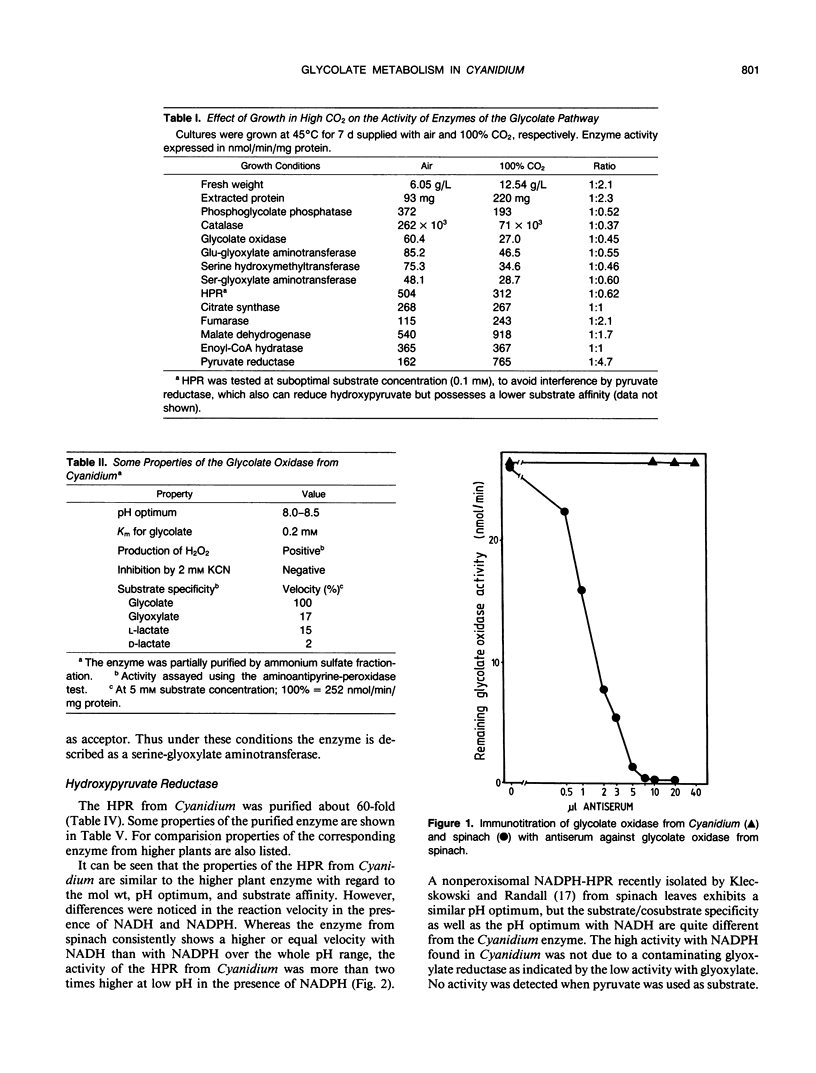
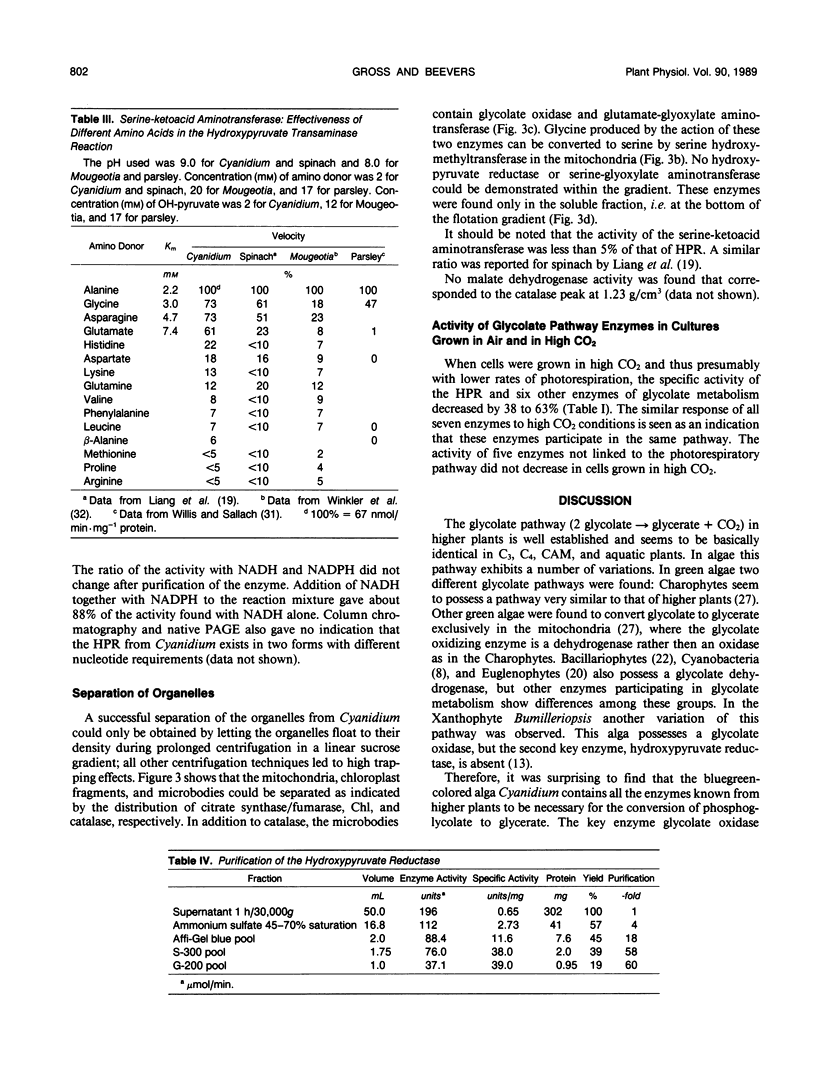
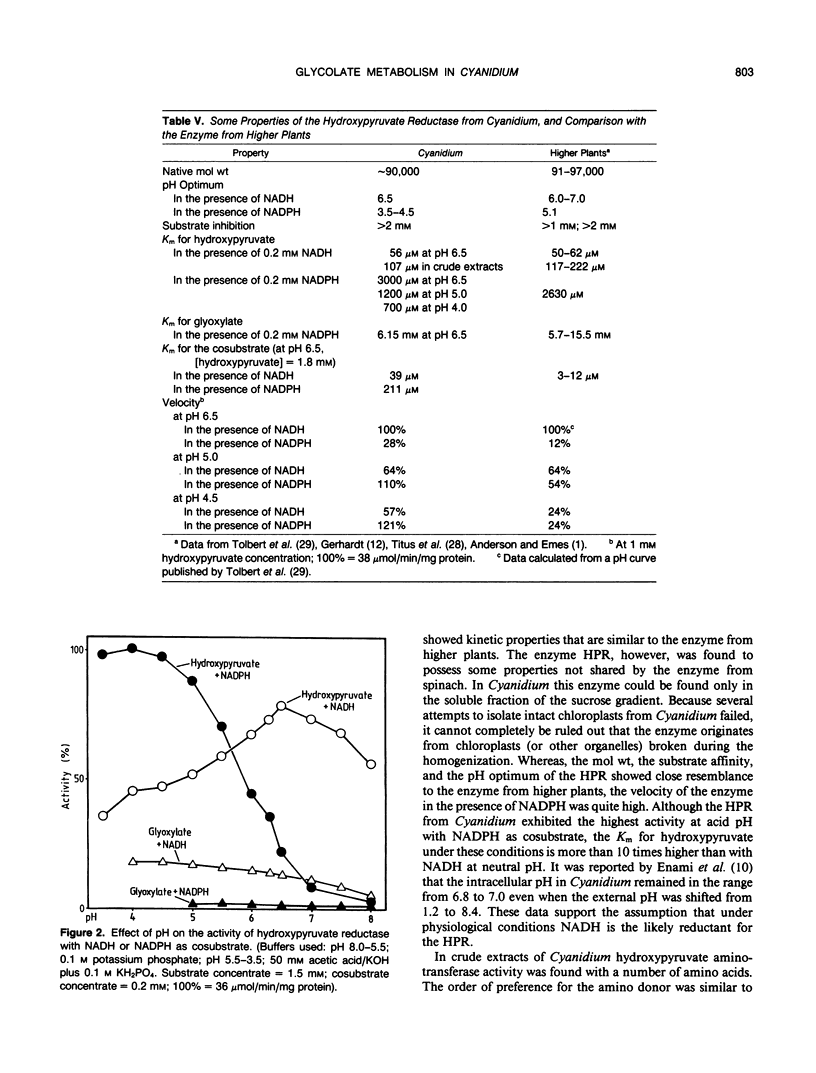
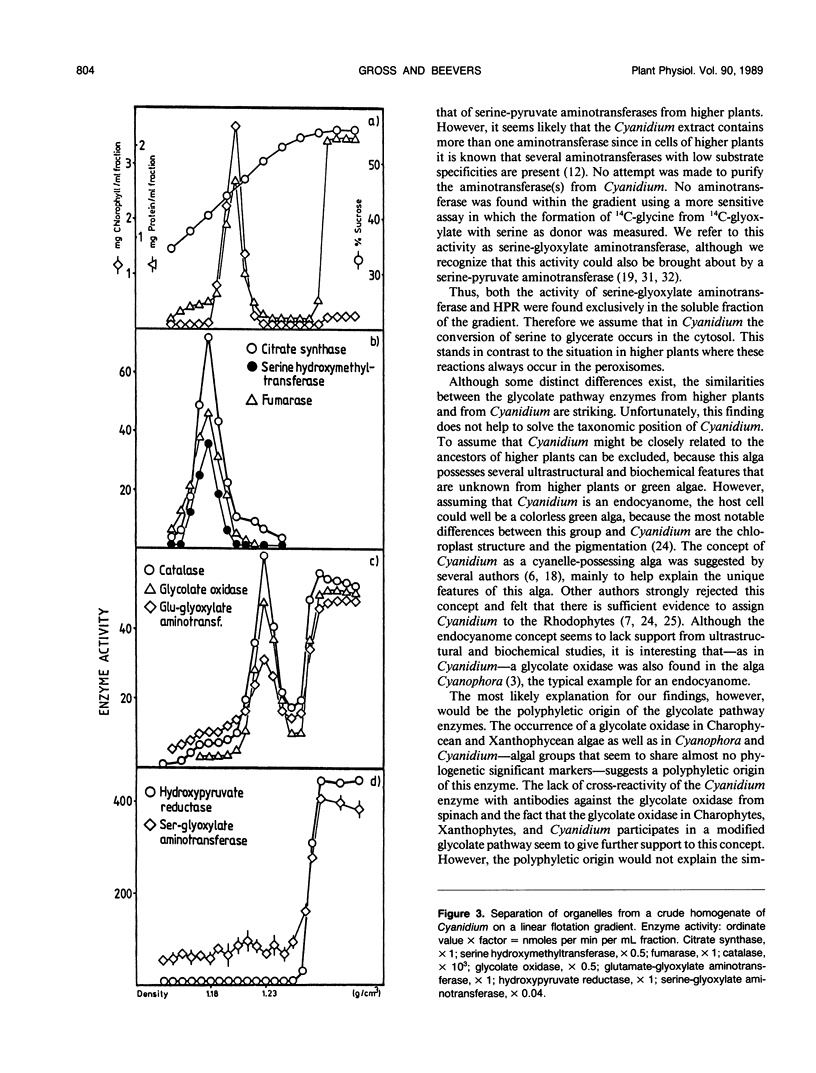
Abstract
The intracellular distribution of enzymes capable of catalyzing the reactions from phosphoglycolate to glycerate in the bluegreen colored eucaryotic alga Cyanidium caldarium has been studied. After separating the organelles from a crude homogenate on a linear flotation gradient, the enzymes glycolate oxidase and glutamate-glyoxylate aminotransferase along with catalase were present in the peroxisomal fraction (density: 1.23 grams per cubic centimeter). Serine hydroxymethyltransferase was found in the mitochondrial fraction (density: 1.18 grams per cubic centimeter). In contrast to the observations in green leaves of higher plants, the enzymes for the conversion of serine to glycerate (serine-glyoxylate aminotransferase and hydroxypyruvate reductase) were found only in the soluble fraction of the gradient. The partial characterization of enzymes from Cyanidium participating in glycolate metabolism revealed only slight differences from the corresponding enzymes from higher plants. The phylogenetic implications of the observed similarities between the enigmatic alga Cyanidium and higher plants are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Codd G. A., Stewart W. D. Pathways of glycollate metabolism in the blue-green alga Anabaena cylindrica. Arch Mikrobiol. 1973 Dec 4;94(1):11–28. doi: 10.1007/BF00414075. [DOI] [PubMed] [Google Scholar]
- Ford T. W. Ribulose 1,5-bisphosphate carboxylase from the thermophilic, acidophilic alga, Cyanidium caldarium (Geitler). Purification, characterisation and thermostability of the enzyme. Biochim Biophys Acta. 1979 Aug 15;569(2):239–248. doi: 10.1016/0005-2744(79)90059-7. [DOI] [PubMed] [Google Scholar]
- Gross W., Winkler U., Stabenau H. Characterization of Peroxisomes from the Alga Bumilleriopsis filiformis. Plant Physiol. 1985 Feb;77(2):296–299. doi: 10.1104/pp.77.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Bowman P. D., Beevers H. Immunological and biochemical studies on isozymes of malate dehydrogenase and citrate synthetase in castor bean glyoxysomes. Plant Physiol. 1974 Sep;54(3):364–367. doi: 10.1104/pp.54.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husic D. W., Tolbert N. E. NADH:hydroxypyruvate reductase and NADPH:glyoxylate reductase in algae: partial purification and characterization from Chlamydomonas reinhardtii. Arch Biochem Biophys. 1987 Feb 1;252(2):396–408. doi: 10.1016/0003-9861(87)90046-4. [DOI] [PubMed] [Google Scholar]
- Kleczkowski L. A., Randall D. D. Purification and characterization of a novel NADPH(NADH)-dependent hydroxypyruvate reductase from spinach leaves. Comparison of immunological properties of leaf hydroxypyruvate reductases. Biochem J. 1988 Feb 15;250(1):145–152. doi: 10.1042/bj2500145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., Yu C., Huang A. H. Conversion of glycerate to serine in intact spinach leaf peroxisomes. Arch Biochem Biophys. 1984 Sep;233(2):393–401. doi: 10.1016/0003-9861(84)90460-0. [DOI] [PubMed] [Google Scholar]
- Paul J. S., Volcani B. E. Photorespiration in diatoms. IV. Two pathways of glycolate metabolism in synchronized cultures of Cylindrotheca fusiformis. Arch Microbiol. 1976 Nov 2;110(23):247–252. doi: 10.1007/BF00690234. [DOI] [PubMed] [Google Scholar]
- Redgwell R. J. Fractionation of plant extracts using ion-exchange Sephadex. Anal Biochem. 1980 Sep 1;107(1):44–50. doi: 10.1016/0003-2697(80)90489-3. [DOI] [PubMed] [Google Scholar]
- Seckbach J., Hammerman I. S., Hanania J. Ultrastructural studies of cyanidium caldarium: contribution to phylogenesis. Ann N Y Acad Sci. 1981;361:409–425. [PubMed] [Google Scholar]
- Stabenau H. Localization of Enzymes of Glycolate Metabolism in the Alga Chlorogonium elongatum. Plant Physiol. 1974 Dec;54(6):921–924. doi: 10.1104/pp.54.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus D. E., Hondred D., Becker W. M. Purification and characterization of hydroxypyruvate reductase from cucumber cotyledons. Plant Physiol. 1983 Jun;72(2):402–408. doi: 10.1104/pp.72.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]
- Winkler U., Säftel W., Stabenau H. Studies on the aminotransferases participating in the glycolate metabolism of the alga mougeotia. Plant Physiol. 1982 Aug;70(2):340–343. doi: 10.1104/pp.70.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]


