Abstract
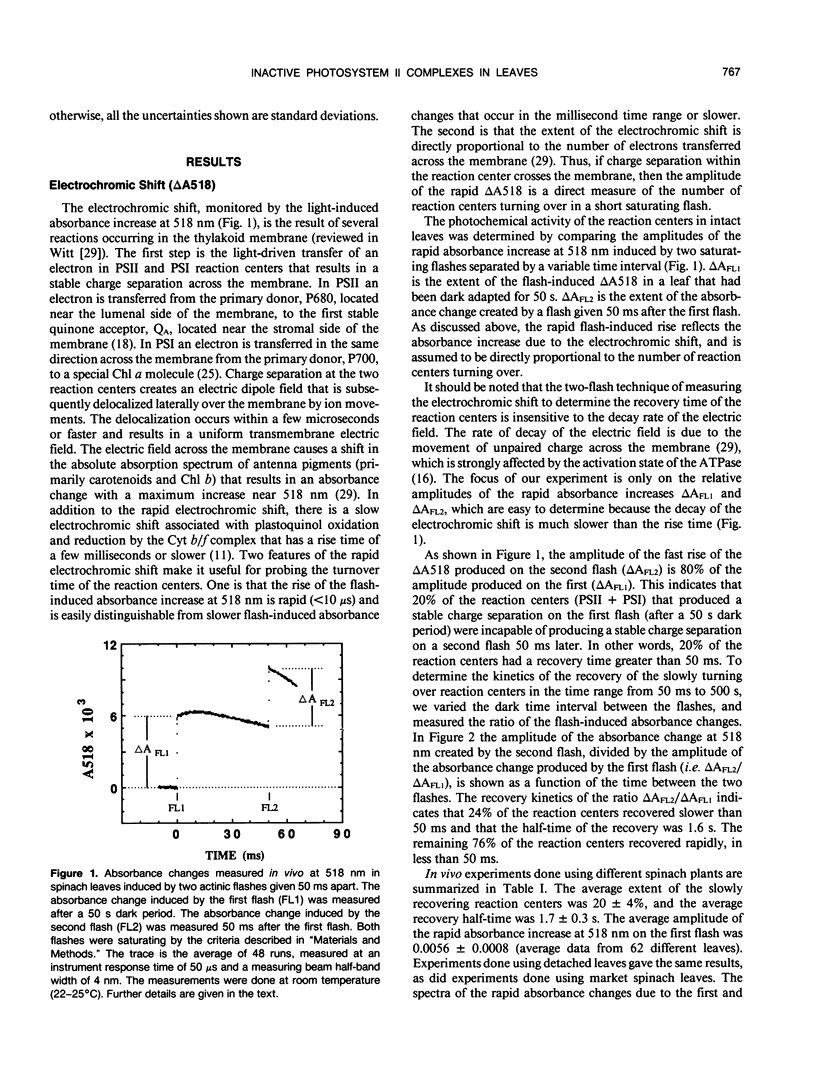
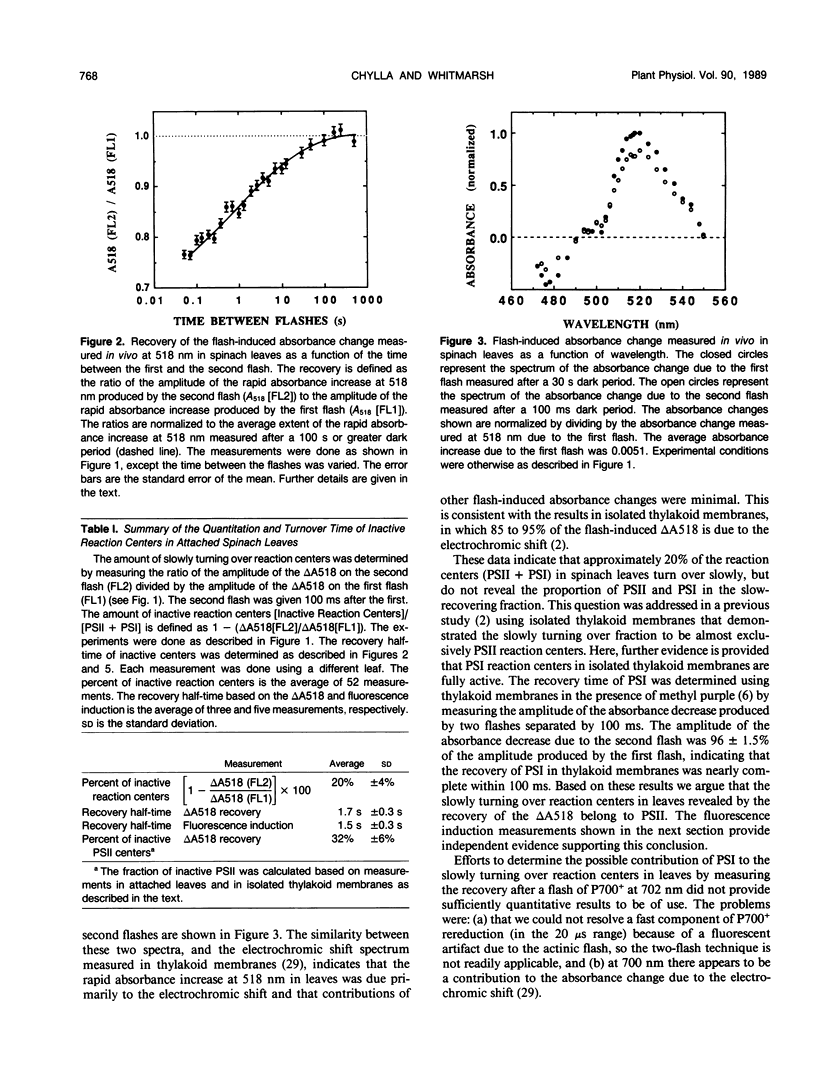
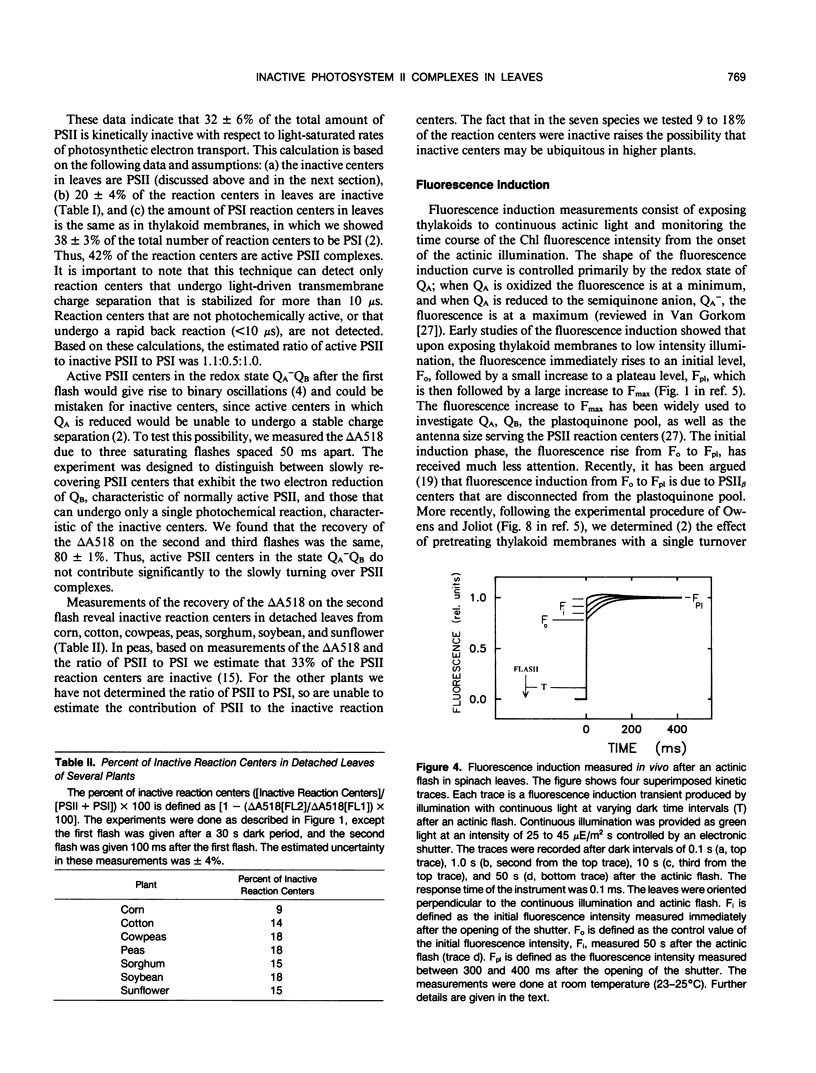
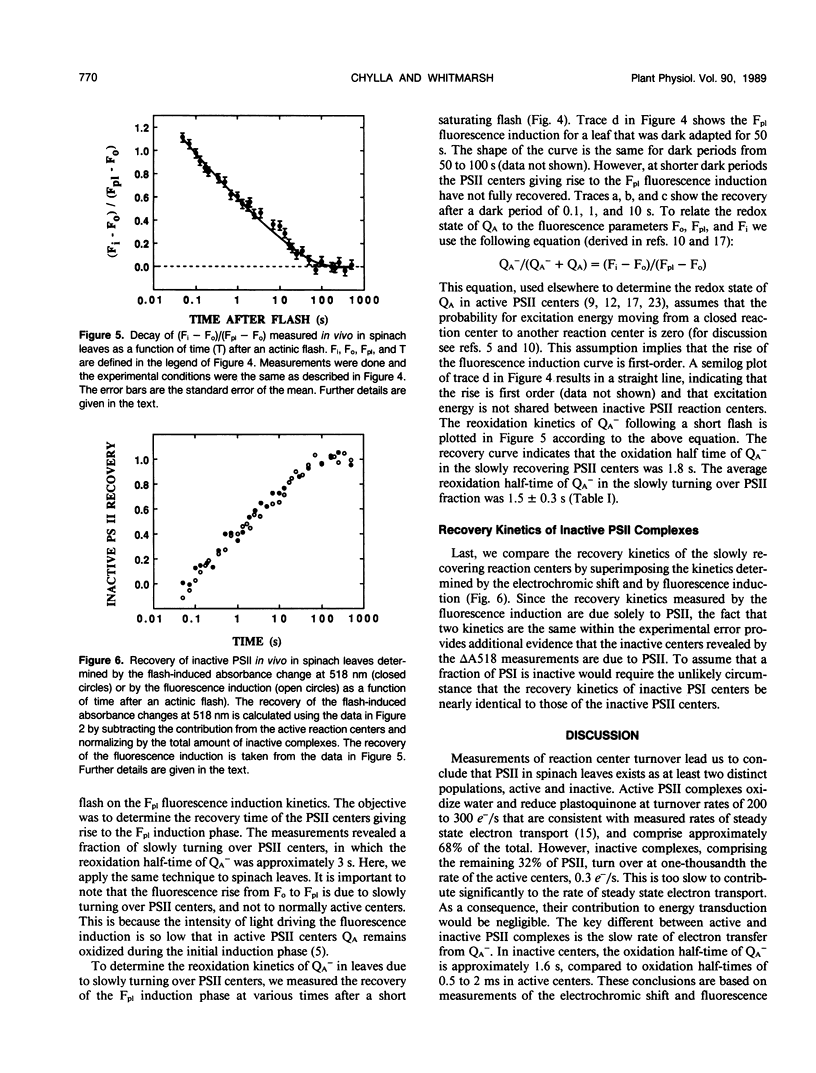
The flash-induced electrochromic shift, measured by the amplitude of the rapid absorbance increase at 518 nanometers (ΔA518), was used to determine the amount of charge separation within photosystems II and I in spinach (Spinacia oleracea L.) leaves. The recovery time of the reaction centers was determined by comparing the amplitudes of ΔA518 induced by two flashes separated by a variable time interval. The recovery of the ΔA518 on the second flash revealed that 20% of the reaction centers exhibited a recovery half-time of 1.7 ± 0.3 seconds, which is 1000 times slower than normally active reaction centers. Measurements using isolated thylakoid membranes showed that photosystem I constituted 38% of the total number of reaction centers, and that the photosystem I reaction centers were nearly fully active, indicating that the slowly turning over reaction centers were due solely to photosystem II. The results demonstrate that in spinach leaves approximately 32% of the photosystem II complexes are effectively inactive, in that their contribution to energy conversion is negligible. Additional evidence for inactive photosystem II complexes in spinach leaves was provided by fluorescence induction measurements, used to monitor the oxidation kinetics of the primary quinone acceptor of photosystem II, QA, after a short flash. The measurements showed that in a fraction of the photosystem II complexes the oxidation of QA− was slow, displaying a half-time of 1.5 ± 0.3 seconds. The kinetics of QA− oxidation were virtually identical to the kinetics of the recovery of photosystem II determined from the electrochromic shift. The key difference between active and inactive photosystem II centers is that in the inactive centers the oxidation rate of QA− is slow compared to active centers. Measurements of the electrochromic shift in detached leaves from several different species of plants revealed a significant fraction of slowly turning over reaction centers, raising the possibility that reaction centers that are inefficient in energy conversion may be a common feature in plants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Forbush B., Kok B. Reaction between primary and secondary electron acceptors of photosystem II of photosynthesis. Biochim Biophys Acta. 1968 Aug 20;162(2):243–253. doi: 10.1016/0005-2728(68)90106-0. [DOI] [PubMed] [Google Scholar]
- Graan T., Ort D. R. Quantitation of the rapid electron donors to P700, the functional plastoquinone pool, and the ratio of the photosystems in spinach chloroplasts. J Biol Chem. 1984 Nov 25;259(22):14003–14010. [PubMed] [Google Scholar]
- Itoh S. Membrane surface potential and the reactivity of the system II primary electron acceptor to charged electron carriers in the medium. Biochim Biophys Acta. 1978 Nov 9;504(2):324–340. doi: 10.1016/0005-2728(78)90180-9. [DOI] [PubMed] [Google Scholar]
- JOLIOT A., JOLIOT P. ETUDE CIN'ETIQUE DE LA R'EACTION PHOTOCHIMIQUE LIB'ERANT L'OXYG'ENE AU COURS DE LA PHOTOSYNTH'ESE. C R Hebd Seances Acad Sci. 1964 May 4;258:4622–4625. [PubMed] [Google Scholar]
- Lee W. J., Whitmarsh J. Photosynthetic apparatus of pea thylakoid membranes : response to growth light intensity. Plant Physiol. 1989 Mar;89(3):932–940. doi: 10.1104/pp.89.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin S., Kok B. Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta. 1966 Nov 8;126(3):413–432. doi: 10.1016/0926-6585(66)90001-x. [DOI] [PubMed] [Google Scholar]
- Melis A., Homann P. H. Heterogeneity of the photochemical centers in system II of chloroplasts. Photochem Photobiol. 1976 May;23(5):343–350. doi: 10.1111/j.1751-1097.1976.tb07259.x. [DOI] [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Portis A. R. Involvement of stromal ATP in the light activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in intact isolated chloroplasts. Plant Physiol. 1988 Jan;86(1):293–298. doi: 10.1104/pp.86.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh J., Ort D. R. Stoichiometries of electron transport complexes in spinach chloroplasts. Arch Biochem Biophys. 1984 Jun;231(2):378–389. doi: 10.1016/0003-9861(84)90401-6. [DOI] [PubMed] [Google Scholar]
- Witt H. T. Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The central role of the electric field. Biochim Biophys Acta. 1979 Mar 14;505(3-4):355–427. doi: 10.1016/0304-4173(79)90008-9. [DOI] [PubMed] [Google Scholar]


