Abstract
Temperature appears to be an important factor affecting the occurrence and distribution of aquatic hyphomycetes, the dominant leaf litter-decomposing fungi in streams. We compared conidium production by eight species of aquatic hyphomycetes grown on yellow poplar leaves in stream-simulating microcosms at three temperatures (15, 20, and 25°C). The greatest conidium production occurred at 15°C for one species, 20°C for two species, and 25°C for two species. Two species produced similar numbers of conidia at 20 and 25°C, and one species produced similar numbers of conidia at all three temperatures. Linear growth rates were determined on malt extract agar. Six species had the same pattern of temperature responses for growth on malt extract agar as for sporulation on leaves, as shown by the positive correlations between the two parameters at the three temperatures. The species examined also exhibited differences in number of conidia produced from a similar amount of leaf material at a given temperature. These differences appeared to be due primarily to differences in individual conidium mass (determined by weighing conidia produced from cultures), as shown by the relationship of the type Y = k/X (r2 = 0.96), where Y is the number of conidia produced, X is the individual conidium mass in milligrams, and k is a constant empirically determined to be 2.11. This finding supports the hypothesis that aquatic hyphomycetes allocate similar amounts of their resources to reproduction but vary with respect how these resources are partitioned into reproductive units (conidia).
Terrestrial leaf litter is a major energy source in woodland streams (4, 8). Aquatic hyphomycetes are the predominant microorganisms that colonize leaves in streams, and their activity is affected by a number of environmental variables (1, 13). Temperature appears to be an important factor affecting the occurrence and distribution of these fungi (11). Some species are more common in temperate climates, and others are more common in the tropics (1, 9). In temperate climates, seasonal shifts in species composition can occur, with species common in the tropics becoming dominant during the summer and absent during the winter (3, 11). The effect of temperature on the growth and sporulation of aquatic hyphomycetes has received relatively little attention, and in all instances, fungi were grown on agar media containing relatively high concentrations of nutrients (7, 11, 17). Recently, the activity of aquatic hyphomycetes growing on leaf litter has been studied in laboratory microcosms (12). In these microcosms, aquatic hyphomycetes cause changes to leaves that are similar to changes that occur in the stream environment (5, 12). In addition, concentrations of nutrients similar to those occurring naturally in streams can be maintained. By counting conidia released from fungi growing on leaf material in microcosms, sporulation rates during the degradation of leaf material under controlled conditions can be determined.
Our major objective was to determine the effect of temperature on the sporulation of eight common aquatic hyphomycetes grown on leaf litter in stream-simulating microcosms and to compare these results with the effect of temperature on growth as determined from rates of radial extension on agar media. In the course of carrying out these studies, it became evident that fungal species exhibited large differences in the numbers of conidia produced from a given amount of leaf litter. The total number of conidia produced by a fungal species appeared to be related to the size of the conidia. A secondary objective of this study was to determine the individual conidial mass for each species and to investigate potential relationships between the total number of conidia produced and the individual conidial mass.
MATERIALS AND METHODS
Fungal strains.
Cultures of aquatic hyphomycetes were obtained from single-spore isolates and maintained on 1 or 2% malt extract agar (pH 5.6). The strains used in the experiments originated from Alabama (referenced as AL) and southwestern France (referenced as CERR). These regions exhibit slight differences in their temperature regime: water temperature in Alabama streams ranges from 2 to 25°C, whereas a typical range for French mountain streams is about 2 to 18°C. The species chosen were among the most common and representative in either region (3, 6, 12, 14) and included Anguillospora filiformis Greathead AL 5, Anguillospora longissima (Sacc. et Sydow) Ingold AL 4, Articulospora tetracladia Ingold AL 101, Flagellospora curvula Ingold CERR 30.67, Lunulospora curvula Ingold AL 83, Tetrachaetum elegans Ingold CERR 28.74, Tetracladium marchalianum De Wild. AL 103, and Tricladium chaetocladium Ingold AL 2. Most of these species are widely distributed, with L. curvula being more common in warmer streams and F. curvula being more common in colder streams.
Effect of temperature on sporulation.
Sporulation rates were determined in stream-simulating microcosms as previously described (12). The microcosms are glass aeration chambers containing the fungi growing axenically on leaf disks in a liquid medium. Each chamber is aerated at a controlled flow rate (100 ml min−1) which causes continuous agitation of the leaf disks in the medium. In each microcosm, 15 sterilized leaf disks of yellow poplar (Liriodendron tulipifera L.) and 40 ml of a sterile liquid medium (0.1 g of CaCl2 · 2H2O, 10 mg of MgSO4 · 7H2O, 10 mg of KNO3, 0.55 mg of K2HPO4, and 0.5 g of MOPS [3-morpholinopropanesulfonic acid] buffer in 1 liter of water, adjusted to pH 7 with KOH) were inoculated with a suspension containing ca. 3,000 conidia. The medium was replaced after the first day and then every 2 days, and the number of conidia released for each 2-day period was determined. For the first 7 days, all the microcosms were incubated at 15°C to minimize the effect of temperature on the initial mycelial growth. The end of this period coincided with the beginning of sporulation. The microcosms were then moved to different incubators set at 15, 20, and 25°C to examine the effect of these temperatures on sporulation. Three replicate microcosms per species and per temperature were sampled during the subsequent 18 to 28 days. Numbers of conidia were determined by filtering (5-μm-pore-size membrane filter) aliquots of the conidial suspension or dilutions thereof, fixing and staining them with lactic acid containing 0.1% trypan blue, and counting the conidia present in 25 fields at a magnification of ×160. Duplicate filters were counted for each replicate.
Effect of temperature on mycelial growth.
Petri dishes (diameter, 90 mm) filled with 25 ml of 2% malt extract agar (pH 5.6) were inoculated with cylinders (diameter, 6 mm) of fungal mycelium cut from the growing edge of colonies. The radius of each colony (three measurements per plate, evenly spaced out to fill 360°) was measured at intervals of 2 to 3 days. Five replicate plates were used for each species and each incubation temperature (15, 20, and 25°C). Growth was expressed as rates of radial extension (millimeters per day).
Determination of individual conidial mass.
Conidia from each of the eight species were produced by aerating fungal mycelium growing on 1% malt agar in six microcosms containing distilled water. After sporulation began, the conidial suspensions produced from all microcosms during 24 h were combined and the conidial concentrations were determined by filtering aliquots and counting as described above. Known volumes of the conidial suspension were then filtered through tared membrane filters, dried at 60°C, and weighed (12).
Statistical analyses.
Statistical analyses and calculations were performed with the Systat computer package (19).
RESULTS
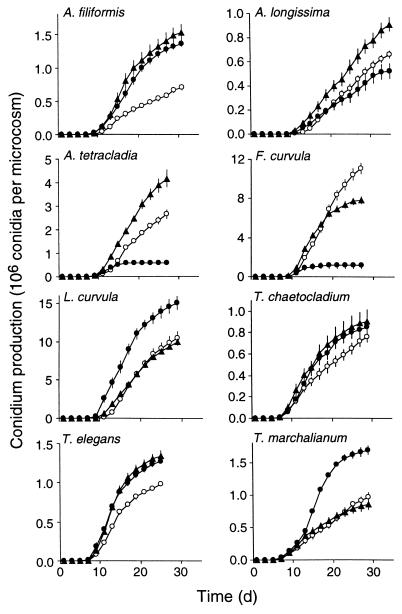
As the fungi grew on leaf disks in the microcosms, most exhibited similar patterns of growth and sporulation (Fig. 1) that consisted of four phases: (i) a period of 7 to 9 days following inoculation without production of conidia, (ii) a period of a few days of increasing sporulation rate, (iii) 6 to 10 days or more of constant and high sporulation rates, and (iv) a phase of decreasing sporulation rate. An exception was Anguillospora longissima at 15 and 20°C, in which the rate of sporulation remained more or less constant until the end of the study (35 days for this species). The fungal species differed both in the pattern of their sporulation response to temperature and in their overall sporulation rate (Fig. 1). In all species except Tricladium chaetocladium, the temperature significantly affected the sporulation rate (analysis of variance [Table 1]). As shown in Fig. 1 and as shown by the F values (Table 1), F. curvula exhibited the greatest differences in response to this range of temperatures. In this species, conidium production was highest at 15°C and lowest at 25°C. Conidium production by Articulospora tetracladia was also low at 25°C. This species, together with Anguillospora longissima, exhibited the highest conidium production at 20°C. For L. curvula and Tetracladium marchalianum, the highest conidium production occurred at 25°C, whereas total conidium production by Anguillospora filiformis and Tetrachaetum elegans was not significantly different at 20 and 25°C. When the highest sporulation rates of each species were compared by analysis of variance (data not shown), the effect of temperature was the same as noted for conidium production.
FIG. 1.
Cumulative number of conidia produced per microcosm at the three temperatures. ○, 15°C; ▴, 20°C; ▪, 25°C. Vertical bars indicate ±1SE (n = 3).
TABLE 1.
Analysis of variance of the cumulative conidium production at the three temperaturesa
| Species | F | P |
|---|---|---|
| Anguillospora filiformis | 30 | <0.001 |
| Anguillospora longissima | 13 | <0.010 |
| Articulospora tetracladia | 57 | <0.001 |
| Flagellospora curvula | 136 | <0.001 |
| Lunulospora curvula | 14 | <0.010 |
| Tricladium chaetocladium | 1 | 0.416 |
| Tetrachaetum elegans | 23 | <0.010 |
| Tetracladium marchalianum | 36 | <0.001 |
n = 3 for each species and each temperature.
The relationship between mycelial growth and sporulation responses to temperature varied with species (Table 2). Six of the eight species exhibited significant linear relationships with positive slopes between growth rate and conidium production at the temperatures examined (P < 0.01). For Anguillospora longissima, no simple linear relation was found, due to decreased sporulation activity coinciding with an increased growth rate at 25°C. For Tetracladium marchalianum, the linear regression was negative because of greater sporulation and minimum growth rates at the highest temperature.
TABLE 2.
Radial growth rates on malt extract agar at the three temperatures
| Species | Radial growth rate (mm/day) ata:
|
Signb | ||
|---|---|---|---|---|
| 15°C | 20°C | 25°C | ||
| Anguillospora filiformis | 0.63 ± 0.04 | 0.85 ± 0.02 | 0.88 ± 0.02 | + |
| Anguillospora longissima | 0.79 ± 0.01 | 1.28 ± 0.01 | 1.51 ± 0.02 | ∼0 |
| Articulospora tetracladia | 1.10 ± 0.01 | 1.72 ± 0.01 | 0.70 ± 0.02 | + |
| Flagellospora curvula | 0.80 ± 0.01 | 0.97 ± 0.02 | 0.11 ± 0.01 | + |
| Lunulospora curvula | 0.29 ± 0.01 | 0.39 ± 0.02 | 0.68 ± 0.01 | + |
| Tricladium chaetocladium | 0.61 ± 0.01 | 0.81 ± 0.01 | 0.90 ± 0.01 | + |
| Tetrachaetum elegans | 0.54 ± 0.02 | 0.75 ± 0.02 | 0.59 ± 0.01 | + |
| Tetracladium marchalianum | 0.73 ± 0.01 | 1.00 ± 0.01 | 0.46 ± 0.01 | − |
Mean ± SE.
The sign refers to the slope of the linear regression between growth rate and conidium production.
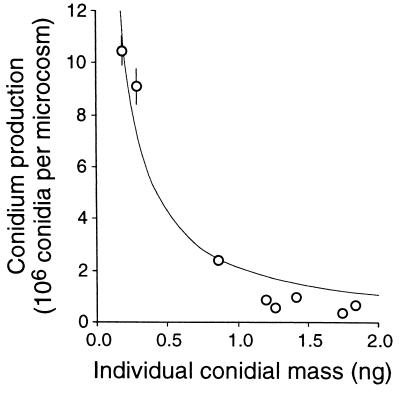
The number of conidia produced per microcosm after 25 days at 15°C ranged from 0.38 × 106 (Anguillospora longissima) to 10.5 × 106 (F. curvula). Similar ranges occurred at the other temperatures. The individual conidial mass of the eight species varied over an order of magnitude from 0.19 ng/conidium for F. curvula to 1.83 ng/conidium for Tricladium chaetocladium (Table 3). The species with the smallest conidia exhibited the highest conidium production, while the species with the second highest individual conidial mass showed the lowest conidium production (Fig. 2; Table 3). Since individual conidial mass and number produced appeared to be inversely proportional, the relationship chosen to fit the data was of the type Y = k/X, where Y is the cumulative number of conidia produced per microcosm and X is the individual conidial mass expressed in milligrams. The equation for the nonlinear regression of these data was Y = 2.11/X (asymptotic standard error [SE] = 0.09, r2 = 0.96).
TABLE 3.
Individual conidial mass of the eight species of aquatic hyphomycetes
| Species | Mass (ng)a | n |
|---|---|---|
| Anguillospora filiformis | 1.27 ± 0.11b | 5 |
| Anguillospora longissima | 1.74 ± 0.12 | 5 |
| Articulospora tetracladia | 0.86 ± 0.11 | 8 |
| Flagellospora curvula | 0.19 ± 0.04 | 4 |
| Lunulospora curvula | 0.29 ± 0.04b | 7 |
| Tricladium chaetocladium | 1.83 ± 0.19 | 6 |
| Tetrachaetum elegans | 1.42 ± 0.07 | 7 |
| Tetracladium marchalianum | 1.21 ± 0.18 | 9 |
Mean ± SE.
From reference 12.
FIG. 2.
Cumulative number of conidia produced per microcosm at 15°C as a function of the individual conidial mass of the eight species. Vertical bars indicate ±1SE (n = 3 per species). The curve indicates the regression Y = 2.11/X (r2 = 0.96).
DISCUSSION
Under the conditions of this study, the period of sporulation for all species extended over 15 to 20 days except in the case of unfavorable temperatures (Articulospora tetracladia and F. curvula at 25°C). Anguillospora longissima was the only species whose rate of sporulation did not decrease after 35 days (15 and 20°C only). This was possibly due to an exceptionally long period of sporulation activity or to a continuous sporulation-germination-growth cycle caused by insufficient conidia in the inoculum at the beginning of the experiment. The effect of temperature on growth was similar to its effect on sporulation for most of the species examined (Table 2). However, this was not the case for two species (Anguillospora longissima and Tetracladium marchalianum). While this may be due simply to differences in the methods (e.g., different substrates and media) used for the two measurements, we cannot rule out the possibility that these fungi possess different strategies for mycelial growth and for allocation of resources to reproductive structures. A temperature unfavorable for growth may stimulate sporulation, as was observed for T. marchalianum at 25°C.
To induce the sporulation of aquatic hyphomycetes, many species must be submerged (15), and sporulation rates are generally stimulated by turbulence or flow (10, 18). Previously, the effect of temperature on sporulation activity of aquatic hyphomycete species has been based on conidium production by colonies grown on agar containing relatively high concentrations of nutrients (7, 17). Koske and Duncan (7) examined species isolated from terrestrial environments and determined the number of spores produced on the surface of agar cultures without submersing the mycelia. They found small numbers of conidia, making their results difficult to compare with the results of the present study. Webster et al. (17) found that sporulation by L. curvula at 25°C was 1,000 times that at 15°C whereas conidium production by Tricladium chaetocladium was similar at 15 and 20°C and slightly depressed at 25°C. In both cases, they aerated agar disks containing mycelium in water. In the present study, conidium production by L. curvula at 25°C was only 1.5 times that at 15°C and sporulation of T. chaetocladium was not significantly different at the three temperatures. Such differences between studies, particularly with respect to the magnitude of the changes in the sporulation rate of L. curvula, appear to be due to the differences in nutrient content available to the fungi. Note that leaf material such as that used in the present study simulates natural situations more closely. However, variations due to the use of different strains in these studies, with these strains possibly corresponding to different ecotypes, cannot be precluded.
The temperatures we examined in the present study (15 to 25°C) represent the upper range for many streams in temperate zones of the world. It appears that as the temperature approaches 25°C, the sporulation of some species like F. curvula and Articulospora tetracladia would be inhibited. The decrease in sporulation activity by these species at temperatures between 20 and 25°C agrees with previous observations on the seasonal occurrence of these species in nature (3, 6, 11). However, the effect of temperature may be modified in situations where two or more species are competing. When L. curvula and Tricladium chaetocladium were grown in mixed cultures, the optimum temperatures for sporulation were lower than when the fungi were grown in pure cultures (17). These observations suggest that the fungal response to temperature under natural conditions might be more complex than illustrated by the results of this study. Currently, the relative importance of the direct effect of temperature on the success of a species and how this may be modified as a result of interspecific interactions remains unknown.
Although aquatic hyphomycetes have been essentially studied through their anamorphic stage (i.e., that producing asexual spores, or conidia), the existence of the teleomorphic stage in these fungi must not be ignored (16). About 1/10 of the currently described anamorphs of these fungi (three of the eight species in the present study) are connected to ascomycetous or, less frequently, basidiomycetous teleomorphs. However, the extent to which the sexual stage of aquatic hyphomycetes is relevant in the natural life cycle of these fungi remains largely unknown. While anamorphic spores are typically produced from ephemeral leaf litter, teleomorphs generally seem to occur on wood, i.e., on a substratum providing nutrients over a longer period, which allows the development of sexual structures (16). Teleomorphic forms could therefore be of interest not only in providing gene recombination through sexual reproduction but also in allowing these fungi to survive during periods unfavorable for growth. This may represent one mechanism by which species producing conidia within narrow temperature ranges can survive in streams exhibiting seasonally unfavorable temperatures.
The total number of conidia released from a given amount of leaf material (ca. 60 mg) in the present study was species dependent. Cumulative conidium production ranged from 0.9 × 106 to 15 × 106 conidia after 30 to 35 days in the present study. Differences among species appeared to be related to the size of the conidium produced. The greater number of conidia produced by species like F. curvula and L. curvula was balanced by the smaller individual conidium size of these species. Bärlocher and Schweizer (2) recognized the importance of size differences in the conidia of aquatic hyphomycete species and calculated the total reproductive output as the product of the number of conidia produced and the conidium volume. The model proposed here supports this concept for total reproductive output on a mass basis. It also supports the idea that the total conidium production expressed on a mass basis, i.e., the product of conidium production and individual conidial mass, is a constant under a particular set of conditions whatever the species. For the conditions used in the present study, the total reproductive output was 2.11 mg. Previous estimates of conidium production made for three species grown individually on leaf material (5, 12) agree with this value, which reinforces such an hypothesis. Consequently, the reproductive output of the eight aquatic hyphomycetes we examined presents a much narrower range when conidium production was expressed on a mass basis (minimum/maximum ratio, 1:4) than on a number basis (minimum/maximum ratio, 1:28). The remaining differences in reproductive output among species can be attributed to differences in basic strategies of resource allocation, which have also been demonstrated to vary with the species (5, 12, 15). Although the results in this study were obtained from a limited number of pure cultures, they seem to support some generalization. The species with more and smaller offspring, such as F. curvula and L. curvula, clearly contrast with the species producing fewer and larger conidia, such as Tricladium chaetocladium and Anguillospora longissima. The first group of species appears to include rapidly colonizing species which frequently dominate the fungal assemblages, especially during the initial stage of leaf colonization (6, 12). Whether the success of these species is due mainly to the large numbers of conidia (offspring) they produce or to other traits that comprise their life history strategies is not known. However, since aquatic hyphomycetes allocate an average of 50% or more of their production to the formation of conidia (5, 12), reproduction appears to be important in the colonization of detritus in streams by these fungi.
ACKNOWLEDGMENT
This work was supported by an exchange grant from the Centre National de la Recherche Scientifique and the National Science Foundation.
REFERENCES
- 1.Bärlocher F. The ecology of aquatic hyphomycetes, Ecol. Stud Anal Synth. 1992;94:1–225. [Google Scholar]
- 2.Bärlocher F, Schweizer M. Effects of leaf size and decay rate on colonization by aquatic hyphomycetes. Oikos. 1983;41:205–210. [Google Scholar]
- 3.Chauvet E. Aquatic hyphomycete distribution in South-Western France. J Biogeogr. 1991;18:699–706. [Google Scholar]
- 4.Cummins K W. The study of stream ecosystems: a functional view. In: Pomeroy L R, Alberts J J, editors. Concepts of ecosystem ecology. New York, N.Y: Springer Verlag; 1988. pp. 247–262. [Google Scholar]
- 5.Gessner M O, Chauvet E. Growth and production of aquatic hyphomycetes in decomposing leaf litter. Limnol Oceanogr. 1997;42:496–505. [Google Scholar]
- 6.Gessner M O, Thomas M, Jean-Louis A-M, Chauvet E. Stable successional patterns of aquatic hyphomycetes on leaves decaying in a summer cool stream. Mycol Res. 1993;97:163–173. [Google Scholar]
- 7.Koske R E, Duncan I W. Temperature effects on growth, sporulation and germination of some aquatic hyphomycetes. Can J Bot. 1974;52:1387–1391. [Google Scholar]
- 8.Maltby L. Detritus processing. In: Calow P, Petts G E, editors. The rivers handbook. Hydrological and ecological principles. Vol. 1. Oxford, United Kingdom: Blackwell Scientific Publications Ltd.; 1992. pp. 331–353. [Google Scholar]
- 9.Nilsson S. Freshwater hyphomycetes. Taxonomy, morphology and ecology. Symb Bot Ups. 1964;18:1–130. [Google Scholar]
- 10.Sanders P F, Webster J. Sporulation responses of some ‘aquatic hyphomycetes’ in flowing water. Trans Br Mycol Soc. 1980;74:601–605. [Google Scholar]
- 11.Suberkropp K. Effect of temperature on seasonal occurrence of aquatic hyphomycetes. Trans Br Mycol Soc. 1984;82:53–62. [Google Scholar]
- 12.Suberkropp K. Relationships between growth and sporulation of aquatic hyphomycetes on decomposing leaf litter. Mycol Res. 1991;95:843–850. [Google Scholar]
- 13.Suberkropp K. Aquatic hyphomycetes communities. In: Carroll G C, Wicklow D T, editors. The fungal community. Its organization and role in the ecosystem. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1992. pp. 729–747. [Google Scholar]
- 14.Suberkropp K, Chauvet E. Regulation of leaf breakdown by fungi in streams: influences of water chemistry. Ecology. 1995;76:1433–1445. [Google Scholar]
- 15.Suberkropp K, Weyers H S. Application of fungal and bacterial production methodologies to decomposing leaves in streams. Appl Environ Microbiol. 1996;62:1610–1615. doi: 10.1128/aem.62.5.1610-1615.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster J. Anamorph-teleomorph relationships. Ecol Stud Anal Synth. 1992;94:99–117. [Google Scholar]
- 17.Webster J, Moran S T, Davey R A. Growth and sporulation of Tricladium chaetocladium and Lunulospora curvula in relation to temperature. Trans Br Mycol Soc. 1976;67:491–495. [Google Scholar]
- 18.Webster J, Towfik F H. Sporulation of aquatic hyphomycetes in relation to aeration. Trans Br Mycol Soc. 1972;59:353–364. [Google Scholar]
- 19.Wilkinson L. Systat: the system for statistics. Evanston, Ill: Systat Inc.; 1990. [Google Scholar]