Abstract
The serotonin hypothesis of depression is still influential. We aimed to synthesise and evaluate evidence on whether depression is associated with lowered serotonin concentration or activity in a systematic umbrella review of the principal relevant areas of research. PubMed, EMBASE and PsycINFO were searched using terms appropriate to each area of research, from their inception until December 2020. Systematic reviews, meta-analyses and large data-set analyses in the following areas were identified: serotonin and serotonin metabolite, 5-HIAA, concentrations in body fluids; serotonin 5-HT1A receptor binding; serotonin transporter (SERT) levels measured by imaging or at post-mortem; tryptophan depletion studies; SERT gene associations and SERT gene-environment interactions. Studies of depression associated with physical conditions and specific subtypes of depression (e.g. bipolar depression) were excluded. Two independent reviewers extracted the data and assessed the quality of included studies using the AMSTAR-2, an adapted AMSTAR-2, or the STREGA for a large genetic study. The certainty of study results was assessed using a modified version of the GRADE. We did not synthesise results of individual meta-analyses because they included overlapping studies. The review was registered with PROSPERO (CRD42020207203). 17 studies were included: 12 systematic reviews and meta-analyses, 1 collaborative meta-analysis, 1 meta-analysis of large cohort studies, 1 systematic review and narrative synthesis, 1 genetic association study and 1 umbrella review. Quality of reviews was variable with some genetic studies of high quality. Two meta-analyses of overlapping studies examining the serotonin metabolite, 5-HIAA, showed no association with depression (largest n = 1002). One meta-analysis of cohort studies of plasma serotonin showed no relationship with depression, and evidence that lowered serotonin concentration was associated with antidepressant use (n = 1869). Two meta-analyses of overlapping studies examining the 5-HT1A receptor (largest n = 561), and three meta-analyses of overlapping studies examining SERT binding (largest n = 1845) showed weak and inconsistent evidence of reduced binding in some areas, which would be consistent with increased synaptic availability of serotonin in people with depression, if this was the original, causal abnormaly. However, effects of prior antidepressant use were not reliably excluded. One meta-analysis of tryptophan depletion studies found no effect in most healthy volunteers (n = 566), but weak evidence of an effect in those with a family history of depression (n = 75). Another systematic review (n = 342) and a sample of ten subsequent studies (n = 407) found no effect in volunteers. No systematic review of tryptophan depletion studies has been performed since 2007. The two largest and highest quality studies of the SERT gene, one genetic association study (n = 115,257) and one collaborative meta-analysis (n = 43,165), revealed no evidence of an association with depression, or of an interaction between genotype, stress and depression. The main areas of serotonin research provide no consistent evidence of there being an association between serotonin and depression, and no support for the hypothesis that depression is caused by lowered serotonin activity or concentrations. Some evidence was consistent with the possibility that long-term antidepressant use reduces serotonin concentration.
Subject terms: Diagnostic markers, Depression
Introduction
The idea that depression is the result of abnormalities in brain chemicals, particularly serotonin (5-hydroxytryptamine or 5-HT), has been influential for decades, and provides an important justification for the use of antidepressants. A link between lowered serotonin and depression was first suggested in the 1960s [1], and widely publicised from the 1990s with the advent of the Selective Serotonin Reuptake Inhibitor (SSRI) antidepressants [2–4]. Although it has been questioned more recently [5, 6], the serotonin theory of depression remains influential, with principal English language textbooks still giving it qualified support [7, 8], leading researchers endorsing it [9–11], and much empirical research based on it [11–14]. Surveys suggest that 80% or more of the general public now believe it is established that depression is caused by a ‘chemical imbalance’ [15, 16]. Many general practitioners also subscribe to this view [17] and popular websites commonly cite the theory [18].
It is often assumed that the effects of antidepressants demonstrate that depression must be at least partially caused by a brain-based chemical abnormality, and that the apparent efficacy of SSRIs shows that serotonin is implicated. Other explanations for the effects of antidepressants have been put forward, however, including the idea that they work via an amplified placebo effect or through their ability to restrict or blunt emotions in general [19, 20].
Despite the fact that the serotonin theory of depression has been so influential, no comprehensive review has yet synthesised the relevant evidence. We conducted an ‘umbrella’ review of the principal areas of relevant research, following the model of a similar review examining prospective biomarkers of major depressive disorder [21]. We sought to establish whether the current evidence supports a role for serotonin in the aetiology of depression, and specifically whether depression is associated with indications of lowered serotonin concentrations or activity.
Methods
Search strategy and selection criteria
The present umbrella review was reported in accordance with the 2009 PRISMA statement [22]. The protocol was registered with PROSPERO in December 2020 (registration number CRD42020207203) (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=207203). This was subsequently updated to reflect our decision to modify the quality rating system for some studies to more appropriately appraise their quality, and to include a modified GRADE to assess the overall certainty of the findings in each category of the umbrella review.
In order to cover the different areas and to manage the large volume of research that has been conducted on the serotonin system, we conducted an ‘umbrella’ review. Umbrella reviews survey existing systematic reviews and meta-analyses relevant to a research question and represent one of the highest levels of evidence synthesis available [23]. Although they are traditionally restricted to systematic reviews and meta-analyses, we aimed to identify the best evidence available. Therefore, we also included some large studies that combined data from individual studies but did not employ conventional systematic review methods, and one large genetic study. The latter used nationwide databases to capture more individuals than entire meta-analyses, so is likely to provide even more reliable evidence than syntheses of individual studies.
We first conducted a scoping review to identify areas of research consistently held to provide support for the serotonin hypothesis of depression. Six areas were identified, addressing the following questions: (1) Serotonin and the serotonin metabolite 5-HIAA–whether there are lower levels of serotonin and 5-HIAA in body fluids in depression; (2) Receptors - whether serotonin receptor levels are altered in people with depression; (3) The serotonin transporter (SERT) - whether there are higher levels of the serotonin transporter in people with depression (which would lower synaptic levels of serotonin); (4) Depletion studies - whether tryptophan depletion (which lowers available serotonin) can induce depression; (5) SERT gene – whether there are higher levels of the serotonin transporter gene in people with depression; (6) Whether there is an interaction between the SERT gene and stress in depression.
We searched for systematic reviews, meta-analyses, and large database studies in these six areas in PubMed, EMBASE and PsycINFO using the Healthcare Databases Advanced Search tool provided by Health Education England and NICE (National Institute for Health and Care Excellence). Searches were conducted until December 2020.
We used the following terms in all searches: (depress* OR affective OR mood) AND (systematic OR meta-analysis), and limited searches to title and abstract, since not doing so produced numerous irrelevant hits. In addition, we used terms specific to each area of research (full details are provided in Table S1, Supplement). We also searched citations and consulted with experts.
Inclusion criteria were designed to identify the best available evidence in each research area and consisted of:
Research synthesis including systematic reviews, meta-analysis, umbrella reviews, individual patient meta-analysis and large dataset analysis.
Studies that involve people with depressive disorders or, for experimental studies (tryptophan depletion), those in which mood symptoms are measured as an outcome.
Studies of experimental procedures (tryptophan depletion) involving a sham or control condition.
Studies published in full in peer reviewed literature.
Where more than five systematic reviews or large analyses exist, the most recent five are included.
Exclusion criteria consisted of:
Animal studies.
Studies exclusively concerned with depression in physical conditions (e.g. post stroke or Parkinson’s disease) or exclusively focusing on specific subtypes of depression such as postpartum depression, depression in children, or depression in bipolar disorder.
No language or date restrictions were applied. In areas in which no systematic review or meta-analysis had been done within the last 10 years, we also selected the ten most recent studies at the time of searching (December 2020) for illustration of more recent findings. We performed this search using the same search string for this domain, without restricting it to systematic reviews and meta-analyses.
Data analysis
Each member of the team was allocated one to three domains of serotonin research to search and screen for eligible studies using abstract and full text review. In case of uncertainty, the entire team discussed eligibility to reach consensus.
For included studies, data were extracted by two reviewers working independently, and disagreement was resolved by consensus. Authors of papers were contacted for clarification when data was missing or unclear.
We extracted summary effects, confidence intervals and measures of statistical significance where these were reported, and, where relevant, we extracted data on heterogeneity. For summary effects in the non-genetic studies, preference was given to the extraction and reporting of effect sizes. Mean differences were converted to effect sizes where appropriate data were available.
We did not perform a meta-analysis of the individual meta-analyses in each area because they included overlapping studies [24]. All extracted data is presented in Table 1. Sensitivity analyses were reported where they had substantial bearing on interpretation of findings.
Table 1.
Study characteristics and results.
| Study | Design | N total: studies, participants (cases; controls where relevant) | Serotonin measure | Medication status | Summary effect (95% CI), p Heterogeneity-p, I2 (95% CI), N for analysis (if different from total sample size) | Quality rating (AMSTAR-2/ *STREGA) % satisfactory (of applicable terms) |
|---|---|---|---|---|---|---|
| Serotonin and 5HIAA: case (depression) vs. healthy control studies comparing concentration of serotonin in plasma and CSF | ||||||
| Huang et al., 2020 | Meta-analysis of cohort studies | 3 studies, 663 women with depression; 1806 controls | 5-HT in plasma | 18.3% of patients were taking antidepressants. |
β = −0.26 (−0.48 to −0.03), p > 0.05 after correction for multiple testing Heterogeneity not reported Sensitivity analysis: No difference in levels of 5-HT in women with depression not taking antidepressants compared to controls (women without depression not taking antidepressants) (p = 0.528) Lower levels of 5-HT in women with depression taking antidepressants compared to controls (p < 0.0001) and in women taking antidepressants without depression compared to controls (p < 0.0001) |
60% |
| Ogawa et al., 2018 | Systematic review and meta-analysis | 11 studies, 435 people with depression; 380 controls | 5-HIAA in CSF | All participants drug free – washout: 4–57 days (mode 14 days) | g = 0.042 (−0.26 to 0.17), p = 0.70, heterogeneity: I2 = 48.24 (p = 0.026) | 48% |
| Pech et al., 2018 | Systematic review and meta-analysis | 13 studies, 529 people with depression; 473 controls | 5-HIAA in CSF |
10 studies: patients were drug free - washout: 4 days-6 weeks (mostly 2–4 weeks). 2 studies: some patients used occasional antidepressants. 1 study: majority of patients taking imipramine and chlorpromazine |
Mean difference = −3.85 (−8.89 to 1.19) (g = 0.06), p = 0.14, heterogeneity – not reported | 38% |
| Receptors: case (depression) vs. healthy control studies comparing 5-HT1 receptor binding | ||||||
| Nikolaus et al., 2016 | Systematic review and meta-analysis | 14 studies, 245 people with depression; 316 controls | 5-HT1A receptor binding |
2 studies conducted with drug naïve patients (total N = 19). 11 studies - prior treatment with antidepressants and/or other psychiatric drugs – washout: 1–1825 days (mode 14 days) 1 study medication not specified |
Trend for increased 5-HT1 in parahippocampal gyrus (+23%), p = 0.096. Trend for reduced 5-HT1 in midbrain (−17%), p = 0.076. N and heterogeneity – not reported for any brain areas. |
19% |
| Wang et al., 2016 | Systematic review and meta-analysis | 10 studies, 218 people with depression; 261 controls | 5-HT1A receptor binding |
9 studies: drug-free – washout: 2–26 weeks (mainly 2–3 weeks). 1 study: medication naïve. |
Reduced 5-HT1A binding mesiotemporal cortex SMD = −0.8 (−1.36, −0.24), p = 0.005, heterogeneity: I2 = 87% (P < 0.00001) 218 patients, 261 controls** Reduced 5-HT1A binding hippocampus SMD = −0.29 (−0.51, −0.07), P = 0.010, heterogeneity: I2 = 33%, p = 0.18 148 patients, 203 controls. Reduced 5-HT1A binding raphe nucleus SMD = −0.60 (−1.17, −0.04), p = 0.04 Heterogeneity: I2 = 88%, p < 0.00001 218 patients, 261 controls Reduced 5-HT1A binding insular cortex SMD = −0.79 (−1.54, to −0.05), p = 0.04. Heterogeneity: I2 = 91%, p < 0.00001 180 patients, 225 controls. There was no significant difference in the occipital cortex and the anterior cingulate cortex. |
52% |
| Serotonin transporter (SERT): case (depression) vs. healthy control studies comparing SERT binding | ||||||
| Nikolaus et al., 2016 | Systematic review and meta-analysis | 35 studies, 694 people with depression; 700 controls | SERT binding |
26 studies: drug-free - washout: 3–900 days. 5 studies: drug naive 2 studies: patients medicated when scanned. 2 studies: did not report medication. |
Reduced SERT in thalamus −12%, p = 0.004 Reduced SERT in amygdala −15%, p = 0.04. Reduced SERT in midbrain/ pons −8%, p = 0.005 Increased SERT in insula +9%, p = 0.037 N, CI and heterogeneity – not reported for any brain areas. |
19% |
| Kambeitz & Howes., 2015 | Systematic review and meta-analysis |
25 in vivo imaging studies, 25 post-mortem studies, 877 people with depression; 968 controls |
SERT binding |
In vivo studies: three studies – antidepressant naïve patients. Post Mortem studies: medication status not reported |
In vivo Reduced SERT in brainstem g = −0.31 (−0.55 to −0.08), p = 0.01, heterogeneity - I2 = 60.69% (95% CI: 34.39 to 83.35%), N = 880 Reduced SERT in midbrain g = −0.28 (−0.49 to −0.07), p = 0.01, heterogeneity - I2 = 49.68% (95% CI: 14.34% to 78.72%), N = 827 Reduced SERT in amygdala g = −0.37*** (−0.61 to −0.13), p<0.01, heterogeneity - I2 = 0% (95%-CI: 0% to 75.38%), N = 318 Reduced SERT in striatum g = −0.39 (−0.62 to −0.17), P<.001, heterogeneity - I2 = 6.7% (0% to 78.1%), N = 370 No difference: hippocampus, thalamus, cingulate cortex, frontal cortex. Post-mortem: Reduced SERT in hippocampus g = −0.63 (−1.12 to −0.15), p = 0.01 heterogeneity - I2 = 43.97% (0% to 93.34), N = 141 Non-significant after correction for publication bias; g = 0.32, p = 0.32 No difference: brainstem, frontal lobe. Meta-analysis not possible for other areas |
48% |
| Gryglewski et al., 2014 | Systematic review and meta-analysis | 18 studies, 364 people with depression; 372 controls | SERT binding | 149/364 patients - drug naïve; others drug free: 5 days to > 1 year (median 7 weeks) |
Reduced SERT in midbrain: g = −0.49 (−0.84 to −0.14), heterogeneity - I2 = 68.7%, 313 patients, 321 controls. Reduced SERT in amygdala g = −0.50 (−0.78 to −0.22), heterogeneity - I2 = 0%, 96 patients, 128 controls. No difference: brainstem, thalamus, striatum, frontal cortex, cingulate cortex. |
48% |
| Tryptophan depletion studies: effect of acute tryptophan depletion (ATD) on mood in healthy volunteers; healthy volunteers with family history of depression; drug-free patients with MDD in remission | ||||||
| Ruhé et al., 2007 | Systematic review and meta-analysis |
Included in meta-analysis: 32 healthy volunteer studies, N = 566; 19 patient studies N = 322 |
ATD |
6 studies involved patients with prior use of antidepressants: 1 study: <3 months; 2 studies 1–3 months 3 studies >6 months 7 studies involved patients with current use of antidepressants. Remainder unspecified. |
No effect of ATD in healthy volunteers in parallel group studies (negative for family history of depression): Pooled g = −0.63 (−1.95 to 0.70), N = 151. Sensitivity analysis excluding an outlier study reduced Hedges g to 0.16 (−0.43 to 0.76), N = 125. No effect of ATD in cross-over studies with volunteers with no family history of depression, g = −0.19 (−0.43 to 0.05), p = 0.13, heterogeneity - I2 = 65.6%, p < 0.001, N = 259# ATD lowered mood in cross-over studies with healthy controls with family history of depression: Hedge’s g = −0.56 (−1.00 to −0.13), p = 0.01, heterogeneity - I2 = 50.8%, p = 0.06, N = 75. ATD lowered mood in cross-over studies in drug-free people with remitted MDD: g = −1.90 (−3.02 to −0.78), p = 0.0009, heterogeneity - I2 = 89.4%, P< 0.00001, N = 97. ATD lowered mood in cross-over studies with people with remitted MDD currently using antidepressants : g = −0.49 (−0.89 to −0.10), p = 0.01, heterogeneity - I2 = 53.3%, P = 0.04, N = 83. |
71% |
| Fusar-Poli et al., 2006 | Systematic review and narrative synthesis | 22 studies (23 cohorts), 64 people with remitted depression; 278 controls | ATD | A portion of the remitted depressed group were taking antidepressants but exact proportion not reported | Results reported narratively. 17/19 studies involving healthy volunteers showed no effect of tryptophan depletion on mood. 4 studies in patients with remitted depression, an unspecified portion of whom were taking antidepressants, found a decrease in mood following tryptophan depletion. Effect sizes or statistical significance were not reported. | 22% |
| SERT gene: association between SERT gene and depression | ||||||
| Border et al., 2019 | Genetic association study | Data from two genetic data banks, 48,190–115,257 individuals | Association between 5-HTTLPR polymorphism and depression | N/A |
No relationship between 5-HTTLPR polymorphism and estimated lifetime MDD diagnosis OR = 1.000, p = 0.994 (N = 115,257) None of seven other depression outcomes (e.g. estimated lifetime diagnosis, current depression severity) showed an association with the 5-HTTLPR polymorphism. N = 48,190–115,257 |
88%* |
| Culverhouse et al., 2018 | Collaborative meta-analysis | 31 data-sets, 43,165 Individuals | 5-HTTLPR polymorphism association with depression | N/A |
No significant effect of number of copies of s-allele of 5HTTLPR on lifetime depression. OR 1.00 (0.95 to 1.05), p = 0.95, N = 21,135. Heterogeneity- not reported All other analyses with variations of stress exposure and depression evaluation were non-significant with OR 1.00–1.08, p = 0.36–0.97, N = 13,835–28,252 |
100% |
| Oo et al., 2016 | Systematic review and meta-analysis |
23 studies, 3392 people with depression; 5093 controls |
5-HTTLPR polymorphism association with depression | N/A |
5-HTTLPR polymorphism associated with depression (per S allele): OR = 1.16 (1.08 to 1.23), p-value. Heterogeneity- I2 = 29.3%, p = 0.09 Homozygote carriers of the S allele of 5HTTLPR polymorphism compared with heterozygote and non-carriers combined (SS vs SL+LL genotype): OR = 1.33 (1.19 to 1.48) for major depressive disorder. Heterogeneity- I2 = 0.1%, p = 0.46 |
62% |
| Gatt et al., 2015 | Umbrella review | 11 meta-analyses included 1014–14,250 participants |
7 meta-analyses of case control studies of 5-HTTLPR polymorphism and depression. 4 meta-analyses of GWAS |
N/A |
Individual gene meta-analyses: 3 studies found the S allele or SS genotype associated with depression in mixed and Caucasian samples (OR 1.11 (1.04–1.19), N = 9459 OR 1.23 (1.01–1.52), N = 1014 OR 1.40 (1.19–1.65), N = 6884) 3 studies reported no effect of the S allele in mixed and Caucasian samples 2 studies reported no effect of the SS genotype in Caucasian samples 3 studies found that the S allele or SS genotype had no effect in Asian samples No evidence of association in all 4 GWAS meta-analyses (n = 6566 to n = 12,664) |
17% |
| Kiyohara & Yoshimasu, 2010 | Systematic review and meta-analysis | 22 studies, 2934 people with depression; 4985 controls | 5-HTTLPR polymorphism association with depression | N/A |
5-HTTLPR polymorphism (SS vs LL) associated with depression in Caucasian samples (but not Asian samples): OR = 1.41 (1.15 to 1.72), Heterogeneity p = 0.23 (N = 5756) |
57% |
| Gene-stress interaction | ||||||
| Border et al., 2019 | See Border et al., 2019 (above) | See Border et al., 2019 (above) | 5-HTTLPR Polymorphism x environmental interaction effect on depression | N/A |
No significant interaction between the 5-HTTLPR polymorphism and childhood trauma. Exp(β) = 0.998, p = 0.919, N = 115,249. Heterogeneity not given as not a meta-analysis. 31 other analyses using different measures of stress or depression found no significant interaction. |
See Border et al., 2019 (above) |
| Bleys et al 2018 | Systematic review and meta-analysis | 51 studies, N = 51,449 | Interaction between stress and 5-HTTLPR polymorphism in depression | N/A |
OR = 1.18 (1.09 to 1.28). Heterogeneity- I2 = 52.4%, p < 0.0001 |
33% |
| Culverhouse et al., 2018 | See Culverhouse et al., 2018 (above) | See Culverhouse et al., 2018 (above) | Interaction between stressful life events and 5-HTTLPR polymorphism for risk of depression | N/A |
No significant interaction between number of copies of the s-allele for 5-HTTLPR and stress on risk of depression. OR = 1.05 (0.91 to 1.21), p = 0.49, N = 21,135 I2 = 0.0, p = 0.69 All other analyses with variations of stress and depression evaluation (including continuous measures of stress) were non-significant, with ORs from 0.85 to 1.06, p = 0.17–0.50, N = 13,835-28,252 I2 = 0–16.7, p = 0.26-0.87 |
See Culverhouse et al., 2018 (above) |
| Sharpley et al 2014 | Systematic review and meta-analysis | 81 studies, N = 55,269 | Interaction between stress and 5-HTTLPR polymorphism in depression | N/A |
OR not reported - Liptak-Stouffer Z-score method was used which combined p-values across studies, p = 9 × 10−7 Heterogeneity- not reported (N = 55,269) |
48% |
| Karg et al 2011 | Systematic review and meta-analysis | 54 studies, N = 40,749 | Whether the 5-HTTLPR polymorphism moderates the relationship between stress and depression | N/A |
OR not reported - Liptak-Stouffer Z-score method was used which combined p-values across studies, p = 0.00002. Heterogeneity- not reported (N = 40,749) |
29% |
Extracted summary effects, confidence intervals and measures of statistical significance are reported. In reviews in which multiple meta-analyses were conducted, only summary effect sizes that were statistically significant are reported, with relevant negative findings that did not reach statistical significance listed. Where no relevant analysis was statistically significant, the results of the principal analysis (e.g., main gene effect) is reported. Data on heterogeneity is reported where relevant. Where sample size for a particular analysis was different from the total sample size, this is reported. For summary effects in the non-genetic studies, preference was given to the extraction and reporting of effect sizes. Mean differences were converted to effect sizes where appropriate data were available [25]. Where these data were not available the most relevant measure, such as the beta co-efficient, or average percentage change in ligand binding was reported. For genetic association studies, and gene-stress interactions studies we odds ratios are reported.
CSF Cerebrospinal fluid, g Hedges g, GWAS Genome Wide Association Studies, MDD Major Depressive Disorder, N/A not applicable, OR Odds Ratio, SMD Standard Mean Difference.
*STREGA rating; **where there were internal discrepancies between reported numbers in text or figures we obtained data from the original authors; ***where there were internal discrepancies between reported numbers in the text, and authors did not respond to queries, details from figures were prioritised, and statistics calculated from other data.
#sample size has a different meaning in crossover studies since each participant contributes data twice.
The quality rating of systematic reviews and meta-analyses was assessed using AMSTAR-2 (A MeaSurement Tool to Assess systematic Reviews) [25]. For two studies that did not employ conventional systematic review methods [26, 27] we used a modified version of the AMSTAR-2 (see Table S3). For the genetic association study based on a large database analysis we used the STREGA assessment (STrengthening the REporting of Genetic Association Studies) (Table S4) [28]. Each study was rated independently by at least two authors. We report ratings of individual items on the relevant measure, and the percentage of items that were adequately addressed by each study (Table 1, with further detail in Tables S3 and S4).
Alongside quality ratings, two team members (JM, MAH) rated the certainty of the results of each study using a modified version of the GRADE guidelines [29]. Following the approach of Kennis et al. [21], we devised six criteria relevant to the included studies: whether a unified analysis was conducted on original data; whether confounding by antidepressant use was adequately addressed; whether outcomes were pre-specified; whether results were consistent or heterogeneity was adequately addressed if present; whether there was a likelihood of publication bias; and sample size. The importance of confounding by effects of current or past antidepressant use has been highlighted in several studies [30, 31]. The results of each study were scored 1 or 0 according to whether they fulfilled each criteria, and based on these ratings an overall judgement was made about the certainty of evidence across studies in each of the six areas of research examined. The certainty of each study was based on an algorithm that prioritised sample size and uniform analysis using original data (explained more fully in the supplementary material), following suggestions that these are the key aspects of reliability [27, 32]. An assessment of the overall certainty of each domain of research examining the role of serotonin was determined by consensus of at least two authors and a direction of effect indicated.
Results
Search results and quality rating
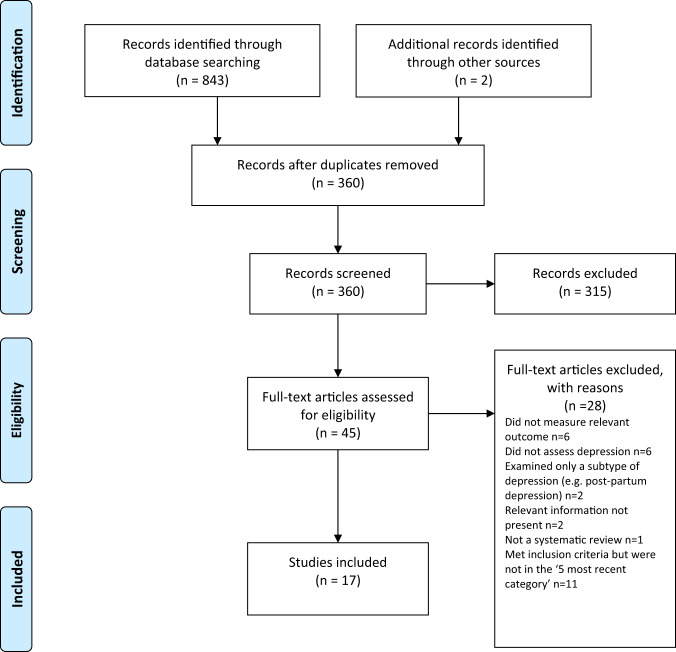
Searching identified 361 publications across the 6 different areas of research, among which seventeen studies fulfilled inclusion criteria (see Fig. 1 and Table S1 for details of the selection process). Included studies, their characteristics and results are shown in Table 1. As no systematic review or meta-analysis had been performed within the last 10 years on serotonin depletion, we also identified the 10 latest studies for illustration of more recent research findings (Table 2).
Fig. 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagramme.
Table 2.
Recent depletion studies comparing acute tryptophan depletion drink with amino acid balance drink (sham drink) - characteristics and results.
| Study | Randomised controlled trial design | Participants: Healthy volunteers (HV) or people with depression* | Medication status | Summary effect (95% CI), p |
|---|---|---|---|---|
| Frey & McCabe, 2020 | Parallel group, double blind | 46 HV | No psychotropic medication for at least 3 months | No effect |
| Bar et al., 2020 | Crossover trial, double blind | 30 HV | Not specified | No effect |
| Deza-Araujo et al., 2018 | Crossover trial, double blind | 85 HV | Not specified | No effect |
| Martin et al., 2017 | Crossover trial, double blind | 15 HV | No history of neurological or psychiatric disorders | No effect |
| Eisner et al., 2016 | Crossover trial, double blind | 64 HV | No history of neurological or psychiatric disorders | No effect |
| Trotter et al., 2016 | Parallel group, double blind | 30 HV | Not specified | No effect |
| Hogenelst 2016 | Crossover trial, double blind | 40 HV with or without a family history of MDD | ‘No use of psychotropics’ | No effect |
| Weinstein et al., 2015 | Crossover trial, double blind | 15 people with depression | Treated with sertraline for 12 weeks prior to study | No effect |
| Moreno et al., 2015 | Crossover trial, double blind | 64 people with personal and family history of MDD; in remission | No ‘psychotropic medication’ for at least 3 months prior to test | A significant main effect of test condition (χ 2 = 5.14, d.f. = 1, p = 0.023) mainly due to improvement of HDRS scores following sham drink |
| Hembold et al., 2013 | Crossover trial, double blind | 18 HV | Not specified | No effect |
MDD Major Depressive disorder, HDRS Hamilton depression rating scale.
*sample size has a different meaning in crossover studies since each participant contributes data twice.
Quality ratings are summarised in Table 1 and reported in detail in Tables S2–S3. The majority (11/17) of systematic reviews and meta-analyses satisfied less than 50% of criteria. Only 31% adequately assessed risk of bias in individual studies (a further 44% partially assessed this), and only 50% adequately accounted for risk of bias when interpreting the results of the review. One collaborative meta-analysis of genetic studies was considered to be of high quality due to the inclusion of several measures to ensure consistency and reliability [27]. The large genetic analysis of the effect of SERT polymorphisms on depression, satisfied 88% of the STREGA quality criteria [32].
Serotonin and 5-HIAA
Serotonin can be measured in blood, plasma, urine and CSF, but it is rapidly metabolised to 5-hydroxyindoleacetic acid (5-HIAA). CSF is thought to be the ideal resource for the study of biomarkers of putative brain diseases, since it is in contact with brain interstitial fluid [33]. However, collecting CSF samples is invasive and carries some risk, hence large-scale studies are scarce.
Three studies fulfilled inclusion criteria (Table 1). One meta-analysis of three large observational cohort studies of post-menopausal women, revealed lower levels of plasma 5-HT in women with depression, which did not, however, reach statistical significance of p < 0.05 after adjusting for multiple comparisons. Sensitivity analyses revealed that antidepressants were strongly associated with lower serotonin levels independently of depression.
Two meta-analyses of a total of 19 studies of 5-HIAA in CSF (seven studies were included in both) found no evidence of an association between 5-HIAA concentrations and depression.
Receptors
Fourteen different serotonin receptors have been identified, with most research on depression focusing on the 5-HT1A receptor [11, 34]. Since the functions of other 5-HT receptors and their relationship to depression have not been well characterised, we restricted our analysis to data on 5-HT1A receptors [11, 34]. 5-HT1A receptors, known as auto-receptors, inhibit the release of serotonin pre-synaptically [35], therefore, if depression is the result of reduced serotonin activity caused by abnormalities in the 5-HT1A receptor, people with depression would be expected to show increased activity of 5-HT1A receptors compared to those without [36].
Two meta-analyses satisfied inclusion criteria, involving five of the same studies [37, 38] (see Table 1). The majority of results across the two analyses suggested either no difference in 5-HT1A receptors between people with depression and controls, or a lower level of these inhibitory receptors, which would imply higher concentrations or activity of serotonin in people with depression. Both meta-analyses were based on studies that predominantly involved patients who were taking or had recently taken (within 1–3 weeks of scanning) antidepressants or other types of psychiatric medication, and both sets of authors commented on the possible influence of prior or current medication on findings. In addition, one analysis was of very low quality [37], including not reporting on the numbers involved in each analysis and using one-sided p-values, and one was strongly influenced by three studies and publication bias was present [38].
The serotonin transporter (SERT)
The serotonin transporter protein (SERT) transports serotonin out of the synapse, thereby lowering the availability of serotonin in the synapse [39, 40]. Animals with an inactivated gene for SERT have higher levels of extra-cellular serotonin in the brain than normal [41–43] and SSRIs are thought to work by inhibiting the action of SERT, and thus increasing levels of serotonin in the synaptic cleft [44]. Although changes in SERT may be a marker for other abnormalities, if depression is caused by low serotonin availability or activity, and if SERT is the origin of that deficit, then the amount or activity of SERT would be expected to be higher in people with depression compared to those without [40]. SERT binding potential is an index of the concentration of the serotonin transporter protein and SERT concentrations can also be measured post-mortem.
Three overlapping meta-analyses based on a total of 40 individual studies fulfilled inclusion criteria (See Table 1) [37, 39, 45]. Overall, the data indicated possible reductions in SERT binding in some brain areas, although areas in which effects were detected were not consistent across the reviews. In addition, effects of antidepressants and other medication cannot be ruled out, since most included studies mainly or exclusively involved people who had a history of taking antidepressants or other psychiatric medications. Only one meta-analysis tested effects of antidepressants, and although results were not influenced by the percentage of drug-naïve patients in each study, numbers were small so it is unlikely that medication-related effects would have been reliably detected [45]. All three reviews cited evidence from animal studies that antidepressant treatment reduces SERT [46–48]. None of the analyses corrected for multiple testing, and one review was of very low quality [37]. If the results do represent a positive finding that is independent of medication, they would suggest that depression is associated with higher concentrations or activity of serotonin.
Depletion studies
Tryptophan depletion using dietary means or chemicals, such as parachlorophenylalanine (PCPA), is thought to reduce serotonin levels. Since PCPA is potentially toxic, reversible tryptophan depletion using an amino acid drink that lacks tryptophan is the most commonly used method and is thought to affect serotonin within 5–7 h of ingestion. Questions remain, however, about whether either method reliably reduces brain serotonin, and about other effects including changes in brain nitrous oxide, cerebrovascular changes, reduced BDNF and amino acid imbalances that may be produced by the manipulations and might explain observed effects independent of possible changes in serotonin activity [49].
One meta-analysis and one systematic review fulfilled inclusion criteria (see Table 1). Data from studies involving volunteers mostly showed no effect, including a meta-analysis of parallel group studies [50]. In a small meta-analysis of within-subject studies involving 75 people with a positive family history, a minor effect was found, with people given the active depletion showing a larger decrease in mood than those who had a sham procedure [50]. Across both reviews, studies involving people diagnosed with depression showed slightly greater mood reduction following tryptophan depletion than sham treatment overall, but most participants had taken or were taking antidepressants and participant numbers were small [50, 51].
Since these research syntheses were conducted more than 10 years ago, we searched for a systematic sample of ten recently published studies (Table 2). Eight studies conducted with healthy volunteers showed no effects of tryptophan depletion on mood, including the only two parallel group studies. One study presented effects in people with and without a family history of depression, and no differences were apparent in either group [52]. Two cross-over studies involving people with depression and current or recent use of antidepressants showed no convincing effects of a depletion drink [53, 54], although one study is reported as positive mainly due to finding an improvement in mood in the group given the sham drink [54].
SERT gene and gene-stress interactions
A possible link between depression and the repeat length polymorphism in the promoter region of the SERT gene (5-HTTLPR), specifically the presence of the short repeats version, which causes lower SERT mRNA expression, has been proposed [55]. Interestingly, lower levels of SERT would produce higher levels of synaptic serotonin. However, more recently, this hypothesis has been superseded by a focus on the interaction effect between this polymorphism, depression and stress, with the idea that the short version of the polymorphism may only give rise to depression in the presence of stressful life events [55, 56]. Unlike other areas of serotonin research, numerous systematic reviews and meta-analyses of genetic studies have been conducted, and most recently a very large analysis based on a sample from two genetic databanks. Details of the five most recent studies that have addressed the association between the SERT gene and depression, and the interaction effect are detailed in Table 1.
Although some earlier meta-analyses of case-control studies showed a statistically significant association between the 5-HTTLPR and depression in some ethnic groups [57, 58], two recent large, high quality studies did not find an association between the SERT gene polymorphism and depression [27, 32]. These two studies consist of by far the largest and most comprehensive study to date [32] and a high-quality meta-analysis that involved a consistent re-analysis of primary data across all conducted studies, including previously unpublished data, and other comprehensive quality checks [27, 59] (see Table 1).
Similarly, early studies based on tens of thousands of participants suggested a statistically significant interaction between the SERT gene, forms of stress or maltreatment and depression [60–62], with a small odds ratio in the only study that reported this (1.18, 95% CI 1.09 to 1.28) [62]. However, the two recent large, high-quality studies did not find an interaction between the SERT gene and stress in depression (Border et al [32] and Culverhouse et al.) [27] (see Table 1).
Overall results
Table 3 presents the modified GRADE ratings for each study and the overall rating of the strength of evidence in each area. Areas of research that provided moderate or high certainty of evidence such as the studies of plasma serotonin and metabolites and the genetic and gene-stress interaction studies all showed no association between markers of serotonin activity and depression. Some other areas suggested findings consistent with increased serotonin activity, but evidence was of very low certainty, mainly due to small sample sizes and possible residual confounding by current or past antidepressant use. One area - the tryptophan depletion studies - showed very low certainty evidence of lowered serotonin activity or availability in a subgroup of volunteers with a family history of depression. This evidence was considered very low certainty as it derived from a subgroup of within-subject studies, numbers were small, and there was no information on medication use, which may have influenced results. Subsequent research has not confirmed an effect with numerous negative studies in volunteers.
Table 3.
Modified GRADE ratings for each study and the overall rating of strength of evidence.
| Study | Unified statistical analysis on original data | Confounding by antidepressant use adequately excluded (where effect found)* | Outcomes of interest pre-specified | Consistent results (or heterogeneity adequately addressed if present) | Little likelihood of publication bias (in funnel plots or tests) | Large sample (>500 for non-genetic and >10,000 for genetic) | Certainty of evidence | Overall certainty of domain |
|---|---|---|---|---|---|---|---|---|
| Serotonin and 5HIAA: case (depression) vs. healthy control studies comparing concentration of serotonin in plasma and CSF | ||||||||
| Huang et al., 2020 | 1 | 1 | 1 | 0 | 1 | 1 | Moderate certainty evidence of no effect (lowered serotonin explained by antidepressants) | Moderate certainty evidence of no effect |
| Ogawa et al., 2018 | 0 | N/A | 1 | 1 | 1 | 1 | Moderate certainty evidence of no effect | |
| Pech et al., 2018 | 0 | N/A | 1 | 1 | 0 | 1 | Low certainty evidence of no effect | |
| Receptors: case (depression) vs. healthy control studies comparing 5-HT1 receptor binding | ||||||||
| Nikolaus et al., 2016 | 0 | 0 | 0 | 0 | 0 | 0 | Very low certainty evidence of increased serotonin activity | Very low certainty evidence of increased serotonin activity |
| Wang et al., 2016 | 0 | 0 | 1 | 0 | 0 | 0 | Very low certainty evidence of increased serotonin activity | |
| Serotonin transporter (SERT): case (depression) vs. healthy control studies comparing SERT binding | ||||||||
| Nikolaus et al., 2016 | 0 | 0 | 0 | 0 | 0 | 0 | Very low certainty evidence of increased serotonin activity | Very low certainty evidence of increased serotonin activity |
| Kambeitz & Howes., 2015 | 0 | 0 | 1 | 1 | 0 | 1 | Very low certainty evidence of increased serotonin activity | |
| Gryglewski et al., 2014 | 0 | 0 | 1 | 1 | 0 | 1 | Very low certainty evidence of increased serotonin activity | |
| Tryptophan depletion studies: effect of acute tryptophan depletion (ATD) on mood in healthy volunteers; healthy volunteers with family history of depression; drug-free patients with MDD in remission | ||||||||
| Ruhe et al., 2007 | 0 | 0 | 1 | 1 | 0 | 0 | Very low certainty evidence of no effect for people with no family history of depression. Very low certainty evidence of lowered serotonin activity in those with a family history and people with depression in remission. | Very low certainty evidence of no effect or lowered serotonin activity in vulnerable populations |
| Fusar-Poli et al., 2006 | 0 | N/A | 1 | 0 | 0 | 0 | Very low certainty evidence of lowered serotonin activity | |
| SERT gene: association between SERT gene and depression | ||||||||
| Border et al., 2019 | 1 | N/A | 1 | N/A | 1 | 1 | High certainty evidence of no effect of the SERT gene | High certainty evidence of no effect for SERT gene |
| Culverhouse et al., 2018 | 1 | N/A | 1 | 1 | 1 | 1 | High certainty evidence of no effect of the SERT gene | |
| Oo et al., 2016 | 0 | N/A | 0 | 1 | 1 | 0 | Very low certainty evidence of an effect for SERT gene | |
| Gatt et al., 2015 | 0 | N/A | 0 | 0 | 0 | 0 | Very low certainty evidence of an effect for SERT gene | |
| Kiyohara & Yoshimasu, 2010 | 0 | N/A | 1 | 0 | 1 | 0 | Very low certainty evidence of an effect for SERT gene | |
| Gene-stress interaction | ||||||||
| Border et al., 2019 | 1 | N/A | 1 | N/A | 1 | 1 | High certainty evidence of no effect for gene-stress interaction | High certainty evidence of no effect for gene-stress interaction |
| Bleys et al., 2018 | 0 | N/A | 1 | 0 | 1 | 1 | Low certainty evidence of an effect for gene-stress interaction | |
| Culverhouse et al., 2018 | 1 | N/A | 1 | 1 | 1 | 1 | High certainty evidence of no effect for gene-stress interaction | |
| Sharpley et al., 2014 | 0 | N/A | 1 | 0 | 1 | 1 | Low certainty evidence of an effect for gene-stress interaction | |
| Karg et al., 2011 | 0 | N/A | 1 | 0 | 1 | 1 | Low certainty evidence of an effect for gene-stress interaction | |
N/A not applicable
*Where no effect found or where prior use of medication is not relevant (as in genetic studies), item was rated as ‘not applicable’.
Discussion
Our comprehensive review of the major strands of research on serotonin shows there is no convincing evidence that depression is associated with, or caused by, lower serotonin concentrations or activity. Most studies found no evidence of reduced serotonin activity in people with depression compared to people without, and methods to reduce serotonin availability using tryptophan depletion do not consistently lower mood in volunteers. High quality, well-powered genetic studies effectively exclude an association between genotypes related to the serotonin system and depression, including a proposed interaction with stress. Weak evidence from some studies of serotonin 5-HT1A receptors and levels of SERT points towards a possible association between increased serotonin activity and depression. However, these results are likely to be influenced by prior use of antidepressants and its effects on the serotonin system [30, 31]. The effects of tryptophan depletion in some cross-over studies involving people with depression may also be mediated by antidepressants, although these are not consistently found [63].
The chemical imbalance theory of depression is still put forward by professionals [17], and the serotonin theory, in particular, has formed the basis of a considerable research effort over the last few decades [14]. The general public widely believes that depression has been convincingly demonstrated to be the result of serotonin or other chemical abnormalities [15, 16], and this belief shapes how people understand their moods, leading to a pessimistic outlook on the outcome of depression and negative expectancies about the possibility of self-regulation of mood [64–66]. The idea that depression is the result of a chemical imbalance also influences decisions about whether to take or continue antidepressant medication and may discourage people from discontinuing treatment, potentially leading to lifelong dependence on these drugs [67, 68].
As with all research synthesis, the findings of this umbrella review are dependent on the quality of the included studies, and susceptible to their limitations. Most of the included studies were rated as low quality on the AMSTAR-2, but the GRADE approach suggested some findings were reasonably robust. Most of the non-genetic studies did not reliably exclude the potential effects of previous antidepressant use and were based on relatively small numbers of participants. The genetic studies, in particular, illustrate the importance of methodological rigour and sample size. Whereas some earlier, lower quality, mostly smaller studies produced marginally positive findings, these were not confirmed in better-conducted, larger and more recent studies [27, 32]. The identification of depression and assessment of confounders and interaction effects were limited by the data available in the original studies on which the included reviews and meta-analyses were based. Common methods such as the categorisation of continuous measures and application of linear models to non-linear data may have led to over-estimation or under-estimation of effects [69, 70], including the interaction between stress and the SERT gene. The latest systematic review of tryptophan depletion studies was conducted in 2007, and there has been considerable research produced since then. Hence, we provided a snapshot of the most recent evidence at the time of writing, but this area requires an up to date, comprehensive data synthesis. However, the recent studies were consistent with the earlier meta-analysis with little evidence for an effect of tryptophan depletion on mood.
Although umbrella reviews typically restrict themselves to systematic reviews and meta-analyses, we aimed to provide the most comprehensive possible overview. Therefore, we chose to include meta-analyses that did not involve a systematic review and a large genetic association study on the premise that these studies contribute important data on the question of whether the serotonin hypothesis of depression is supported. As a result, the AMSTAR-2 quality rating scale, designed to evaluate the quality of conventional systematic reviews, was not easily applicable to all studies and had to be modified or replaced in some cases.
One study in this review found that antidepressant use was associated with a reduction of plasma serotonin [26], and it is possible that the evidence for reductions in SERT density and 5-HT1A receptors in some of the included imaging study reviews may reflect compensatory adaptations to serotonin-lowering effects of prior antidepressant use. Authors of one meta-analysis also highlighted evidence of 5-HIAA levels being reduced after long-term antidepressant treatment [71]. These findings suggest that in the long-term antidepressants might produce compensatory changes [72] that are opposite to their acute effects [73, 74]. Lowered serotonin availability has also been demonstrated in animal studies following prolonged antidepressant administration [75]. Further research is required to clarify the effects of different drugs on neurochemical systems, including the serotonin system, especially during and after long-term use, as well as the physical and psychological consequences of such effects.
This review suggests that the huge research effort based on the serotonin hypothesis has not produced convincing evidence of a biochemical basis to depression. This is consistent with research on many other biological markers [21]. We suggest it is time to acknowledge that the serotonin theory of depression is not empirically substantiated.
Supplementary information
Author contributions
JM conceived the idea for the study. JM, MAH, MPH, TS and SA designed the study. JM, MAH, MPH, TS, and SA screened articles and abstracted data. JM drafted the first version of the manuscript. JM, MAH, MPH, TS, SA, and REC contributed to the manuscript’s revision and interpretation of findings. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
There was no specific funding for this review. MAH is supported by a Clinical Research Fellowship from North East London NHS Foundation Trust (NELFT). This funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Data availability
All extracted data is available in the paper and supplementary materials. Further information about the decision-making for each rating for categories of the AMSTAR-2 and STREGA are available on request.
Competing interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). SA declares no conflicts of interest. MAH reports being co-founder of a company in April 2022, aiming to help people safely stop antidepressants in Canada. MPH reports royalties from Palgrave Macmillan, London, UK for his book published in December, 2021, called “Evidence-biased Antidepressant Prescription.” JM receives royalties for books about psychiatric drugs, reports grants from the National Institute of Health Research outside the submitted work, that she is co-chairperson of the Critical Psychiatry Network (an informal group of psychiatrists) and a board member of the unfunded organisation, the Council for Evidence-based Psychiatry. Both are unpaid positions. TS is co-chairperson of the Critical Psychiatry Network. RC is an unpaid board member of the International Institute for Psychiatric Drug Withdrawal.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-022-01661-0.
References
- 1.Coppen A. The biochemistry of affective disorders. Br J Psychiatry. 1967;113:1237–64. doi: 10.1192/bjp.113.504.1237. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. What Is Psychiatry? 2021. https://www.psychiatry.org/patients-families/what-is-psychiatry-menu.
- 3.GlaxoSmithKline. Paxil XR. 2009. www.Paxilcr.com (site no longer available). Last accessed 27th Jan 2009.
- 4.Eli Lilly. Prozac - How it works. 2006. www.prozac.com/how_prozac/how_it_works.jsp?reqNavId=2.2. (site no longer available). Last accessed 10th Feb 2006.
- 5.Healy D. Serotonin and depression. BMJ: Br Med J. 2015;350:h1771. doi: 10.1136/bmj.h1771. [DOI] [PubMed] [Google Scholar]
- 6.Pies R. Psychiatry’s New Brain-Mind and the Legend of the “Chemical Imbalance.” 2011. https://www.psychiatrictimes.com/view/psychiatrys-new-brain-mind-and-legend-chemical-imbalance. Accessed March 2, 2021.
- 7.Geddes JR, Andreasen NC, Goodwin GM. New Oxford Textbook of Psychiatry. Oxford, UK: Oxford University Press; 2020. [Google Scholar]
- 8.Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. 10th Editi. Lippincott Williams & Wilkins (LWW); 2017.
- 9.Cowen PJ, Browning M. What has serotonin to do with depression? World Psychiatry. 2015;14:158–60. doi: 10.1002/wps.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry. 2017;4:409–18. doi: 10.1016/S2215-0366(17)30015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yohn CN, Gergues MM, Samuels BA. The role of 5-HT receptors in depression. Mol Brain. 2017;10:28. doi: 10.1186/s13041-017-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn A, Haeusler D, Kraus C, Höflich AS, Kranz GS, Baldinger P, et al. Attenuated serotonin transporter association between dorsal raphe and ventral striatum in major depression. Hum Brain Mapp. 2014;35:3857–66. doi: 10.1002/hbm.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amidfar M, Colic L, Kim MWAY-K. Biomarkers of major depression related to serotonin receptors. Curr Psychiatry Rev. 2018;14:239–44. doi: 10.2174/1573400514666181016115747. [DOI] [Google Scholar]
- 14.Albert PR, Benkelfat C, Descarries L. The neurobiology of depression—revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philos Trans R Soc Lond B Biol Sci. 2012;367:2378–81. doi: 10.1098/rstb.2012.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilkington PD, Reavley NJ, Jorm AF. The Australian public’s beliefs about the causes of depression: associated factors and changes over 16 years. J Affect Disord. 2013;150:356–62. doi: 10.1016/j.jad.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Pescosolido BA, Martin JK, Long JS, Medina TR, Phelan JC, Link BG. A disease like any other? A decade of change in public reactions to schizophrenia, depression, and alcohol dependence. Am J Psychiatry. 2010;167:1321–30. doi: 10.1176/appi.ajp.2010.09121743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Read J, Renton J, Harrop C, Geekie J, Dowrick C. A survey of UK general practitioners about depression, antidepressants and withdrawal: implementing the 2019 Public Health England report. Therapeutic Advances in. Psychopharmacology. 2020;10:204512532095012. doi: 10.1177/2045125320950124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demasi M, Gøtzsche PC. Presentation of benefits and harms of antidepressants on websites: A cross-sectional study. Int J Risk Saf Med. 2020;31:53–65. doi: 10.3233/JRS-191023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakobsen JC, Gluud C, Kirsch I. Should antidepressants be used for major depressive disorder? BMJ Evidence-Based. Medicine. 2020;25:130–130. doi: 10.1136/bmjebm-2019-111238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moncrieff J, Cohen D. Do antidepressants cure or create abnormal brain states? PLoS Med. 2006;3:e240. doi: 10.1371/journal.pmed.0030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. 2020;25:321–38. doi: 10.1038/s41380-019-0585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018;21:95–100. doi: 10.1136/ebmental-2018-300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2,. version 6.Cochrane; 2021.
- 25.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang T, Balasubramanian R, Yao Y, Clish CB, Shadyab AH, Liu B, et al. Associations of depression status with plasma levels of candidate lipid and amino acid metabolites: a meta-analysis of individual data from three independent samples of US postmenopausal women. Mol Psychiatry. 2020;2020. 10.1038/s41380-020-00870-9. [DOI] [PMC free article] [PubMed]
- 27.Culverhouse RC, Saccone NL, Horton AC, Ma Y, Anstey KJ, Banaschewski T, et al. Collaborative meta-analysis finds no evidence of a strong interaction between stress and 5-HTTLPR genotype contributing to the development of depression. Mol Psychiatry. 2018;23:133–42. doi: 10.1038/mp.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Little J, Higgins JPT, Ioannidis JPA, Moher D, Gagnon F, von Elm E, et al. STrengthening the REporting of Genetic Association Studies (STREGA)— An Extension of the STROBE Statement. PLoS Med. 2009;6:e1000022. doi: 10.1371/journal.pmed.1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is quality of evidence and why is it important to clinicians? BMJ. 2008;336:995–8. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon HS, Hattori K, Ogawa S, Sasayama D, Ota M, Teraishi T, et al. Relationships of cerebrospinal fluid monoamine metabolite levels with clinical variables in major depressive disorder. J Clin Psychiatry. 2017;78:e947–56. doi: 10.4088/JCP.16m11144. [DOI] [PubMed] [Google Scholar]
- 31.Kugaya A, Seneca NM, Snyder PJ, Williams SA, Malison RT, Baldwin RM, et al. Changes in human in vivo serotonin and dopamine transporter availabilities during chronic antidepressant administration. Neuropsychopharmacology. 2003;28:413–20. doi: 10.1038/sj.npp.1300036. [DOI] [PubMed] [Google Scholar]
- 32.Border R, Johnson EC, Evans LM, Smolen A, Berley N, Sullivan PF, et al. No support for historical candidate gene or candidate gene-by-interaction hypotheses for major depression across multiple large samples. Am J Psychiatry. 2019;176:376–87. doi: 10.1176/appi.ajp.2018.18070881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogawa S, Tsuchimine S, Kunugi H. Cerebrospinal fluid monoamine metabolite concentrations in depressive disorder: A meta-analysis of historic evidence. J Psychiatr Res. 2018;105:137–46. doi: 10.1016/j.jpsychires.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 34.Nautiyal KM, Hen R. Serotonin receptors in depression: from A to B. F1000Res. 2017;6:123. doi: 10.12688/f1000research.9736.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rojas PS, Neira D, Muñoz M, Lavandero S, Fiedler JL. Serotonin (5‐HT) regulates neurite outgrowth through 5‐HT1A and 5‐HT7 receptors in cultured hippocampal neurons. J Neurosci Res. 2014;92:1000–9. doi: 10.1002/jnr.23390. [DOI] [PubMed] [Google Scholar]
- 36.Kaufman J, DeLorenzo C, Choudhury S, Parsey RV. The 5-HT1A receptor in Major Depressive Disorder. Eur Neuropsychopharmacol. 2016;26:397–410. doi: 10.1016/j.euroneuro.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikolaus S, Müller H-W, Hautzel H. Different patterns of 5-HT receptor and transporter dysfunction in neuropsychiatric disorders – a comparative analysis of in vivo imaging findings. Rev Neurosci. 2016;27:27–59. doi: 10.1515/revneuro-2015-0014. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Zhou C, Zhu D, Wang X, Fang L, Zhong J, et al. Serotonin-1A receptor alterations in depression: A meta-analysis of molecular imaging studies. BMC Psychiatry. 2016;16:1–9. doi: 10.1186/s12888-016-1025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kambeitz JP, Howes OD. The serotonin transporter in depression: Meta-analysis of in vivo and post mortem findings and implications for understanding and treating depression. J Affect Disord. 2015;186:358–66. doi: 10.1016/j.jad.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 40.Meyer JH. Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J Psychiatry Neurosci. 2007;32:86–102. [PMC free article] [PubMed] [Google Scholar]
- 41.Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–81. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Shen H-W, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, et al. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–9. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- 43.Hagino Y, Takamatsu Y, Yamamoto H, Iwamura T, Murphy DL, Uhl GR, et al. Effects of MDMA on extracellular dopamine and serotonin levels in mice lacking dopamine and/or serotonin transporters. Curr Neuropharmacol. 2011;9:91–5. doi: 10.2174/157015911795017254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Z, Zhen J, Karpowich NK, Law CJ, Reith MEA, Wang D-N. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat Struct Mol Biol. 2009;16:652–7. doi: 10.1038/nsmb.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gryglewski G, Lanzenberger R, Kranz GS, Cumming P. Meta-analysis of molecular imaging of serotonin transporters in major depression. J Cereb Blood Flow Metab. 2014;34:1096–103. doi: 10.1038/jcbfm.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benmansour S, Owens WA, Cecchi M, Morilak DA, Frazer A. Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of this transporter. J Neurosci. 2002;22:6766–72. doi: 10.1523/JNEUROSCI.22-15-06766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, et al. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci. 1999;19:10494–501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horschitz S, Hummerich R, Schloss P. Down-regulation of the rat serotonin transporter upon exposure to a selective serotonin reuptake inhibitor. Neuroreport. 2001;12:2181–4. doi: 10.1097/00001756-200107200-00027. [DOI] [PubMed] [Google Scholar]
- 49.Young SN. Acute tryptophan depletion in humans: a review of theoretical, practical and ethical aspects. J Psychiatry Neurosci. 2013;38:294–305. doi: 10.1503/jpn.120209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–59. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 51.Fusar-Poli P, Allen P, McGuire P, Placentino A, Cortesi M, Perez J. Neuroimaging and electrophysiological studies of the effects of acute tryptophan depletion: A systematic review of the literature. Psychopharmacology. 2006;188:131–43. doi: 10.1007/s00213-006-0493-1. [DOI] [PubMed] [Google Scholar]
- 52.Hogenelst K, Schoevers RA, Kema IP, Sweep FCGJ, aan het Rot M. Empathic accuracy and oxytocin after tryptophan depletion in adults at risk for depression. Psychopharmacology. 2016;233:111–20. doi: 10.1007/s00213-015-4093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinstein JJ, Rogers BP, Taylor WD, Boyd BD, Cowan RL, Shelton KM, et al. Effects of acute tryptophan depletion on raphé functional connectivity in depression. Psychiatry Res. 2015;234:164–71. doi: 10.1016/j.pscychresns.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreno FA, Erickson RP, Garriock HA, Gelernter J, Mintz J, Oas-Terpstra J, et al. Association study of genotype by depressive response during tryptophan depletion in subjects recovered from major depression. Mol. Neuropsychiatry. 2015;1:165–74. doi: 10.1159/000439114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munafò MR. The serotonin transporter gene and depression. Depress Anxiety. 2012;29:915–7. doi: 10.1002/da.22009. [DOI] [PubMed] [Google Scholar]
- 56.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 57.Kiyohara C, Yoshimasu K. Association between major depressive disorder and a functional polymorphism of the 5-hydroxytryptamine (serotonin) transporter gene: A meta-analysis. Psychiatr Genet. 2010;20:49–58. doi: 10.1097/YPG.0b013e328335112b. [DOI] [PubMed] [Google Scholar]
- 58.Oo KZ, Aung YK, Jenkins MA, Win AK. Associations of 5HTTLPR polymorphism with major depressive disorder and alcohol dependence: A systematic review and meta-analysis. Aust N. Z J Psychiatry. 2016;50:842–57. doi: 10.1177/0004867416637920. [DOI] [PubMed] [Google Scholar]
- 59.Culverhouse RC, Bowes L, Breslau N, Nurnberger JI, Burmeister M, Fergusson DM, et al. Protocol for a collaborative meta-analysis of 5-HTTLPR, stress, and depression. BMC Psychiatry. 2013;13:1–12. doi: 10.1186/1471-244X-13-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited. Arch Gen Psychiatry. 2011;68:444. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharpley CF, Palanisamy SKA, Glyde NS, Dillingham PW, Agnew LL. An update on the interaction between the serotonin transporter promoter variant (5-HTTLPR), stress and depression, plus an exploration of non-confirming findings. Behav Brain Res. 2014;273:89–105. doi: 10.1016/j.bbr.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 62.Bleys D, Luyten P, Soenens B, Claes S. Gene-environment interactions between stress and 5-HTTLPR in depression: A meta-analytic update. J Affect Disord. 2018;226:339–45. doi: 10.1016/j.jad.2017.09.050. [DOI] [PubMed] [Google Scholar]
- 63.Delgado PL. Monoamine depletion studies: implications for antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67:22–26. [PubMed] [Google Scholar]
- 64.Kemp JJ, Lickel JJ, Deacon BJ. Effects of a chemical imbalance causal explanation on individuals’ perceptions of their depressive symptoms. Behav Res Ther. 2014;56:47–52. doi: 10.1016/j.brat.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 65.Lebowitz MS, Ahn W-K, Nolen-Hoeksema S. Fixable or fate? Perceptions of the biology of depression. J Consult Clin Psychol. 2013;81:518. doi: 10.1037/a0031730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zimmermann M, Papa A. Causal explanations of depression and treatment credibility in adults with untreated depression: Examining attribution theory. Psychol Psychother. 2020;93:537–54. doi: 10.1111/papt.12247. [DOI] [PubMed] [Google Scholar]
- 67.Maund E, Dewar-Haggart R, Williams S, Bowers H, Geraghty AWA, Leydon G, et al. Barriers and facilitators to discontinuing antidepressant use: A systematic review and thematic synthesis. J Affect Disord. 2019;245:38–62. doi: 10.1016/j.jad.2018.10.107. [DOI] [PubMed] [Google Scholar]
- 68.Eveleigh R, Speckens A, van Weel C, Oude Voshaar R, Lucassen P. Patients’ attitudes to discontinuing not-indicated long-term antidepressant use: barriers and facilitators. Therapeutic Advances in. Psychopharmacology. 2019;9:204512531987234. doi: 10.1177/2045125319872344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Springer, Cham; 2015.
- 70.Schafer JL, Kang J. Average causal effects from nonrandomized studies: a practical guide and simulated example. Psychol Methods. 2008;13:279–313. doi: 10.1037/a0014268. [DOI] [PubMed] [Google Scholar]
- 71.Pech J, Forman J, Kessing LV, Knorr U. Poor evidence for putative abnormalities in cerebrospinal fluid neurotransmitters in patients with depression versus healthy non-psychiatric individuals: A systematic review and meta-analyses of 23 studies. J Affect Disord. 2018;240:6–16. doi: 10.1016/j.jad.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 72.Fava GA. May antidepressant drugs worsen the conditions they are supposed to treat? The clinical foundations of the oppositional model of tolerance. Therapeutic Adv Psychopharmacol. 2020;10:2045125320970325. doi: 10.1177/2045125320970325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kitaichi Y, Inoue T, Nakagawa S, Boku S, Kakuta A, Izumi T, et al. Sertraline increases extracellular levels not only of serotonin, but also of dopamine in the nucleus accumbens and striatum of rats. Eur J Pharm. 2010;647:90–6. doi: 10.1016/j.ejphar.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 74.Gartside SE, Umbers V, Hajós M, Sharp T. Interaction between a selective 5‐HT1Areceptor antagonist and an SSRI in vivo: effects on 5‐HT cell firing and extracellular 5‐HT. Br J Pharmacol. 1995;115:1064–70. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bosker FJ, Tanke MAC, Jongsma ME, Cremers TIFH, Jagtman E, Pietersen CY, et al. Biochemical and behavioral effects of long-term citalopram administration and discontinuation in rats: role of serotonin synthesis. Neurochem Int. 2010;57:948–57. doi: 10.1016/j.neuint.2010.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All extracted data is available in the paper and supplementary materials. Further information about the decision-making for each rating for categories of the AMSTAR-2 and STREGA are available on request.