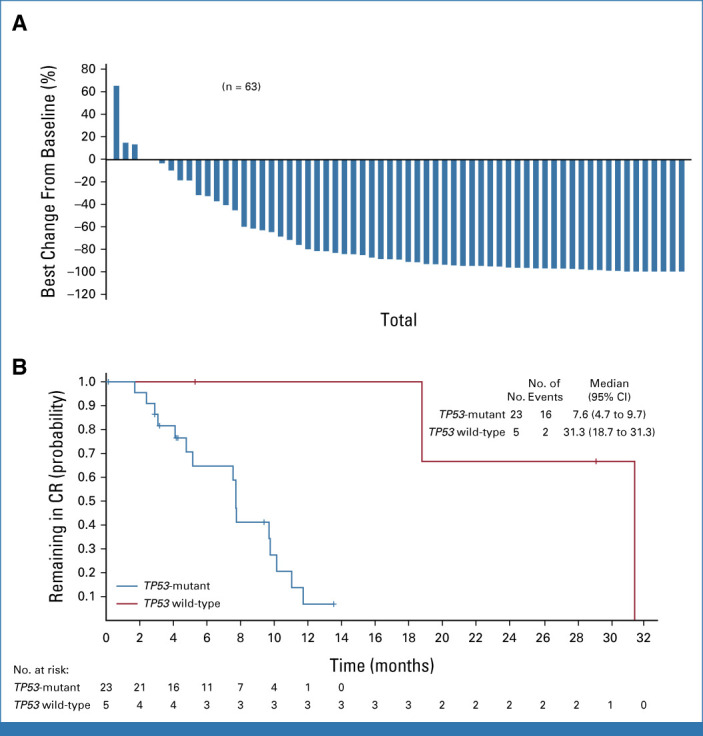
Responses to magrolimab + azacitidine in patients with untreated AML who were unfit for intensive chemotherapy. (A) Waterfall plot of best change from baseline in percent of bone marrow blasts in evaluable patients. (B) Kaplan-Meier curve of the duration of complete remission by TP53 mutation status. Censored patients are represented by vertical tick marks in the Kaplan-Meier curve. CR, complete remission.
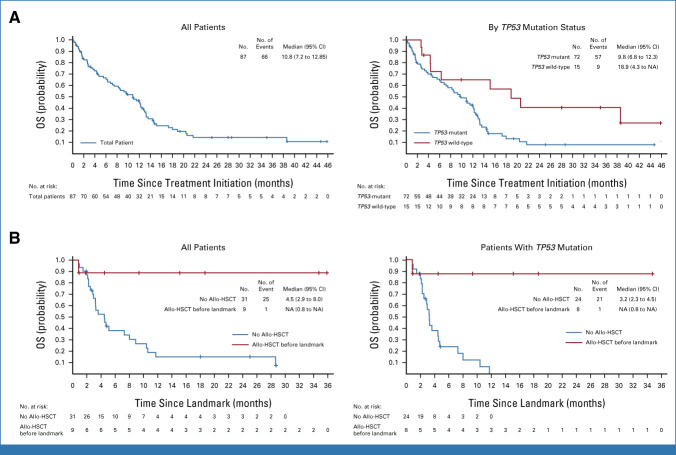
With a median follow-up for survival of 8.9 (IQR, 2.3-12.9) months in patients with TP53 mutations, the median protocol-defined EFS was 3.7 (95% CI, 2.0 to 9.2) months (Data Supplement, Fig S4B) and the OS was 9.8 (6.8 to 12.3) months (Fig 3A). The OS in patients with TP53 mutations with poor-risk ELN cytogenetics (n = 55) was 10.8 (95% CI, 7.2 to 12.8) months.
FIG 3.
Kaplan-Meier curves. (A) OS of all patients (left) and by TP53 mutation status (right); (B) OS of patients who received allo-HSCT and patients who did not, including all patients (left), and patients with TP53 mutations (right) using landmark analysis to control for lead-time bias, with 10 months as the landmark time point to include all patients with TP53 mutations who received allo-HSCT. Patients who discontinued or were lost to follow-up before the landmark time point were excluded from the analyses. The allo-HSCT group includes all patients who had allo-HSCT by the landmark time point. The no allo-HSCT group includes all patients who did not receive allo-HSCT or received allo-HSCT after the landmark time point (n = 1). Among patients bridged to allo-HSCT, the median (range) age was 68 (51-73) years, and ECOG performance status at screening was 0 in 3 of 10 (30%), 1 in 5 of 10 (50%), and 2 in 2 of 10 (20%). Eight patients had adverse-risk cytogenetics, one had intermediate-I risk, and one had unknown risk; three had AML related to previous chemotherapy or radiation; and seven were RBC transfusion-independent at baseline. Allo-HSCT was entirely at the discretion of the investigator, and patients took off-trial treatment before conditioning, so no additional information is available. Censored patients are represented by vertical tick marks in the Kaplan-Meier curve. Allo-HSCT, allogeneic hematopoietic stem-cell transplantation; ECOG, Eastern Cooperative Oncology Group; NA, not available; OS, overall survival.
Thirty-nine patients with TP53 mutations had VAF assessed at screening; the median TP53 VAF was 61.0% (range, 9.8-98.7). MRD was assessed longitudinally by central multiparametric flow cytometry in all 72 TP53-mutant patients. Among 23 patients who achieved CR, 52.2% (12 of 23) demonstrated MRD negativity.
The median OS for 14 patients with TP53 mutations who achieved CR, CRi, or CR with partial hematologic recovery and flow MRD negativity was 14.5 (95% CI, 12.1 to 21.7) months versus 7.5 (4.5 to 10.8) months in the 53 patients who remained MRD-positive, regardless of response (Data Supplement, Fig S6).
Among TP53-wildtype patients (n = 15), the CR rate was 33.3% (95% CI, 11.8 to 61.6) and the median CR duration was 31.3 (95% CI, 18.7 to 31.3) months (Table 3; Fig 2B); the median OS was 18.9 (95% CI, 4.3 to not reached [NR]) months (Fig 3A). Of five patients who achieved CR, 80.0% (4 of 5) demonstrated MRD negativity (Table 3). The PR rate and ORR were 6.7% and 46.7% (95% CI, 21.3 to 73.4), respectively; no patient achieved CRi. A subset of 13 patients who had a baseline TP53 mutation detected by WES and achieved CR had TP53 clearance (Data Supplement, Fig S7).
Additional efficacy data of the ITT population are shown in Table 3, Figure 3, and the Data Supplement (Fig S4).
Ten (11.5%) patients proceeded to allo-HSCT (Table 3) after a median of 6.1 (range, 4.2-11.5) months, including eight TP53-mutant and two TP53 wild-type patients. In the landmark analysis at 10 months after treatment initiation, among patients who had TP53 mutations, the median (95% CI) OS was NR (0.8 months to NR) in those with allo-HSCT and 3.2 (2.3 to 4.5) months in those without (Fig 3B). In TP53-mutant patients, the 1-year survival estimate from the landmark time point was 87.5% with allo-HSCT and 0.0% without allo-HSCT.
DISCUSSION
This study reports the use of magrolimab + azacitidine in patients with untreated AML who were not candidates for IC. Magrolimab + azacitidine was well tolerated and, in patients with TP53-mutant AML, demonstrated promising preliminary efficacy (true CR rate of 31.9%) and OS (9.8 months). Notably, median OS was NR in patients bridged to allo-HSCT.
Anemia is a known side effect of magrolimab on the basis of its mechanism of action (MOA). The mechanism of anemia is not fully understood and is under investigation. This could potentially be an acute extravascular drug-related anemia because of removal of aged RBCs by phagocytic cells of the reticuloendothelial system25 and possible hemagglutination. The proportion of patients with anemia in this study was consistent with observations in previous magrolimab studies.20,25,26 Aged RBCs exhibit increased expression of eat me signals in parallel with a gradual decrease in CD47 expression, leading to a natural removal by splenic and hepatic macrophages.27-29 Transient anemia and corresponding reticulocytosis were observed with magrolimab treatment in nonhuman primates and mitigated with a low initial dose.19 Anemia related to magrolimab was common (reported in 28.7% of patients), but with a single grade 4 event occurring at treatment initiation. In this study, 59.8% of patients had a Hb level of <9 g/dL at baseline and Hb decreases were observed most commonly after the first one to two doses, with a ≥3 g/dL drop reported in 5.7% of patients. A requirement for adequate pretreatment Hb value and a repeat Hb check after initial infusions of magrolimab have been incorporated into current magrolimab trials to help mitigate observed initial drops in Hb (Data Supplement, Methods). Notably, anemia in later cycles was uncommon, with Hb increasing and need for RBC/whole blood transfusions decreasing over time on treatment (Fig 1).
Tolerability of azacitidine alone has varied widely across trials. Here, proportions of grade 3/4 TEAEs, including neutropenia and thrombocytopenia, were comparable with those noted in the frontline prospective randomized trial of azacitidine in patients with AML (febrile neutropenia was most frequent at 28%)30 and lower than the 90.6% thrombocytopenia and 94.3% neutropenia rates reported in the AZA-001 study.31 Overall, tolerability of magrolimab + azacitidine was consistent with the known profiles of the individual treatments20,21,30,31 and no new safety signals were identified. The 16.1% 60-day mortality is comparable with 22% to 26% in TP53-mutant patients treated with venetoclax + azacitidine in other published studies.8,23 The safety and tolerability profile of azacitidine + magrolimab appears to be distinct from that of azacitidine and venetoclax, where prolonged myelosuppression (especially neutropenia) and infections are common, leading to significant cycle delays and dose reductions. In our study, dose reductions occurred in 1.1% for magrolimab and in 10.3% of patients for azacitidine, suggesting that the regimen may be easier to deliver in continuous cycles without need for frequent dose/duration modifications. Efficacy data from this phase Ib study support the magrolimab/azacitidine synergy observed in preclinical studies. Magrolimab + azacitidine showed encouraging efficacy in TP53-mutant AML and preliminary activity in a small subset of patients with TP53 wild-type AML who were enrolled during the initial study period. Importantly, 52.2% of TP53-mutant patients with CR also achieved MRD negativity, and responses deepened over time with 26.1% of TP53-mutant patients without initial CR ultimately achieving CR beyond 4 months on therapy, suggesting that patients who do not achieve initial CR could still benefit over time and potentially proceed to allo-HSCT.
The median OS of 9.8 months in patients with TP53 mutations compares favorably with published outcomes with HMA alone (median OS, 4.9-7.2 months),10,32 venetoclax + HMA combinations (median OS, 5.2-7.2 months),22,23 and IC (median OS, 4.1-6.8 months).1,6,7,9 In a retrospective study, median OS in patients with newly diagnosed TP53-mutant AML was similar to that in patients treated with venetoclax-based regimens (5.7 months) versus nonvenetoclax regimens (6.6 months), suggesting that venetoclax may not improve survival outcomes in TP53-mutant patients.8 Median DORs with magrolimab + azacitidine in patients with TP53 mutations (DOR, 7.7 months; CR/CRi duration, 7.7 months) also compare favorably with those with venetoclax + HMA in TP53-mutant patients (median DOR, 3.5 months in a phase II trial23; median CR/CRi duration, 5.6 months in a phase Ib study).22 Other agents such as eprenetapopt (APR-246) are being evaluated for frontline TP53-mutant MDS/AML treatment33 and maintenance after allo-HSCT,34 engaging divergent MOAs such as restoration of TP53 wild-type function, but at this time, none has shown clear benefit in frontline TP53-mutant myeloid malignancies.
Median OS was longer in patients who achieved a flow-based MRD-negative response on treatment than those who did not. Median OS in TP53-mutant patients who received allo-HSCT was also longer than that in those who did not, suggesting that magrolimab + azacitidine could be an effective and tolerable bridge to allo-HSCT.
Limitations to this study are that it is a single-arm, nonrandomized study conducted at larger academic centers consistent with the phase Ib design; lack of centralized serial TP53 NGS assessments; insufficient numbers to definitively delineate efficacy and impact in individual molecular/cytogenetic subgroups; and enrollment of a predominately White population (86.2%), a shortcoming that continues to be noted across a majority of clinical trials conducted in the United States, and conclusions may not at this time be generalizable to other populations.
In conclusion, magrolimab + azacitidine has an acceptable safety profile with promising efficacy in patients with TP53-mutant AML unfit for standard induction chemotherapy. On the basis of the results presented here, two phase III trials of magrolimab and azacitidine have been initiated in patients with newly diagnosed TP53-mutant AML (ENHANCE-2; ClinicalTrials.gov identifier: NCT04778397) and in patients with newly diagnosed AML ineligible for IC (ENHANCE-3; ClinicalTrials.gov identifier: NCT05079230). Future clinical and translational investigations are also focused on evaluating potential differential activity in molecular subsets, including patients with TP53 mutations in AML and their mechanisms. The ongoing and future larger trials will help determine whether magrolimab combinations can address the urgent unmet need for new treatments for these patients.
ACKNOWLEDGMENT
Dr Mark Chao and Dr Indu Lal (employees of Gilead Sciences, Inc during the time of the study) contributed to study methodology, data curation, and data validation; Dr Chao also contributed to the conception and design of the study. Marsha Scott, PhD (Impact Communication Partners, Inc, New York, NY) provided medical writing support for the outline and the content of the manuscript under the guidance of the authors. She and her colleagues at Impact assisted in the preparation of the manuscript for submission.
Naval G. Daver
Consulting or Advisory Role: Celgene, Agios, Jazz Pharmaceuticals, Pfizer, AbbVie, Astellas Pharma, Daiichi Sankyo, Novartis, Bristol Myers Squibb, Amgen, Immunogen, Genentech, Servier, Syndax, Trillium Therapeutics, Gilead Sciences, Arog, Shattuck Labs, Kite, a Gilead company, Stemline/Menarini
Research Funding: Bristol Myers Squibb (Inst), Pfizer (Inst), Immunogen (Inst), Genentech (Inst), AbbVie (Inst), Astellas Pharma (Inst), Servier (Inst), Daiichi Sankyo (Inst), Gilead Sciences (Inst), Amgen (Inst), Trillium Therapeutics (Inst), Hanmi (Inst), Trovagene (Inst), Fate Therapeutics (Inst), Novimmune (Inst), Glycomimetics (Inst), Kite, a Gilead company (Inst)
Paresh Vyas
Honoraria: Celgene, Pfizer, Jazz Pharmaceuticals, AbbVie, Daiichi Sankyo, Astellas Pharma
Research Funding: Celgene
Patents, Royalties, Other Intellectual Property: Patent for flow cytometric detection of leukemic stem cells
Monzr M. Al Malki
Consulting or Advisory Role: Gilead/Forty Seven, Incyte, NexImmune, CareDX
Research Funding: NexImmune, Gilead/Forty Seven, Incyte, Stemline Therapeutics
Richard A. Larson
Consulting or Advisory Role: Novartis, CVS Caremark, Epizyme, Actinium Pharmaceuticals, Servier, Immunogen, Kling Biotherapeutics, Curis, Jazz Pharmaceuticals, AbbVie, Takeda Science Foundation, Rigel
Research Funding: Daiichi Sankyo (Inst), Celgene (Inst), Astellas Pharma (Inst), Novartis (Inst), Rafael Pharmaceuticals (Inst), Cellectis (Inst), Gilead/Forty Seven
Patents, Royalties, Other Intellectual Property: UpToDate
Adam S. Asch
Research Funding: Forty Seven (Inst), Gilead Sciences (Inst)
Patents, Royalties, Other Intellectual Property: Provisional patent submitted
Gabriel Mannis
Consulting or Advisory Role: AbbVie/Genentech, Astellas Pharma, Bristol Myers Squibb/Celgene, Stemline Therapeutics, Macrogenics, Servier
Research Funding: Astex Pharmaceuticals (Inst), Glycomimetics (Inst), Jazz Pharmaceuticals (Inst), Forty Seven (Inst), Gilead Sciences (Inst), Syndax (Inst), Immune-Onc Therapeutics (Inst), Immunogen (Inst)
Wanxing Chai-Ho
Consulting or Advisory Role: Servier
Research Funding: Neoleukin Therapeutics, Shattuck Labs, Gilead/Forty Seven, Kadmon, Replimune, Syros Pharmaceuticals, Syndax, AbbVie, Sun Pharma
Tiffany N. Tanaka
Honoraria: Gilead Sciences, Survivornet
Consulting or Advisory Role: CTI BioPharma Corp
Research Funding: Function Oncology
Terrence J. Bradley
Consulting or Advisory Role: AbbVie, Novartis, Gilead Sciences
Speakers' Bureau: Novartis, AbbVie
Deepa Jeyakumar
Research Funding: Pfizer, Jazz Pharmaceuticals
Eunice S. Wang
Consulting or Advisory Role: AbbVie, Pfizer, Jazz Pharmaceuticals, Astellas Pharma, Kite/Gilead, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Novartis, Genentech, Gilead Sciences, Pharmaessentia, Janssen, Kura Oncology
Speakers' Bureau: Stemline Therapeutics, Pfizer, Dava Oncology, Kite, a Gilead company, Astellas Pharma
Kendra Sweet
Leadership: Immundex
Honoraria: Curio Science
Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Gilead Sciences, BerGenBio, Curis, Pfizer, Mablytics, Daiichi Sankyo/Lilly, Jazz Pharmaceuticals, Nkarta
Research Funding: Incyte (Inst), Jazz Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Hagop M. Kantarjian
Honoraria: AbbVie, Amgen, Pfizer, Ascentage Pharma Group, Astellas Pharma, AstraZeneca/MedImmune, Ipsen, KAHR Medical, Novartis, Precision Biosciences, Shenzhen Target Rx, Taiho Pharmaceutical, Daiichih-Sankyo (Inst), Immunogen (Inst), Jazz Pharmaceuticals (Inst)
Consulting or Advisory Role: AbbVie
Research Funding: Amgen (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), AbbVie (Inst), Immunogen (Inst), Jazz Pharmaceuticals (Inst), Ascentage Pharma (Inst), Daiichi Sankyo/Lilly (Inst)
Guillermo Garcia-Manero
Honoraria: Astex Pharmaceuticals, Acceleron Pharma, AbbVie, Novartis, Gilead Sciences, Curis, Genentech, Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Astex Pharmaceuticals, Acceleron Pharma, Bristol Myers Squibb
Research Funding: Astex Pharmaceuticals, Novartis, AbbVie, Bristol Myers Squibb, Genentech, Aprea Therapeutics, Curis, Gilead Sciences
Rami Komrokji
Stock and Other Ownership Interests: AbbVie
Consulting or Advisory Role: Novartis, Bristol Myers Squibb, Jazz Pharmaceuticals, AbbVie, Geron, CTI BioPharma Corp, Pharmaessentia, Taiho Oncology, Takeda, Gilead/Forty Seven
Speakers' Bureau: Jazz Pharmaceuticals, Servier, AbbVie, Pharmaessentia, CTI BioPharma Corp
Research Funding: Bristol Myers Squibb/Celgene (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Bristol Myers Squibb, Pharmaessentia
Guan Xing
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Giridharan Ramsingh
Employment: Gilead Sciences, Obsidian Therapeutics
Stock and Other Ownership Interests: Gilead Sciences, Genentech/Roche, Obsidian Therapeutics
Camille Renard
Employment: Gilead Sciences, Alphabet
Stock and Other Ownership Interests: Gilead Sciences, Alphabet
Joshua F. Zeidner
Consulting or Advisory Role: AbbVie, Takeda, Genentech, Bristol Myers Squibb/Celgene, Shattuck Labs, Servier, Gilead Sciences, Immunogen, Daiichi Sankyo/Lilly, Foghorn Therapeutics
Research Funding: Takeda (Inst), Merck (Inst), Gilead Sciences (Inst), Arog (Inst), Astex Pharmaceuticals (Inst), Sumitomo Dainippon Pharma Oncology (Inst), AbbVie (Inst), Stemline Therapeutics (Inst), Shattuck Labs (Inst)
David A. Sallman
Consulting or Advisory Role: AbbVie, Bristol Myers Squibb, Gilead Sciences, Intellia Therapeutics, Kite, a Gilead company, Novartis, Syndax, Bluebird Bio, Janssen, Servier, Shattuck Labs, Syros Pharmaceuticals, Takeda, Magenta Therapeutics, Molecular Partners, Jasper Therapeutics, Nkarta, Affimed Therapeutics, Intellisphere, Precigen, Zentalis, AvenCell
Speakers' Bureau: Incyte
Research Funding: Jazz Pharmaceuticals, Aprea Therapeutics (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 62nd American Society of Hematology Annual Meeting, virtual, December 5-8, 2020, Virtual; the 2022 ASCO Annual Meeting, Chicago, IL, June 3-7, 2022; and the European Hematology Association 2022 Congress, Vienna, Austria, June 15-17, 2022.
SUPPORT
Supported by Gilead Sciences, Inc.
CLINICAL TRIAL INFORMATION
N.G.D, P.V., and D.A.S. contributed equally to this work.
DATA SHARING STATEMENT
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on submitted curriculum vitae and reflecting nonconflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science's discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.
AUTHOR CONTRIBUTIONS
Conception and design: Naval G. Daver, Paresh Vyas, Guillermo Garcia-Manero, Joshua F. Zeidner, David A. Sallman
Provision of study materials or patients: Naval G. Daver, Paresh Vyas, Suman Kambhampati, Monzr M. Al Malki, Richard A. Larson, Adam S. Asch, Gabriel Mannis, Wanxing Chai-Ho, Tiffany N. Tanaka, Terrence J. Bradley, Deepa Jeyakumar, Eunice S. Wang, Kendra Sweet, Hagop M. Kantarjian, Guillermo Garcia-Manero, Rami Komrokji, Joshua F. Zeidner, David A. Sallman
Collection and assembly of data: Guan Xing, Giridharan Ramsingh, Camille Renard
Data analysis and interpretation: Naval G. Daver, Paresh Vyas, Suman Kambhampati, Monzr M. Al Malki, Richard A. Larson, Adam S. Asch, Gabriel Mannis, Wanxing Chai-Ho, Tiffany N. Tanaka, Terrence J. Bradley, Deepa Jeyakumar, Eunice S. Wang, Kendra Sweet, Hagop M. Kantarjian, Rami Komrokji, Guan Xing, Giridharan Ramsingh, Camille Renard, David A. Sallman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Tolerability and Efficacy of the Anticluster of Differentiation 47 Antibody Magrolimab Combined With Azacitidine in Patients With Previously Untreated AML: Phase Ib Results
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Naval G. Daver
Consulting or Advisory Role: Celgene, Agios, Jazz Pharmaceuticals, Pfizer, AbbVie, Astellas Pharma, Daiichi Sankyo, Novartis, Bristol Myers Squibb, Amgen, Immunogen, Genentech, Servier, Syndax, Trillium Therapeutics, Gilead Sciences, Arog, Shattuck Labs, Kite, a Gilead company, Stemline/Menarini
Research Funding: Bristol Myers Squibb (Inst), Pfizer (Inst), Immunogen (Inst), Genentech (Inst), AbbVie (Inst), Astellas Pharma (Inst), Servier (Inst), Daiichi Sankyo (Inst), Gilead Sciences (Inst), Amgen (Inst), Trillium Therapeutics (Inst), Hanmi (Inst), Trovagene (Inst), Fate Therapeutics (Inst), Novimmune (Inst), Glycomimetics (Inst), Kite, a Gilead company (Inst)
Paresh Vyas
Honoraria: Celgene, Pfizer, Jazz Pharmaceuticals, AbbVie, Daiichi Sankyo, Astellas Pharma
Research Funding: Celgene
Patents, Royalties, Other Intellectual Property: Patent for flow cytometric detection of leukemic stem cells
Monzr M. Al Malki
Consulting or Advisory Role: Gilead/Forty Seven, Incyte, NexImmune, CareDX
Research Funding: NexImmune, Gilead/Forty Seven, Incyte, Stemline Therapeutics
Richard A. Larson
Consulting or Advisory Role: Novartis, CVS Caremark, Epizyme, Actinium Pharmaceuticals, Servier, Immunogen, Kling Biotherapeutics, Curis, Jazz Pharmaceuticals, AbbVie, Takeda Science Foundation, Rigel
Research Funding: Daiichi Sankyo (Inst), Celgene (Inst), Astellas Pharma (Inst), Novartis (Inst), Rafael Pharmaceuticals (Inst), Cellectis (Inst), Gilead/Forty Seven
Patents, Royalties, Other Intellectual Property: UpToDate
Adam S. Asch
Research Funding: Forty Seven (Inst), Gilead Sciences (Inst)
Patents, Royalties, Other Intellectual Property: Provisional patent submitted
Gabriel Mannis
Consulting or Advisory Role: AbbVie/Genentech, Astellas Pharma, Bristol Myers Squibb/Celgene, Stemline Therapeutics, Macrogenics, Servier
Research Funding: Astex Pharmaceuticals (Inst), Glycomimetics (Inst), Jazz Pharmaceuticals (Inst), Forty Seven (Inst), Gilead Sciences (Inst), Syndax (Inst), Immune-Onc Therapeutics (Inst), Immunogen (Inst)
Wanxing Chai-Ho
Consulting or Advisory Role: Servier
Research Funding: Neoleukin Therapeutics, Shattuck Labs, Gilead/Forty Seven, Kadmon, Replimune, Syros Pharmaceuticals, Syndax, AbbVie, Sun Pharma
Tiffany N. Tanaka
Honoraria: Gilead Sciences, Survivornet
Consulting or Advisory Role: CTI BioPharma Corp
Research Funding: Function Oncology
Terrence J. Bradley
Consulting or Advisory Role: AbbVie, Novartis, Gilead Sciences
Speakers' Bureau: Novartis, AbbVie
Deepa Jeyakumar
Research Funding: Pfizer, Jazz Pharmaceuticals
Eunice S. Wang
Consulting or Advisory Role: AbbVie, Pfizer, Jazz Pharmaceuticals, Astellas Pharma, Kite/Gilead, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Novartis, Genentech, Gilead Sciences, Pharmaessentia, Janssen, Kura Oncology
Speakers' Bureau: Stemline Therapeutics, Pfizer, Dava Oncology, Kite, a Gilead company, Astellas Pharma
Kendra Sweet
Leadership: Immundex
Honoraria: Curio Science
Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Gilead Sciences, BerGenBio, Curis, Pfizer, Mablytics, Daiichi Sankyo/Lilly, Jazz Pharmaceuticals, Nkarta
Research Funding: Incyte (Inst), Jazz Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Hagop M. Kantarjian
Honoraria: AbbVie, Amgen, Pfizer, Ascentage Pharma Group, Astellas Pharma, AstraZeneca/MedImmune, Ipsen, KAHR Medical, Novartis, Precision Biosciences, Shenzhen Target Rx, Taiho Pharmaceutical, Daiichih-Sankyo (Inst), Immunogen (Inst), Jazz Pharmaceuticals (Inst)
Consulting or Advisory Role: AbbVie
Research Funding: Amgen (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), AbbVie (Inst), Immunogen (Inst), Jazz Pharmaceuticals (Inst), Ascentage Pharma (Inst), Daiichi Sankyo/Lilly (Inst)
Guillermo Garcia-Manero
Honoraria: Astex Pharmaceuticals, Acceleron Pharma, AbbVie, Novartis, Gilead Sciences, Curis, Genentech, Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Astex Pharmaceuticals, Acceleron Pharma, Bristol Myers Squibb
Research Funding: Astex Pharmaceuticals, Novartis, AbbVie, Bristol Myers Squibb, Genentech, Aprea Therapeutics, Curis, Gilead Sciences
Rami Komrokji
Stock and Other Ownership Interests: AbbVie
Consulting or Advisory Role: Novartis, Bristol Myers Squibb, Jazz Pharmaceuticals, AbbVie, Geron, CTI BioPharma Corp, Pharmaessentia, Taiho Oncology, Takeda, Gilead/Forty Seven
Speakers' Bureau: Jazz Pharmaceuticals, Servier, AbbVie, Pharmaessentia, CTI BioPharma Corp
Research Funding: Bristol Myers Squibb/Celgene (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Bristol Myers Squibb, Pharmaessentia
Guan Xing
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Giridharan Ramsingh
Employment: Gilead Sciences, Obsidian Therapeutics
Stock and Other Ownership Interests: Gilead Sciences, Genentech/Roche, Obsidian Therapeutics
Camille Renard
Employment: Gilead Sciences, Alphabet
Stock and Other Ownership Interests: Gilead Sciences, Alphabet
Joshua F. Zeidner
Consulting or Advisory Role: AbbVie, Takeda, Genentech, Bristol Myers Squibb/Celgene, Shattuck Labs, Servier, Gilead Sciences, Immunogen, Daiichi Sankyo/Lilly, Foghorn Therapeutics
Research Funding: Takeda (Inst), Merck (Inst), Gilead Sciences (Inst), Arog (Inst), Astex Pharmaceuticals (Inst), Sumitomo Dainippon Pharma Oncology (Inst), AbbVie (Inst), Stemline Therapeutics (Inst), Shattuck Labs (Inst)
David A. Sallman
Consulting or Advisory Role: AbbVie, Bristol Myers Squibb, Gilead Sciences, Intellia Therapeutics, Kite, a Gilead company, Novartis, Syndax, Bluebird Bio, Janssen, Servier, Shattuck Labs, Syros Pharmaceuticals, Takeda, Magenta Therapeutics, Molecular Partners, Jasper Therapeutics, Nkarta, Affimed Therapeutics, Intellisphere, Precigen, Zentalis, AvenCell
Speakers' Bureau: Incyte
Research Funding: Jazz Pharmaceuticals, Aprea Therapeutics (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kantarjian H, O'Brien S, Cortes J, et al. : Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer 106:1090-1098, 2006 [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute : Surveillance, Epidemiology, and End Results Program: Acute myeloid leukemia (AML) SEER 5-year relative survival rates, 2013-2019. https://seer.cancer.gov/statistics-network/explorer/application.html?site=96&data_type=4&graph_type=5&compareBy=age_range&chk_age_range_157=157&series=9&sex=1&race=1&hdn_stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=2#resultsRegion1
- 3.Döhner H, Estey E, Grimwade D, et al. : Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129:424-447, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai CH, Hou HA, Tang JL, et al. : Genetic alterations and their clinical implications in older patients with acute myeloid leukemia. Leukemia 30:1485-1492, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Lindsley RC, Mar BG, Mazzola E, et al. : Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 125:1367-1376, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou HA, Chou WC, Kuo YY, et al. : TP53 mutations in de novo acute myeloid leukemia patients: Longitudinal follow-ups show the mutation is stable during disease evolution. Blood Cancer J 5:e331, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rücker FG, Schlenk RF, Bullinger L, et al. : TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood 119:2114-2121, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Venugopal S, Shoukier M, Konopleva M, et al. : Outcomes in patients with newly diagnosed TP53-mutated acute myeloid leukemia with or without venetoclax-based therapy. Cancer 127:3541-3551, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Kadia TM, Jain P, Ravandi F, et al. : TP53 mutations in newly diagnosed acute myeloid leukemia: Clinicomolecular characteristics, response to therapy, and outcomes. Cancer 122:3484-3491, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Short NJ, Kantarjian HM, Loghavi S, et al. : Treatment with a 5-day versus a 10-day schedule of decitabine in older patients with newly diagnosed acute myeloid leukaemia: A randomised phase 2 trial. Lancet Haematol 6:e29-e37, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollyea DA, Pratz KW, Wei AH, et al. : Outcomes in patients with poor-risk cytogenetics with or without TP53 mutations treated with venetoclax combined with hypomethylating agents. Blood 138, 2021. (suppl 1; abstr 224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Short NJ, Montalban-Bravo G, Hwang H, et al. : Prognostic and therapeutic impacts of mutant TP53 variant allelic frequency in newly diagnosed acute myeloid leukemia. Blood Adv 4:5681-5689, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallman DA, Komrokji R, Vaupel C, et al. : Impact of TP53 mutation variant allele frequency on phenotype and outcomes in myelodysplastic syndromes. Leukemia 30:666-673, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grob T, Al Hinai ASA, Sanders MA, et al. : Molecular characterization of mutant TP53 acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood 139:2347-2354, 2022 [DOI] [PubMed] [Google Scholar]
- 15.Jaiswal S, Jamieson CH, Pang WW, et al. : CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138:271-285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majeti R, Chao MP, Alizadeh AA, et al. : CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138:286-299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chao MP, Takimoto CH, Feng DD, et al. : Therapeutic targeting of the macrophage immune checkpoint CD47 in myeloid malignancies. Front Oncol 9:1380, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng D, Gip P, McKenna KM, et al. : Combination treatment with 5F9 and azacitidine enhances phagocytic elimination of acute myeloid leukemia. Blood 132, 2018. (suppl 1; abstr 2729) [Google Scholar]
- 19.Liu J, Wang L, Zhao F, et al. : Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One 10:e0137345, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sikic BI, Lakhani N, Patnaik A, et al. : First-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol 37:946-953, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vyas P, Knapper S, Kelly R, et al. : Initial phase 1 results of the first-in-class anti-CD47 antibody Hu5F9-G4 in relapsed/refractory acute myeloid leukemia patients. Presented at the European Hematology Association, Stockholm, Sweden, June 14-17, 2018
- 22.DiNardo CD, Pratz K, Pullarkat V, et al. : Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133:7-17, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim K, Maiti A, Loghavi S, et al. : Outcomes of TP53-mutant acute myeloid leukemia with decitabine and venetoclax. Cancer 127:3772-3781, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheson BD, Bennett JM, Kopecky KJ, et al. : Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21:4642-4649, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Brierley CK, Staves J, Roberts C, et al. : The effects of monoclonal anti-CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion 59:2248-2254, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Advani R, Flinn I, Popplewell L, et al. : CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's lymphoma. N Engl J Med 379:1711-1721, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosman GJ, Willekens FL, Werre JM: Erythrocyte aging: A more than superficial resemblance to apoptosis? Cell Physiol Biochem 16:1-8, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Khandelwal S, van Rooijen N, Saxena RK: Reduced expression of CD47 during murine red blood cell (RBC) senescence and its role in RBC clearance from the circulation. Transfusion 47:1725-1732, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Oldenborg PA, Zheleznyak A, Fang YF, et al. : Role of CD47 as a marker of self on red blood cells. Science 288:2051-2054, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Dombret H, Seymour JF, Butrym A, et al. : International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126:291-299, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. : Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol 28:562-569, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Tang L, Dolnik A, MacBeth KJ, et al. : Impact of gene mutations on overall survival in older patients with acute myeloid leukemia (AML) treated with azacitidine (AZA) or conventional care regimens (CCR). Blood 128:2859, 2016. 27799161 [Google Scholar]
- 33.Sallman DA, DeZern AE, Garcia-Manero G, et al. : Eprenetapopt (APR-246) and azacitidine in TP53-mutant myelodysplastic syndromes. J Clin Oncol 39:1584-1594, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mishra A, Tamari R, DeZern AE, et al. : Eprenetapopt plus azacitidine after allogeneic hematopoietic stem-cell transplantation for TP53-mutant acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol 40:3985-3993, 2022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on submitted curriculum vitae and reflecting nonconflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science's discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.