Abstract
BACKGROUND
Intraosseous clival arteriovenous fistulas (AVFs), in which the shunt drains extracranially from the posterior and anterior condylar veins rather than from the cavernous sinus (CS), are rare. Targeting embolization of an intraosseous clival AVF is challenging because of its complex venous and skull base anatomy; therefore, a therapeutic strategy based on detailed preoperative radiological findings is required to achieve a favorable outcome. Here, the authors report the successful targeted embolization of an intraosseous clival AVF using an ingenious access route.
OBSERVATIONS
A 74-year-old woman presented with left-sided visual impairment, oculomotor nerve palsy, and right facial pain. A fusion image of three-dimensional rotational angiography and cone-beam computed tomography revealed a left CS dural AVF and a right intraosseous clival AVF. The shunt flow of the clival AVF drained extracranially from the posterior and anterior condylar veins via the intraosseous venous route. Transvenous embolization was performed by devising suboccipital, posterior condylar, and intraosseous access routes. The symptoms resolved after the bilateral AVFs were treated.
LESSONS
Accurate diagnosis and proper transvenous access based on detailed intraosseous and craniocervical venous information obtained from advanced imaging modalities are key to resolving intraosseous clival AVF.
Keywords: intraosseous, clivus, arteriovenous fistula, embolization, endovascular
ABBREVIATIONS: ACV = anterior condylar vein, AVF = arteriovenous fistula, CS = cavernous sinus, CT = computed tomography, DAVF = dural arteriovenous fistula, ECA = external carotid artery, IJV = internal jugular vein, IPS = inferior petrosal sinus, LCV = lateral condylar vein, MRA = magnetic resonance angiography, PCV = posterior condylar vein, SCS = suboccipital cavernous sinus, SOV = superior ophthalmic vein, TVE = transvenous embolization, 3D = three-dimensional, 3D-RA = three-dimensional rotational angiography
Dural arteriovenous fistulas (DAVFs) are conditions in which abnormal shunts form in the arteries, veins, or venous sinuses of the dura mater. Rare arteriovenous fistulas (AVFs) with shunts in the intraosseous region rather than in the dura mater have also been reported. Current imaging modalities allow for the accurate diagnosis of intraosseous AVFs that have been misdiagnosed as DAVFs.
Although intraosseous AVFs and some cases of dorsum sellae, condyle, and jugular tubercle AVFs have been reported,1,2 intraosseous clival AVF is extremely rare.3 Target embolization for an intraosseous clival AVF is challenging because of its complex venous anatomy and approach route. We report a rare case of intraosseous clival AVF treated with target embolization by identifying an accurate shunt point and an optimal access route using a three-dimensional (3D) fusion image. Herein, we describe a case of intraosseous clival AVF and discuss its anatomical characteristics and treatment strategies.
Illustrative Case
A 74-year-old woman with a 6-month history of left eye proptosis, diplopia, visual impairment, and right eye facial pain was referred to our hospital. The patient had left oculomotor nerve palsy, and visual acuity in her left eye was at the level of hand-motion recognition. Magnetic resonance angiography (MRA) showed abnormally high intensity around the cavernous sinus (CS), implying a CS DAVF. Right and left external carotid artery (ECA) angiography revealed an AVF around the CS (Fig. 1A–C). Left ECA angiography revealed retrograde drainage of shunt flow into the bilateral superior ophthalmic veins (SOVs; Fig. 1C); however, the shunt point, as indicated by the right ECA angiography, was slightly lower than that on the left side (Fig. 1A and B). Three-dimensional rotational angiography (3D-RA) of the right ECA angiography revealed multiple feeders clustered at the shunt point, and shunt flow did not drain into the CS but into the caudal side (Fig. 1D and E). The 3D-RA fusion image of the bilateral ECAs showed completely different drainage routes for the right and left AVFs. Specifically, shunt flow from the left ECA drained into the internal jugular vein (IJV) via the left inferior petrosal sinus (IPS). In contrast, shunt flow from the right ECA followed a different drainage route (Fig. 1F). Cone-beam computed tomography (CT) and 3D-RA fusion imaging (Artis Zee Biplane ICT, Siemens) revealed that the AVF feeding the right ECA was located in the right clivus (Fig. 2A). The clival AVF drained into the extracranial vein through the intraosseous venous route from the emissary veins, such as the anterior condylar vein (ACV) and posterior condylar vein (PCV; Fig. 2A). In the volume-rendering fusion image of the skull and right ECA angiography, intraosseous shunt flow was not observed because an AVF was present within the skull (Fig. 2B). However, skull translucency imaging revealed intraosseous venous flow within the clivus and jugular tuberculum (Fig. 2C). In contrast, the left-sided AVF feeding from the left ECA had a shunted pouch in the left CS. The left CS shunt was drained into the right IPS (Fig. 2D).
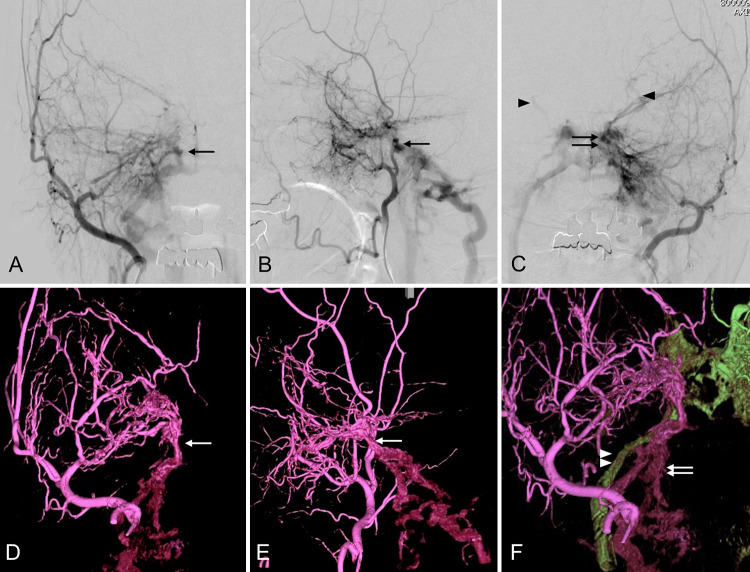
FIG. 1.
Angiography of the right external carotid artery (ECA) in the anteroposterior (A) and lateral (B) projections shows that the right-sided shunt point is slightly lower than the left (arrow). Left ECA angiography (C) shows a cavernous sinus (CS) dural arteriovenous shunt (double arrows) and shunt flow reflux to the bilateral superior ophthalmic veins (arrowheads). Three-dimensional rotational angiography (3D-RA) of the right ECA in the anteroposterior (D) and lateral (E) projections reveals that multiple feeders cluster the shunt point (white arrow) and that the shunt flow does not drain into the CS but into the caudal. The 3D fusion image (F) of the bilateral ECA shows that the right and left arteriovenous fistulas (AVFs) had different drainage routes. The shunt flows from the left ECA draining into the internal jugular vein (IJV) via the left inferior petrosal sinus (IPS; green drainage route, white arrowheads). In contrast, the shunt flows from the right ECA drain into a different route (purple drainage route, white arrows).
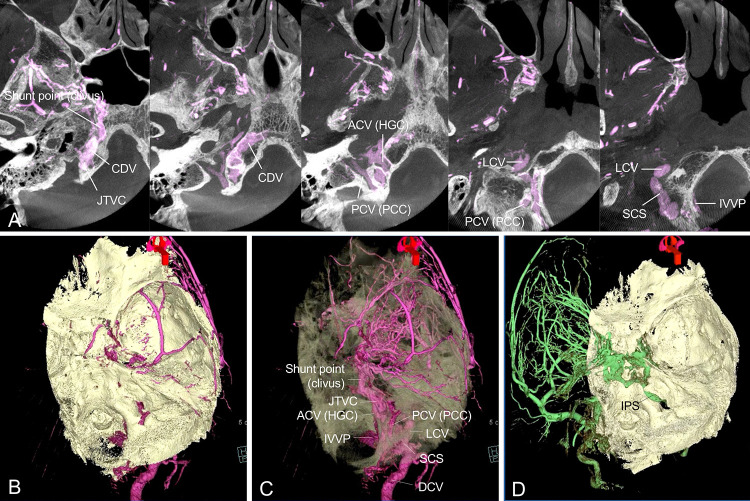
FIG. 2.
The fusion image of cone-beam computed tomography (CT) and 3D-RA of the right ECA. The axial slice image (A) shows the shunt point in the right clivus. The shunt flow drains into extracranial veins such as the lateral condylar vein (LCV), suboccipital cavernous sinus (SCS), internal vertebral venous plexus (IVVP), and deep cervical vein (DCV) through the jugular tuberculum venous complex (JTVC) from the anterior condylar vein (ACV) within the hypoglossal canal (HGC) and the posterior condylar vein (PCV) within the posterior condylar canal (PCC). Volume-rendering fusion image of the right ECA angiography (B and C). Intracranial shunt flow is not observed because the AVF exists within the skull (B). The skull translucency image (C) reveals intraosseous venous flow within the clivus and jugular tuberculum. Volume-rendering fusion image (D) of the left ECA angiography shows the left CS dural arteriovenous fistula (DAVF). CDV = clival diploic vein.
First, we planned to perform transvenous embolization (TVE) for the left-sided CS DAVF, which caused left oculomotor nerve palsy and the visual acuity in her left eye due to reflux into the bilateral SOVs. We performed coil embolization of the CS and left SOV via the left occluded IPS. The left-sided fistula disappeared after initial treatment. However, after initial treatment, the patient complained of pain on the right side of her face. Since the right clival AVF was believed to cause pain, we decided to treat the right clival AVF. As the clival-shunted pouch was not connected to the CS, it was not possible to reach the microcatheter to the intraosseous clival shunt point via the right IPS. Alternatively, we planned to guide the microcatheter via the emissary veins, such as the ACV or PCV, to the clival shunt point. However, if the microcatheter was guided to the shunt point following the shortest course, the transition from the ACV or PCV to the intraosseous venous route would be steeply angled. Therefore, it was believed that the microcatheter would not proceed through the intraosseous venous route, which is a narrow space with septal walls, to reach the shunt point. Therefore, to make the transition from the PCV to the intraosseous venous route straight, we planned to guide the microcatheter into the PCV through the lateral condylar vein (LCV) and suboccipital cavernous sinus (SCS; Fig. 3A). With the patient under general anesthesia, a 7-Fr Cook shuttle sheath (Cook Medical) was inserted into the right IJV. A 4-Fr catheter was inserted into the right ECA for angiographic control. GuidePost (Tokai Medical), a distal access catheter, was placed close to the outlet of the posterior condylar canal through the IJV, anterior condylar confluence, LCV, and SCS to support the microcatheter (Fig. 3B). The Headway DUO microcatheter (Microvention) was guided to the clival shunt point, and coil embolization was performed (Fig. 3C).
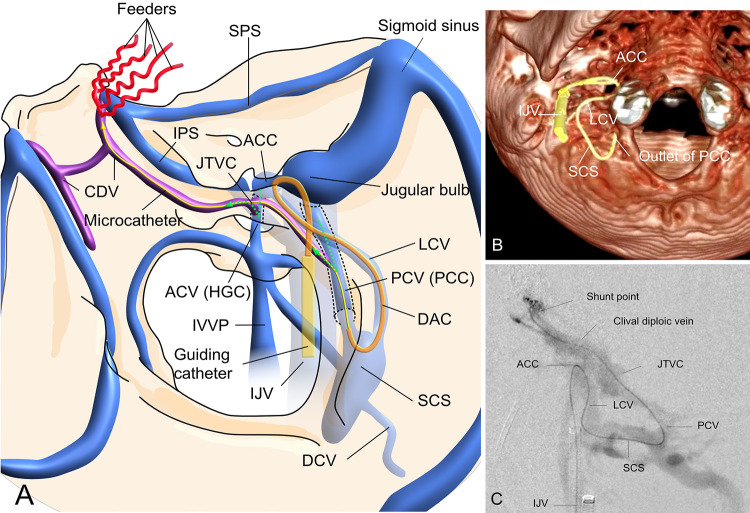
FIG. 3.
Schematic representation (A) of the venous architecture in the present case. The shunt flow of the clival AVF drains into the SCS through the intraosseous venous route (JTVC and CDV) from the ACV and PCV. Guiding the microcatheter to a shunted pouch is difficult because of the steep angle of transition from the ACV or PCV via the IJV to the intraosseous vein (green dotted arrows). The microcatheter can be guided directly to the shunt point through the IJV, anterior condylar confluence (ACC), LCV, SCS, PCV, JTVC, and CDV. The intraoperative volume-rendering image from cone-beam CT (B) shows that the tip of the distal access catheter is located at the outlet of the PCC. Intraoperative microcatheter contrast injection (C) shows that the microcatheter is guided to the shunt point. DAC = distal access catheter; SPS = superior petrosal vein.
Postoperative angiography of the right ECA revealed the disappearance of the AVF (Fig. 4A and B). Postoperative bone CT revealed that the coils were packed within the clivus (Fig. 4C). The symptoms gradually improved after treatment. Follow-up MRA at 14 months did not show recurrent AVFs, and symptoms did not recur.
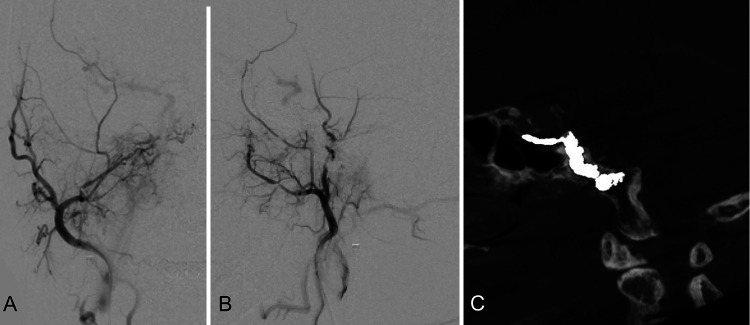
FIG. 4.
Postoperative angiography of the right ECA in the anteroposterior (A) and lateral (B) projections shows that the AVF has disappeared. Postoperative bone CT in sagittal view (C) shows that the coil mass is packed in the clivus.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
This is a case report of a successful transvenous target embolization of an intraosseous clival AVF using a 3D image of the complex venous anatomy of the craniocervical venous system and the intraosseous venous structures at the skull base. To the best of our knowledge, this is the first report of successful targeted embolization of an intraosseous clival AVF by guiding a microcatheter to the shunt point entering the PCV via the LCV and SCS.
Anatomy of the Craniocervical and Intraosseous Venous System
Understanding the craniocervical venous anatomy and intraosseous venous structures is essential for successful transvenous targeted embolization of intraosseous AVFs. The craniocervical venous system by San Millán Ruíz is well established.4 Recently, detailed anatomy of intraosseous venous structures and some cases of intraosseous AVFs within the skull base have been reported.1,2,5,6 Mizutani et al.2,5 reported on intraosseous venous structures, such as the clivus and jugular tubercle. The jugular tubercle venous complex, an intraosseous venous structure adjacent to the jugular tubercle, was connected to the surrounding venous channels, such as the ACV, PCV, IJV, LCV, and clival diploic vein. Knowledge that the ACV and PCV can be used as entry points to access the intraosseous veins adjacent to the jugular tubercle makes transvenous target embolization for intraosseous AVFs possible (Fig. 3A).
Characteristics and Imaging Techniques for Intraosseous Clival AVFs
Intraosseous clival AVFs are rare; only six cases have been reported, including our case (Table 1).1,3,6,7 An intraosseous AVF in the upper clivus, where the shunt drains into the CS, is angiographically similar to a CS DAVF. In four of the six cases of clival AVF, shunt flow drained into the CS.1,6,7 In contrast, intraosseous AVFs located in the middle clivus, where the shunt drains only caudally, are extremely rare and are associated with a complex craniocervical venous system and intraosseous venous structures. Obtaining detailed preoperative 3D information on the skull and vessels can be essential to accurately evaluate the intraosseous venous anatomy of intraosseous clival AVFs. Cone-beam CT helps to obtain detailed information on the skull.8,9 3D-RA can support the assessment of vascular structures to evaluate shunt points and drainage routes.10,11 Therefore, a fusion image of both cone-beam CT and 3D-RA can be suitable for the accurate diagnosis and treatment of intraosseous clival AVF.
TABLE 1.
Cases of intraosseous clival arteriovenous fistulae
| Authors & Year | Age (yrs), Sex | Side | Symptoms | Another AVF | Feeders | Drainers | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Chen et al., 20017 |
59, F |
Lt |
Pulsatile tinnitus & headache, sudden onset of diplopia & lt proptosis |
–– |
Bilat APA, VA |
CS, superior petrosal sinus, & cortical vein |
Incomplete endovascular treatment & radiation |
Symptoms improved |
| 65, F |
Rt |
Pulsatile tinnitus, headache & neck pain, diplopia |
Foramen magnum |
Bilat APA, VA, rt MHT |
CS, IPS, cortical vein. drainage, jugular vein, & suboccipital cervical vein |
Incomplete endovascular treatment |
Lost to FU & died of cerebellar hemorrhage 1 yr later |
|
| Jung et al., 20096 |
— |
— |
Diplopia & ptosis |
Ipsilat CS |
— |
CS, IPS, jugular bulb |
TVE |
Complete occlusion for clival AVF, residual weak shunt of DAVF in ipsilat CS remained |
| Hiramatsu et al., 20211 |
77, F |
Rt |
Double vision due to rt oculomotor nerve palsy |
— |
Bilat IMA |
CS & SMCV |
TVE via IPS & CS |
Complete occlusion |
| Hirata et al., 20213 |
69, M |
Rt |
Pulsatile tinnitus |
Rt PCC |
Rt IMA, rt petrosal branch of MMA, OA, bilat MHT, anterior meningeal artery |
Deep cervical vein & IJV through intraosseous vein in jugular tubercle |
TVE of PCC, TAE of OA |
Pulsatile tinnitus gone, complete occlusion |
| Present case | 79, F | Rt | Lt proptosis & diplopia due to lt oculomotor nerve palsy, lt visual impairment, & rt eye & facial pain | Lt CS DAVF | Rt MMA, AMA, deep temporal artery, AFR, MHT, APA | Suboccipital CS through intraosseous vein, ACV, & PCV | TVE via PCC | Symptoms improved, complete occlusion |
AFR = artery of foramen rotundum; AMA = accessory meningeal artery; APA = ascending pharyngeal artery; FU = follow-up; IMA = internal maxillary artery; MHT = meningohypophyseal artery; MMA = middle meningeal artery; OA = occipital artery; PCC = posterior condylar canal; SMCV = superficial middle cerebral vein; VA = vertebral artery; TAE = transarterial embolization.
Treatment Strategy and Approach to the Shunt Point
Transvenous embolization is suitable for intraosseous clival AVF because transarterial embolization is associated with a high risk of cranial nerve palsy. Intraosseous clival AVFs have two venous drainage directions, the CS and emissary veins. Transvenous embolization via the IPS and CS should be considered in cases of CS drainage. However, when there is no direct connection between the clival-shunted pouch and the CS, as in our case, the shunt flow drains into the emissary veins, such as the PCV or ACV, via the intraosseous venous route. In such cases, TVE via the IPS and CS may not be feasible. Therefore, access to the intraosseous shunt point should be established via emissary veins, such as the ACV or PCV. However, as in the present case, it can be challenging to access the shunt points by selecting the shortest course because the transition from the PCV or ACV into the intraosseous vein is steeply angled (Fig. 3A) and the intraosseous venous route is a narrow space with septum walls.
Proximal outlet occlusion has also been reported in intraosseous clival AVF.3 These authors were unable to guide the microcatheter to the clival diploic vein via the PCV because of acute angulation and a slight trajectory from the PCV to the intraosseous vein in the jugular tubercle. Therefore, we guided the microcatheter to the shunted pouch by entering the PCV via the LCV and SCS to allow a linear approach from the PCV to the clival-shunted pouch, which has not been reported previously (Fig. 3).
Therefore, an accurate diagnosis and optimal treatment may be possible using a 3D fusion image of cone-beam CT and 3D-RA, even in shunt diseases with complex anatomical structures, such as intraosseous clival AVF.
Lessons
A better understanding of the intraosseous and craniocervical venous anatomy and the use of a fusion image of cone-beam CT and 3D-RA may allow the selection of an appropriate access route for intraosseous clival AVF and provide favorable outcomes.
Author Contributions
Conception and design: Iida, Akimoto, Tateishi. Acquisition of data: Iida, Shizawa, Miyake. Analysis and interpretation of data: Iida, Suzuki. Drafting of the article: Iida, Tateishi. Critically revising the article: Suenaga, Iida, Suzuki, Miyake, Hori, Tateishi, Nakai, Yamamoto. Reviewed submitted version of the manuscript: Suenaga, Iida, Shimizu, Shizawa, Miyake, Akimoto, Hori, Nakai. Approved the final version of the manuscript on behalf of all authors: Suenaga. Administrative/technical/material support: Iida, Nakai. Study supervision: Suenaga, Akimoto, Nakai.
References
- 1. Hiramatsu M, Sugiu K, Haruma J, et al. Osseous arteriovenous fistulas in the dorsum sellae, clivus, and condyle. Neuroradiology. 2021;63(1):133–140. doi: 10.1007/s00234-020-02506-9. [DOI] [PubMed] [Google Scholar]
- 2. Mizutani K, Akiyama T, Minami Y, et al. Intraosseous venous structures adjacent to the jugular tubercle associated with an anterior condylar dural arteriovenous fistula. Neuroradiology. 2018;60(5):487–496. doi: 10.1007/s00234-018-1990-8. [DOI] [PubMed] [Google Scholar]
- 3. Hirata K, Kato N, Yamazaki T, Yasuda S, Shiigai M, Matsumaru Y. Arteriovenous fistula of the clival diploic vein associated with arteriovenous fistula of the posterior condylar canal. Interv Neuroradiol. 2021;27(5):672–676. doi: 10.1177/15910199211002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. San Millán Ruíz D, Gailloud P, Rüfenacht DA, Delavelle J, Henry F, Fasel JH. The craniocervical venous system in relation to cerebral venous drainage. AJNR Am J Neuroradiol. 2002;23(9):1500–1508. [PMC free article] [PubMed] [Google Scholar]
- 5. Mizutani K, Toda M, Kurasawa J, et al. Analysis of the venous channel within the clivus using multidetector computed tomography digital subtraction venography. Neuroradiology. 2017;59(3):213–219. doi: 10.1007/s00234-017-1784-4. [DOI] [PubMed] [Google Scholar]
- 6. Jung C, Kwon BJ, Kwon OK, et al. Intraosseous cranial dural arteriovenous fistula treated with transvenous embolization. AJNR Am J Neuroradiol. 2009;30(6):1173–1177. doi: 10.3174/ajnr.A1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen CJ, Wai YY, Wang LJ, Wong YC. MRI of intraosseous dural arteriovenous malformation: findings in two cases. J Comput Assist Tomogr. 2001;25(1):133–136. doi: 10.1097/00004728-200101000-00025. [DOI] [PubMed] [Google Scholar]
- 8. Huang H, Chen D, Lippuner K, Hunziker EB. Human bone typing using quantitative cone-beam computed tomography. Int Dent J. 2023;73(2):259–266. doi: 10.1016/j.identj.2022.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pauwels R, Jacobs R, Singer SR, Mupparapu M. CBCT-based bone quality assessment: are Hounsfield units applicable? Dentomaxillofac Radiol. 2015;44(1):20140238. doi: 10.1259/dmfr.20140238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kakizawa Y, Nagashima H, Oya F, et al. Compartments in arteriovenous malformation nidi demonstrated with rotational three-dimensional digital subtraction angiography by using selective microcatheterization. Report of three cases. J Neurosurg. 2002;96(4):770–774. doi: 10.3171/jns.2002.96.4.0770. [DOI] [PubMed] [Google Scholar]
- 11. Matsubara N, Miyachi S, Izumi T, et al. Usefulness of three-dimensional digital subtraction angiography in endovascular treatment of a spinal dural arteriovenous fistula. J Neurosurg Spine. 2008;8(5):462–467. doi: 10.3171/SPI/2008/8/5/462. [DOI] [PubMed] [Google Scholar]