Abstract
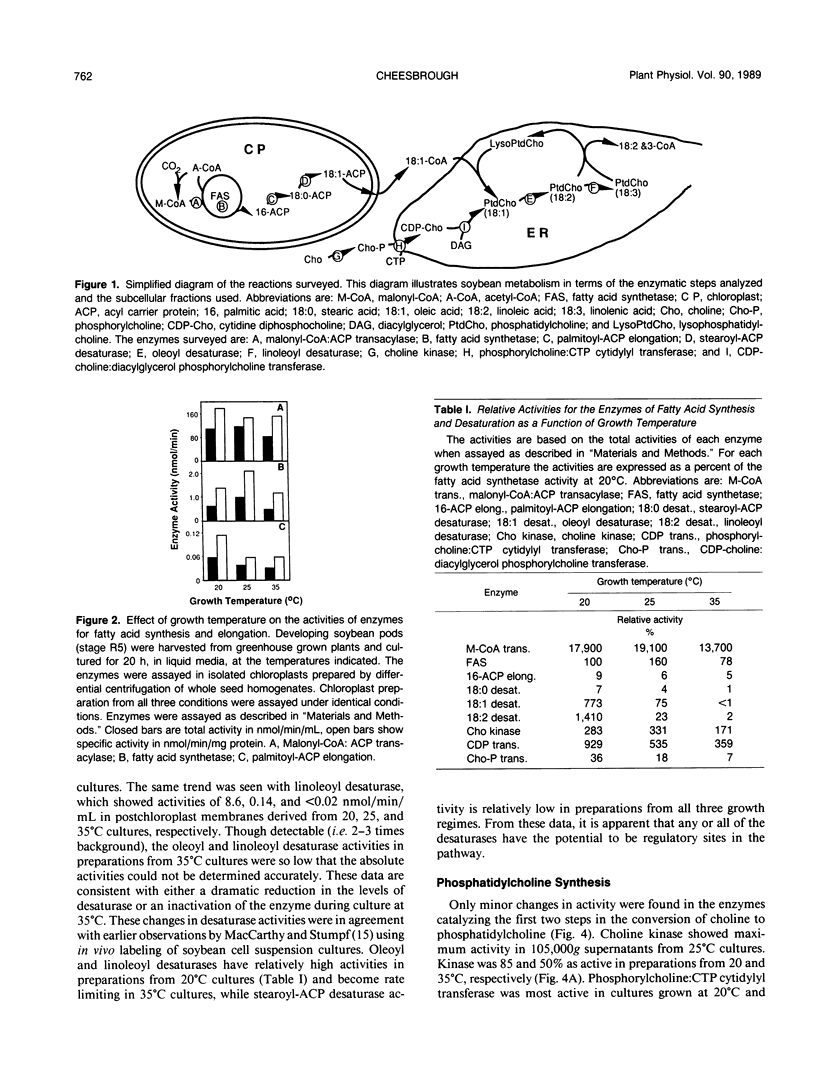
Temperature-induced changes in the enzymes for fatty acid synthesis and desaturation were studied in developing soybean seeds (Glycine max L. var Williams 82). Changes were induced by culture of the seed pods for 20 hours in liquid media at 20, 25, or 35°C. Linoleoyl and oleoyl desaturases were 94 and 10 times as active, respectively, in seeds cultured at 20°C as those cultured at 25°C. Both desaturases had negligible activity in seeds cultured at 35°C compared to seeds cultured at 20°C. Though less dramatic, other enzymes also showed differences in activity after 20 hours in culture at 20, 25, or 35°C. Stearoyl-acyl carrier protein (ACP) desaturase and CDP-choline:diacylglycerol phosphorylcholine transferase were most active in preparations from 20°C cultures. Activities were twofold lower at 25°C and a further threefold lower in 35°C cultures. Cultures from 25 and 35°C had 60 and 40%, respectively, of the phosphorylcholine:CTP cytidylyl transferase activity present in cultures grown at 20°C. Fatty acid synthetase, malonyl-coenzyme A:ACP transacylase, palmitoyl-ACP elongation, and choline kinase were not significantly altered by culture temperature. These data suggest that the enzymes for fatty acid desaturation and phosphatidylcholine synthesis can be rapidly modulated in response to altered growth temperatures, while the enzymes for fatty acid synthesis and elongation are not.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choy P. C., Lim P. H., Vance D. E. Purification and characterization of CTP: cholinephosphate cytidylytransferase from rat liver cytosol. J Biol Chem. 1977 Nov 10;252(21):7673–7677. [PubMed] [Google Scholar]
- Guerra D. J., Ohlrogge J. B. Partial purification and characterization of two forms of malonyl-coenzyme A:acyl carrier protein transacylase from soybean leaf tissue. Arch Biochem Biophys. 1986 Apr;246(1):274–285. doi: 10.1016/0003-9861(86)90473-x. [DOI] [PubMed] [Google Scholar]
- Jaworski J. G., Goldschmidt E. E., Stumpf P. K. Fat metabolism in higher plants. Properties of the palmityl acyl carrier protein: stearyl acyl carrier protein elongation system in maturing safflower seed extracts. Arch Biochem Biophys. 1974 Aug;163(2):769–776. doi: 10.1016/0003-9861(74)90539-6. [DOI] [PubMed] [Google Scholar]
- Kinney A. J., Moore T. S., Jr Phosphatidylcholine synthesis in castor bean endosperm: characteristics and reversibility of the choline kinase reaction. Arch Biochem Biophys. 1988 Jan;260(1):102–108. doi: 10.1016/0003-9861(88)90429-8. [DOI] [PubMed] [Google Scholar]
- Kinney A. J., Moore T. S., Jr Phosphatidylcholine synthesis in castor bean endosperm: the localization and control of CTP: choline-phosphate cytidylyltransferase activity. Arch Biochem Biophys. 1987 Nov 15;259(1):15–21. doi: 10.1016/0003-9861(87)90464-4. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Beevers H. Intracellular distribution of enzymes of the cytidine diphosphate choline pathway in castor bean endosperm. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2429–2432. doi: 10.1073/pnas.69.9.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. A., Rinne R. W. A Comparison of Oleic Acid Metabolism in the Soybean (Glycine max [L.] Merr.) Genotypes Williams and A5, a Mutant with Decreased Linoleic Acid in the Seed. Plant Physiol. 1986 May;81(1):41–44. doi: 10.1104/pp.81.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H., Yamamoto M., Nishida T., Nunoi K., Kikuchi M. High-performance liquid chromatographic analysis of serum long-chain fatty acids by direct derivatization method. J Chromatogr. 1987 May 15;416(2):237–245. doi: 10.1016/0378-4347(87)80507-8. [DOI] [PubMed] [Google Scholar]
- Moore T. S. Phosphatidylcholine synthesis in castor bean endosperm. Plant Physiol. 1976 Mar;57(3):382–386. doi: 10.1104/pp.57.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman H. A., Smith L. A., Lynch D. V., Thompson G. A., Jr Low-temperature-induced changes in intracellular fatty acid fluxes in Dunaliella salina. Arch Biochem Biophys. 1985 Oct;242(1):157–167. doi: 10.1016/0003-9861(85)90489-8. [DOI] [PubMed] [Google Scholar]
- Price-Jones M. J., Harwood J. L. Hormonal regulation of phosphatidylcholine synthesis in plants. The inhibition of cytidylyltransferase activity by indol-3-ylacetic acid. Biochem J. 1983 Dec 15;216(3):627–631. doi: 10.1042/bj2160627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Acyl carrier protein from Escherichia coli. Methods Enzymol. 1981;71(Pt 100):341–351. doi: 10.1016/0076-6879(81)71043-7. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Solubilization, purification, and salt activation of acyl-acyl carrier protein synthetase from Escherichia coli. J Biol Chem. 1979 Aug 10;254(15):7116–7122. [PubMed] [Google Scholar]
- Rock C. O., Garwin J. L. Preparative enzymatic synthesis and hydrophobic chromatography of acyl-acyl carrier protein. J Biol Chem. 1979 Aug 10;254(15):7123–7128. [PubMed] [Google Scholar]
- Shimakata T., Stumpf P. K. The purification and function of acetyl coenzyme A:acyl carrier protein transacylase. J Biol Chem. 1983 Mar 25;258(6):3592–3598. [PubMed] [Google Scholar]
- Thompson G. A., Jr Metabolism and control of lipid structure modification. Biochem Cell Biol. 1986 Jan;64(1):66–69. doi: 10.1139/o86-010. [DOI] [PubMed] [Google Scholar]


