Abstract
Most mental disorders have a typical onset between 12 and 25 years of age, highlighting the importance of this period for the pathogenesis, diagnosis, and treatment of mental ill-health. This perspective addresses interactions between risk and protective factors and brain development as key pillars accounting for the emergence of psychopathology in youth. Moreover, we propose that novel approaches towards early diagnosis and interventions are required that reflect the evolution of emerging psychopathology, the importance of novel service models, and knowledge exchange between science and practitioners. Taken together, we propose a transformative early intervention paradigm for research and clinical care that could significantly enhance mental health in young people and initiate a shift towards the prevention of severe mental disorders.
Subject terms: Psychiatric disorders, Diagnostic markers
Rethinking Mental Health as “Youth Mental Health”
“Mental disorders are chronic diseases of the young.” [1]
Mental disorders constitute a major challenge to both society and science. Syndromes such as schizophrenia, depression, anxiety, and personality disorders comprise some of the largest disease burdens worldwide [2]. Despite the promises of genetics and translational neuroscience, insights into the causal mechanisms of major syndromes remain rudimentary [3], and the search for biomarkers to improve diagnosis and stratification has largely been unsuccessful [4]. Moreover, effect sizes for current pharmacological and psychological treatments are overall modest [5] and a significant number of patients will not respond to treatments [6].
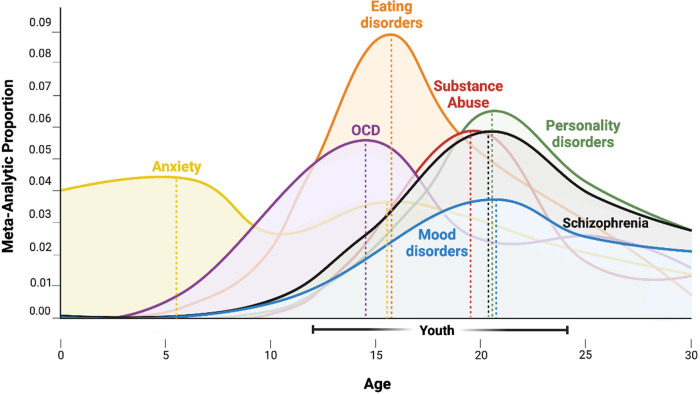
A cardinal feature of the existing paradigm in mental health has been its emphasis on fully established disorders in adulthood while early intervention and prevention have been relatively neglected [7, 8]. However, there is now consistent epidemiological evidence that has highlighted that all major syndromes constituting approximately 75% of mental disorders begin before the age of 25 years [9, 10] (Fig. 1).
Fig. 1. Age of onset of mental disorders.
Distribution of age of onset of mental disorders in the general population based on the meta-analysis by Solmi et al. [9]: Meta-analytic epidemiologic proportion (y-axis) for anxiety disorders (5.5/15.5 years), substance use disorders (19.5 years), schizophrenia/psychotic disorders (20.5 years), eating disorders (15.5 years), personality disorders (20.5 years), obsessive-compulsive (14.5) and mood disorders (20.5 years) (ICD-10 blocks). The dotted horizontal lines represent the peak age of onset for each diagnostic category.
In this perspective, we will make the case for a transformative paradigm in mental health that emphasizes early intervention and prevention of emerging mental disorders during youth, the period between 12 and 25 years of age1, with wide ranging implications for diagnosis, research, and interventions. Our approach is critically informed by the early intervention paradigm in psychosis [11]. Its scope has now been broadened to target emerging mental disorders during youth more generally given that young people with clinical high-risk criteria for psychosis (CHR-P) rarely present solely with signs of psychosis [12] and evidence that early identification and intervention is also potentially effective in personality disorders [13], eating disorders [14], and bipolar disorder [15].
The early intervention paradigm is furthermore motivated by the finding that young people face many barriers to accessing mental health care [16] during a developmental period, which is critical for social and occupational adjustment [17]. Importantly, the COVID pandemic has accelerated this trend with youth reporting a disproportionate increase in mental ill-health [18]. As a result, emerging mental disorders during youth frequently lead to sustained mental health problems during adulthood and lower overall functioning [17, 19].
To address these fundamental challenges, we set out core “pillars” for a youth mental health paradigm. Specifically, we propose that ongoing modifications in behavioral functions and underlying neural circuits suggest the presence of “sensitive periods” for the symptomatic expression of mental ill-health and corresponding “windows of opportunity” for early intervention. Secondly, risk factors interact with these sensitive periods on multiple levels that can be conceptualized as “developmental cascades”. Thirdly, novel diagnostic approaches are needed to facilitate interventions for sub-threshold symptomatic expressions of emerging psychopathology. Finally, the implications of these findings are discussed with respect to novel clinical and policy approaches and models for youth mental health.
Brain development, sensitive periods, and emerging psychopathology
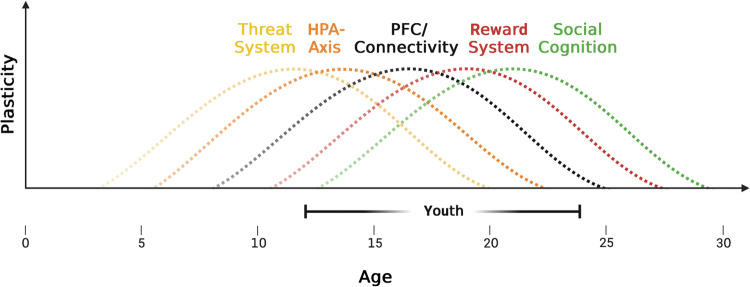
An important principle in brain development is the notion of “sensitive periods” [20, 21]. Sensitive periods can be described as time-limited developmental windows during which environmental exposures have a pronounced effect on the functionality and organization of neural circuits and behavior as a result of heightened plasticity. Originally first described in the visual system [22], there is mounting data that several neural and cognitive systems that are relevant for emerging psychopathology, such as fear and stress regulation, higher cognitive processes, social cognition, and reward-processing, undergo time-limited modifications in circuit properties during youth (Fig. 2).
Fig. 2. Sensitive periods during brain development.
Overview of sensitive periods during brain development: The curves indicate the plastic potential for different neural systems between 0 and 30 years of age: (a) threat-regulation involving cortical-hippocampal-amygdala circuits (b) HPA-axis system (c) PFC/Connectivity subsumes local changes in PFC-properties (E/I-balance, Dopamine) as well as long-range connectivity with cortical-subcortical target regions (d) Reward System comprising striatum and connectivity with PFC and (e) social-Cognitive Processes. HPA-axis hypothalamic-pituitary-adrenal axis, PFC prefrontal cortex, E/I balance Excitation/Inhibition-balance, PFC prefrontal cortex.
A core function that is impaired across many mental disorders is the ability to respond to threats and stress [23]. Importantly, anxiety disorders emerge during the first decade, peak around 15 years of age [9], and often persist into adulthood with significant effects on functioning and quality of life [24]. Recent work has identified unique changes in the neural circuits underlying threat regulation [25]. Specifically, fear extinction during adolescence is reduced in both humans and mice [26] and probing neural circuitry in mice has revealed altered synaptic plasticity and connectivity in prefrontal cortical-hippocampal-amygdala [26]. Interestingly in adolescent mice, if extinction training takes place in the threat-conditioning context that engages hippocampal-based circuits, extinction retention is significantly greater than that in the same-age counterparts that underwent extinction training in a novel context [27]. However, once outside this ‘sensitive period’, this form of context-based extinction has minimal additional effects.
The hypothalamic-pituitary-adrenal (HPA)-axis is the primary site for regulating the body’s stress response through releasing glucocorticoid hormones and HPA-axis dysregulation is involved in several mental disorders and contributes to emerging psychopathology [28]. Evidence from animal studies suggests that a variety of stressors are associated with elevated and prolonged HPA responses in youth compared with adulthood [29] and that differences in the psychopathological phenotypes observed may depend on the timing of the stress exposure during youth [30]. Conversely, environmental enrichment in juvenile animals can reverse the effects of prenatal stress and maternal separation [31, 32]. Together, these findings suggest that both risk and protective factors interact with HPA-functioning during youth [33].
In addition, ongoing modifications in higher-order cognitive functions, such as working memory (WM), response inhibition, and performance monitoring, as well as cognitive control are a central aspect of late brain development [34]. Anatomically, these functions are closely related to the integrity of prefrontal cortical (PFC) circuits, a region that is impaired in a range of mental disorders, including schizophrenia, depression, and bipolar disorder [35].
There is substantial evidence that the composition and interaction of the dopaminergic, glutamatergic, and GABAergic receptors in the PFC undergo profound changes during youth [34]. PV+ interneurons are of particular interest as they contribute towards the opening and closing of sensitive periods [21] and recent evidence suggests that PV+ interneuron expression continues to increase during youth while time-limited downregulation leads to permanent changes in E/I-balance during adulthood [36], a process which may be involved in emerging psychopathology [37].
Modifications of local PFC changes are accompanied by an extensive integration with cortical and subcortical areas through the maturation of long-range connections [38–42]. A core hypothesis underlying brain development is the view that ongoing changes in brain maturation lead to a period when limbic structures, such as the amygdala and striatum, predominate over prefrontal areas during youth [43, 44]. The change to predominance of executive prefrontal regions in adulthood reflect a potential neurobiological basis for improvements in emotion regulation which is critically impaired in affective disorders [45] but also in borderline personality disorders [46].
One important manifestation of the predominance of limbic processing over executive function is risk-taking behavior during youth [47], such as substance abuse. Evidence suggests that the likelihood of developing substance dependence is highest if substance abuse is initiated before age 14 [48], suggesting a sensitive period that is closely related to ongoing changes in incentive salience as well as changes in dopaminergic neurotransmission in the striatum and PFC [49] which is permanently altered by drug abuse [48]. In addition, tetrahydrocannabinol (THC), the principal psychoactive constituent of cannabis, may lead to a permanent disruption of E/I-balance and cognition during youth but not during adulthood [50].
Finally, converging findings have highlighted the possibility of a sensitive period for the development of the social-cognitive processes [51, 52]. Social cognition includes several domains, including theory of mind, emotion processing, and social cue identification, that have been linked to specific brain regions, such as the medial prefrontal cortex (mPFC) and tempo-parietal junction (TPJ) [53]. Recent evidence suggests that self-oriented thinking overlaps with regions required for understanding others, suggesting that the development of identity, a core task for youths, is an overlapping and intertwined process [52].
Reward circuits are particularly sensitive to peer influence during this age group, which can be related to higher levels of risk-taking behavior in social settings [54], and peer evaluations affect the self-image more than during other developmental periods [55]. Social interactions are also a necessary environmental exposure for establishing adult social behavior [56], a particularly pertinent issue given the widespread reduction of social interactions during the COVID-pandemic [57].
Developmental cascades, the environment, and social-cultural context
Youth is a unique period during human development characterized by the onset of puberty, followed by psychosocial milestones, such as the separation from family, developing romantic attachments as well as discovering one’s sexual orientation and identity. The wide-ranging manifestations of youth across and within cultures suggest that this phase is highly shaped by the environmental context. Accordingly, understanding the relationship between sensitive periods, environmental factors, and the emergence of psychopathology necessitates the application of appropriate theoretical and conceptual frameworks that address the highly dynamic and context-dependent nature of this developmental period that in turn can be harnessed to identify risk and resilience factors for emerging mental ill-health.
The population neuroscience approach is well suited to applying a life-course epidemiology paradigm to mental disorders that acknowledges the complexity in time and space of environmental and genomic factors [58, 59]. In this context, it is important to understand and apply the concept of developmental cascades, which is integral to population neuroscience approaches in appreciating how transactions at different timescales (e.g., perinatal, infancy, adolescence, early adulthood), constructs (cognition, mood, behavior), and levels (molecular, physiology, individual, and social) have a domino effect on subsequent development [60]. These developmental cascades refer to the cumulative consequences of the many interactions and transactions occurring during development that result in spreading after-effects across levels, among domains at the same level, and across different systems [61].
Developmental cascades are potentially a useful approach to conceptualizing emerging psychopathology as many mental disorders in adulthood frequently have precursors in non-specific symptomatic manifestations during youth [62] but also in childhood [63, 64]. Recent evidence suggests that the risk of mental disorders is associated with elevated risk for other disorders and that younger age of onset is a predictor of longer duration of symptoms, comorbidity, and worse outcomes [19], highlighting the importance of interventions to target the earliest signs of mental ill-health.
Effects of specific environmental exposures on the outcomes of mental disorders and their behavioral precursors can be potentially mediated or moderated by a broad range of contextual, cultural, and biological factors. Mediators and moderators can, therefore, reveal time-sensitive windows for therapeutic interventions that target remediation of the exposure, mediating, or moderating factors. Thus, social media use has differential effects depending on the developmental timing [65], suggesting that sensitive periods during youth may be particularly malleable by environmental exposures [66].
Until recently, conceptualizing the multitude of environmental exposures and their contribution towards emerging psychopathology has been challenging. The exposome represents the totality of environmental exposures that an individual experiences from conception throughout the lifespan as well as the interaction among these exposures [67]. Choi et al. [68] examined genomic and exposome influences on internalizing and externalizing symptoms in youth, highlighting that additive and interactive influences of the genome and exposome explained over 30% and 60% of the variance in internalizing and externalizing symptoms while a single environmental risk factor accounted for only 1% of the variance.
Emerging psychopathology and diagnostic framework(s)
The onset of the majority of mental disorders during youth [9] as well as the cascading and dynamic nature of psychopathology during development [19] have important implications for diagnostic frameworks. However, current diagnostic systems (DSM-5, ICD-11) have several shortcomings that require novel approaches to enable early detection and intervention. While several alternatives have been suggested, such as the Research Domain Criteria [69] and the Hierarchical Taxonomy of Psychopathology (HiTOP) [70], a developmental perspective on emerging psychopathology is rarely explicitly addressed (but see [71, 72]). In addition, both DSM-5 and ICD-11 do not allow a diagnosis to be made if symptom expression is below a certain cut-off value, even though there is established evidence for prodromal periods for psychosis [73] and bipolar disorder [74] as well as possibly for eating disorders [75], depression [76], and obsessive-compulsive disorders [77] (Table 1).
Table 1.
Overview of early intervention for mental disorders in youth.
| Schizophrenia/ Psychotic disorders |
Mood disorders (BP/MDD) |
Personality disorders | Anxiety disorders | Substance abuse | OCD | Eating disorders | |
|---|---|---|---|---|---|---|---|
| Target Population |
CHR-P: [73]**** FEP: [97]**** |
BD: at-risk states*, prodrome (CHR-BP) [84]* |
BPD subthreshold* Early-Stage BPD* [100] |
At-Risk Populations [137]*** Youth [135]*** |
High-Risk Populations [101]*** Youth [138]*** |
Youth [77]** | Recent-Onset ED [102]** |
|
Detection Screening Instruments (Sensitivity/Specificity, PPV, NPV) Duration of Prodrome (months) Duration of Untreated Illness (months) Relationship to Outcome |
CHR-P: CAARMS, SIPS, SPI-A (67–100%, 39–100%, 24–100%) [139]** CHR-P: 21 [140]* FEP: 28–60 [94]*** Psychosis: 24-26 [141]*** FEP: Negative Symptoms, Self-harm [141]*** |
CHR-BP: BPSS-AS-P [142]* MDD: PHQ-9* [143] BP: 27 [74]*** MDD:1-36 [76]*** BP:60 [144]*** MDD:12-60 [145]*** BP: Onset of Mania, Psychosis [144]*** MDD: Treatment Response, Remission [145]*** |
BPD: MSI-BPD, BPQ, SCID-II BPD module (65-91) [146]* – – – |
CMAS, MASC [147]* – GAD: 108* [148] Phobias: 120* [148] PTSD: 120* [148] – |
CRAFFT (.80-86) [149]*** - Alcohol abuse: 108* [148] Alcohol Dependence: 72* [148] Drug Abuse: 72 [148] Drug Dependence: 30 [148]* - |
SOCS (.67) [150]* - OCD: 84-120 [77]** OCD: Treatment Response [77]** |
ED: SCOFF (100%, 87.5%) [151] EDE-Q (82.8%, 89.7%, 0.94) [152]* AN: 30 [95]** BN: 34 [95]** BED: 67 [95]** AN: Persistence of AN [153]* |
|
Prognosis Assessment instruments (accuracy, AUC) Transition Risk Biomarkers Risk Calculators (AUC, C-Index): |
CAARMS [154]***, SIPS [154]*** (0.85 pooled at 38 months)*** CHR-P: 17% at 1 year; 22% at 3 years) [73]*** CHR-P (Transition): Cognition [82]***, MRI [83]***, EEG [155]*** FEP (Functional Outcomes): [156]* CHR-P (Psychosis Risk): (0.70–0.80) [8]*** |
BPSS-FP (not available) SIBARS (0.7 at 18 months) [157]* 14% at 1 year [85]*; 23% at 2 years [85]* BP At-Risk (Transition): (.70) [158]* BP At-Risk (Onset of BD): (.71) [8] |
– – – – |
– – – – |
– – – – |
– – – – |
– – – – |
|
Interventions (level of evidence) Indicated Prevention Secondary Prevention |
CHR-P: CBT (reduction in transition to psychosis) [98]1b FEP: Specialized EI- Services (psychosocial and pharmacological interventions: significant effects on functional and clinical outcomes) [97] 1b |
BP At-Risk: Pharmacology, Psycho-Social Interventions (no effect on transition, moderate effect on depression) [159]1b MDD: School-based interventions (small effect on symptoms) [160] MDD (Youth): Psycho-Social Interventions (no effect on onset but possible reduction of symptoms) 1b |
– Early-Stage BPD: EI Service Model (psychotherapy, befriending: greater treatment attendance/completion), [13]1c |
School-Based Interventions: (small effect on symptoms) [161]1b Psychological/Educational Interventions: (small, preventive effect) [162]1b – |
Cannabis: School-Based Interventions (small effect on cannabis use) [163]1b Youth with Substance Abuse: Motivational Interviewing (small effects on use) [164]1b Preliminary evidence for self-help/peer and CBT [101]2b |
– – |
Recent Onset ED: Specialized EI Service (improved clinical outcomes, reduction in admissions) [102]2b |
* single study, ** systematic review, *** meta-analysis, **** umbrella review. Level of evidence:
1a) Systematic reviews (with homogeneity) of randomized controlled trials 1b) Individual randomized controlled trials (with narrow confidence interval) 1c) All or none randomized controlled trials 2) a Systematic reviews (with homogeneity) of cohort studies 2b) Individual cohort study or low quality randomized controlled trials (e.g. <80% follow-up) 2c) "Outcomes" Research; ecological studies 3a) Systematic review (with homogeneity) of case-control studies 3b) Individual case-control study 4) Case-series (and poor quality cohort and case-control studies) 5) Expert opinion without explicit critical appraisal, or based on physiology, bench research or "first principles"
AN Anorexia Nervosa, APS attenuated psychotic symptoms, BED Binge Eating Disorders, BLIPS brief limited intermittent psychotic symptoms, BN Bulimia Nervosa, BP Bipolar Disorders, BPD Borderline Personality Disorder, BPSS-AS-P Bipolar Prodrome Symptom Scale - Abbreviated Screen for Patients, BPQ Borderline Personality Questionnaire, BPSS-AP Bipolar Prodrome Symptom Interview and Abbreviated Screen for Patients, BPSS-FP Bipolar Prodrome Symptom Interview and Scale-Full Prospective, CAARMS Comprehensive Assessment of At Risk Mental States, CHR-BP Clinical High-Risk for Bipolar Disorder, CHR-P Clinical High-Risk for Psychosis, CMAS Children’s Manifest Anxiety Scale, CBT Cognitive Behavioral Therapy, CRAFFT Car, Relax, Alone, Forget, Friends, Trouble Questionnaire, ED Eating Disorders, EDE-Q Eating Disorders Examination Questionnaire, EEG Electroencephalography, FEP First Episode Psychosis, GAD Generalized Anxiety Disorder, GRD genetic risk and deterioration syndrome, MASC Multidimensional Anxiety Scale for Children, MDD Major Depressive Disorder, MSI-BPD McLean Screening Instrument for Borderline Personality Disorder, MRI Magnetic Resonance Imaging, OCD Obsessive Compulsive Disorder, PHQ-9 Patient Health Questionnaire, PTSD Post-traumatic stress disorder, SCID-BPD BPD items from the Structured Clinical Interview for DSM-IV Axis II disorders (SCID-II) Personality Questionnaire, SCOFF Sick, Control, One, Fat, Food Questionnaire, SIBARS Semistructured Interview for Bipolar At Risk States, SIPS Structured Interview for Psychosis-Risk Syndromes, SOCS Self-report Short OCD Screener, SPI-A Schizophrenia Proneness Instrument, Adult version
CHR-P criteria were first developed for psychosis over 20 years ago [78] and comprise Attenuated Psychotic Symptoms (APS), Brief (and Limited) Intermittent Psychotic Symptoms (BLIPS or BIPS), and Genetic Risk and Deterioration Syndrome (GRD) (for details see [73]). CHR-P criteria are associated with high prognostic accuracy that is comparable to other paradigms of preventative medicine [79]. Thus, approximately 20% of individuals meeting CHR-P will develop a first episode of psychosis (FEP) in the initial 2 years [80]. There is evidence that clinical [81], cognitive [82], and neuroimaging measures [83] constitute possible biomarkers that significantly increase accuracy for predicting clinical outcomes in CHR-P participants.
Following the CHR-P paradigm, high-risk criteria were developed for bipolar disorders (CHR-BP) that comprise subthreshold mania and depressive symptoms [84]. Cross-sectional studies have indicated good internal reliability and consistency of these instruments [74]. First evidence suggests that CHR-BP criteria are associated with a conversion rate to bipolar disorder of 14.3% within 12 months [85].
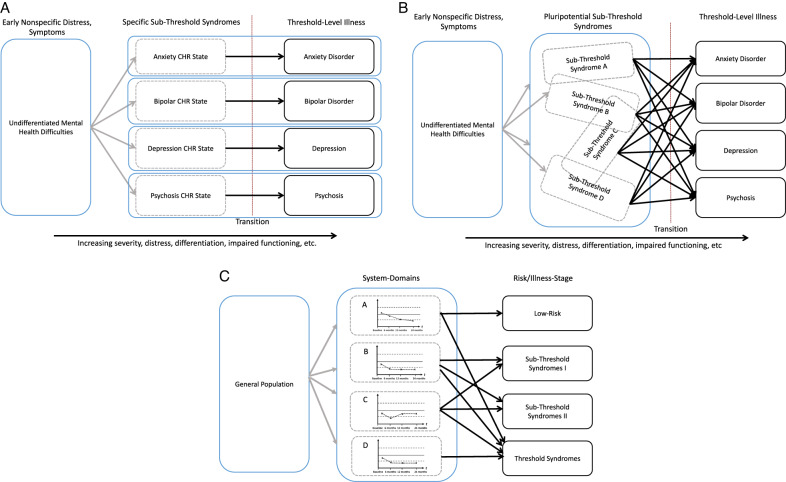
Building on the high-risk paradigm that is oriented along established diagnostic categories, alternative approaches have been advocated that reflect psychopathological dimensions [19, 86]. The clinical staging framework positions individuals along a multidimensional gradient of health to illness, capturing elements of risk, onset, course, and trajectory of illness [87, 88] (Fig. 3). Similar to clinical staging models in other areas of healthcare, staging frameworks hold the promise of guiding treatment selection, with less intensive interventions preferred at earlier stages and interventions with a higher risk/benefit ratio reserved for later stages [89].
Fig. 3. Diagnostic models in youth mental health.
Diagnostic Models in Youth Mental Health: (A) diagnostic staging model focused on symptoms and functioning. B A transdiagnostic, pluripotential staging model in which variable subthreshold symptoms may overlap but give rise to a range of end-stage disorders. CHR indicates clinical high risk. C Growth Charts: Detection of emerging mental disorders in the general population. Four proposed domains of assessment and their age sex- and age-adjusted norms are displayed. Once a threshold of divergence from normative trajectories is reached, individuals could be offered options of closer tracking, more comprehensive assessments, or preventative or clinical interventions. The latter would range from low-risk preventive interventions when such departures begin to manifest clinically (at earlier stages), or treatment of manifest abnormalities that are functionally relevant and/or lead to distress (at later stages). Panels (A, B) are adapted from [165].
Clinical staging models in youth mental health have been described in increasing levels of detail with some organized around specific diagnostic categories while others are transdiagnostic in nature [88]. The latter often aims to bridge the nonspecific, pluripotent nature of early-stage phenomena with more delineated presentations, extension to other dimensions of illness, and added layers of comorbidity seen in later stages. In integrating severity, multidimensionality, and pluripotentiality into a single model, clinical staging should be guided to develop a closer fit with youth-onset clinical syndromes with the ultimate goal of clinical utility [88].
Preventive approaches in the broader population may, however, require alternative approaches that are informed by data about normative development as well as vulnerability and risk exposures. Pediatric growth charts are a clinically valuable tool rooted in normative development and reflecting both physiology and external influences [90, 91]. Importantly, growth charts also allow the prediction of specific traits, such as height and weight, and if a deviation occurs then interventions to correct a possible developmental anomaly can be implemented during sensitive periods (Fig. 3).
A prerequisite for the application of growth charts in youth mental health is the availability of normative developmental data that comprise important domains of emerging psychopathology, such as cognition, emotion regulation, and sleep [92]. These can also be complemented by neuroimaging data, peripheral, digital, and genetic information as well as information about known risk factors and knowledge about sensitive periods. These multidimensional mental health growth charts would determine where an individual is located within normative development that in turn then lead to appropriate stage- and risk-adapted interventions.
Developing and implementing tools for prevention and early intervention
The data on sensitive periods [23, 25, 33, 48, 49] as well as the peak incidence of mental disorders during youth [9, 10] have implications for service provision and treatments. Current approaches and service models, however, remain primarily organized around established diagnostic categories of mental disorders in adulthood [88] that impose further barriers for early intervention by the artificial distinction between child and adolescent as well as adult mental health services [93]. Accordingly, novel clinical approaches for youth mental health are required that emphasize early intervention, low-threshold service delivery, population-based prevention, novel technologies as well as translational and interactional research models.
Sensitive periods and interventions for youth mental health
One important implication of sensitive periods during youth is that the timing of interventions is potentially critical for illness course and prognosis, suggesting that the plastic potential of neural circuits can be harnessed to modify ongoing developmental processes. Currently, however, substantial treatment delays for the majority of syndromes with an onset during youth, such as psychosis, bipolar disorders, and eating disorders, are pervasive [74, 94, 95] (Table 1), ranging from 2-5 years.
Duration of untreated psychosis (DUP) is an important determinant for symptomatic and functional treatment responses in FEP patients [94]. Similar data are available for BP [96], obsessive-compulsive [77] as well as eating disorders [95]. Moreover, there is evidence from indicated prevention in FEP-populations [97], suggesting that specialized psycho-social and pharmacological interventions can improve clinical outcomes compared to standard care [97]. In addition, there is evidence that CBT can reduce transition rates in CHR-P participants [98]. However, a more recent study suggested that clinical gains in FEP may not be sustained beyond the first two years of treatment [99].
Recent studies have extended the early intervention approach to personality disorders [100], substance abuse [101], and eating disorders [102]. Specialized psycho-social interventions for youth with borderline personality disorder (BPD) are effective in improving functioning and access to care [13] and early detection of eating disorders can improve prognosis, and decrease morbidity and mortality [14].
In order to maximize the benefits of the early intervention, we furthermore propose that behavioral and pharmacological interventions may benefit from incorporating data on sensitive periods [103]. Data from animal models of schizophrenia, for example, have shown that different behavioral and neurobiological interventions during the adolescent period but not during earlier or later developmental windows can completely rescue cognitive deficits and associated neural circuit dysfunctions [104–106].
The large majority of pharmacological and psycho-social interventions applied to youths have been developed for adult populations, potentially neglecting important differences in the properties of neural circuits as well as psychological variables. For example, a high proportion of youths with anxiety disorders do not respond to cognitive-behavioral therapy (CBT) [107] which may be due to inherent differences in fronto-amygdala circuitry [108]. Similarly, pharmacological interventions may also need to be adjusted given the ongoing modifications in neurotransmitter systems [34, 36].
A critical consideration in early pharmacologic intervention is minimizing side-effects. For many young people, early adverse experiences with psychotropic medication can reduce long-term adherence with all psychiatric treatments. As a result, the early intervention and the clinical staging models have encouraged investigations into more benign pharmacological treatments including “neutraceuticals”, that is, food or food products with health and medical benefits, such as fish oil [109], N-acetylcysteine [110], and cannabidiol [111], that may be suitable for targeting emerging psychopathology.
Novel service models
The developmental timing of mental disorders and the unique social-cultural embedding of youth highlight the need for novel service models that also address the fact that young people have the most limited access to mental health services across the lifespan [16]. The first, and now most extensive example is the Australian headspace model, a national youth mental health service stream designed to provide highly accessible, youth-friendly centers that promote and support early intervention for mental and substance use disorders in young people [112, 113]. In addition to these face-to-face services, headspace also runs a 24/7 nationwide online support service (eheadspace; www.eheadspace.org.au). This service model has now been implemented in several countries [93].
A recent study investigated outcome data in 58.000 clients examining self-reported psychological distress, quality of life, and clinician-reported social and occupational functioning. The results showed that approximately 70% of young people who attended headspace centers in Australia significantly improved on at least one outcome measure [114]. However, functional improvements were observed in only 1/3 of cases which may reflect the fact that interventions were too short and not very intensive [115]. Headspace is a prominent example but not the only available model; further evaluations of youth mental health services are currently being conducted globally [116–119].
Digital technologies
Early intervention approaches may also critically benefit from incorporating digital mental health technologies with unique opportunities and challenges [120]. However, Lettie et al. [121] highlighted that few studies using digital approaches and technologies targeting youth mental health have been replicated or assessed in real-world clinical settings. Many studies remain challenging to interpret given high rates of bias and few using causal methods to assess impact [122]. Furthermore, currently available commercial apps for youth mental health lack scientific evidence [123].
In contrast, there is emerging evidence for hybrid-interventions for youth mental health, involving both technology and some degree of human support. For example, the Moderated Online Social Therapy (MOST) offers intervention for youth diagnosed with early-stage psychosis, depression, help-seeking young people, and carers in a coherent platform [124–126]. New clinical models offering telehealth visits combined with a smartphone app, mindLAMP, involving both digital phenotyping for personalizing care and digital interventions for practicing skills, also show promise for rapidly reducing anxiety and depression-related symptoms [127]. Furthermore, a new generation of apps, such as EMIcompass, capture digital signals related to daily life (eg sleep patterns, mood) and use that data to respond with personalized and just-in-time support, thereby offering scalable and customized support for youth [128].
Digital technologies can also be used to detect emerging mental disorders outside established clinical pathways, an important prerequisite for population-based preventive approaches. In a recent study, a web-based screening platform allowed the identification of youth with CHR-P status as well as individuals with fully manifested psychosis with good sensitivity and specificity [129]. Digital phenotyping methods can also aid in relapse prediction by detecting changes in symptoms and behavioral patterns unique to each youth that may be associated with clinical deterioration [130]. Moreover, analysis of text messages on social media, in combination with machine and deep-learning techniques, may provide novel ways of identifying emerging mental disorders [131].
Knowledge exchange between science and clinical care
The development of novel interventions for youth mental health requires consideration of the unique possibilities and requirements needed to enable the targeted search for preventive interventions and treatments. Specifically, we propose a bidirectional knowledge exchange that is reframed as a three-way interaction of democratizing research across researchers, clinicians, and youth with lived experience, including patients and carers [132], targeting the discovery of risk factors, mechanisms, and clinical responses to existing interventions in youth mental health [133]. Limiting youth and their families from the knowledge exchange network would be a critical oversight. Their participation in all aspects of youth mental health is thus vital to ensure that care provided is accessible, appropriate and effective. As such, co-design of interventions and services is an important aspect of youth mental health.
Towards a paradigm for youth mental health
The converging findings from epidemiology, and basic and clinical research provide a powerful and complementary imperative for a “youth mental health paradigm” to guide science, practice, and policies. These are motivated by the highly plastic properties of neural circuits and associated cognitive and behavioral processes during youth which coincide with the peak incidence of major mental disorders between 12-25 years of age.
A critical implication of the timing of mental ill-health during youth and from the developmental cascade model is a broader focus on early manifestations of mental disorders as a primary focus for targeted interventions to prevent the occurrence of long-standing and chronic mental health conditions in adulthood [2]. In our view, this will require a shift towards early intervention models for a broad range of syndromes to enable selective and indicative prevention, low-threshold services for youth mental health [93], and population-based preventive approaches [8]. Here, prognostic algorithms that utilize knowledge of risk factors in combination with sensitive periods as well as biomarkers may be important for guiding clinical decision-making (Table 1).
The available evidence that early intervention is effective in psychosis [97, 134] as well as in other syndromes [8] together with the large unmet need in young people that has recently dramatically accelerated [9] provides additional impetus for such an endeavor. Several avenues towards interventions and implementation for youth mental health may follow from the framework outlined here that can be tested in large-scale studies. Firstly, prevention may focus on specific syndromes, such as the CHR-P paradigm vs. broader transdiagnostic phenotypes in youth mental health [88]. Secondly, population-based approaches that target the earliest manifestation of ill-mental health based on normative, developmental data vs. secondary prevention in clinical settings. Finally, the utility of knowledge derived from sensitive periods to guide the development and implementation of interventions in youth mental health remains to be demonstrated but could potentially be more effective for changing developmental trajectories vs. the application of established psycho-social and pharmacological therapies that were developed for adult populations.
While ambitious in scope, the benefits of such a youth mental health paradigm could be substantial and address the urgent need to improve the treatment of the most vulnerable age group for mental ill-health.
Acknowledgements
This perspective is based on an Ernst Strüngmann Forum on Youth Mental Health, which occurred between July 29 and August 3, 2018, in Frankfurt am Main, Germany. A book on this topic was published: Uhlhaas PJ, Wood SJ, eds. Youth Mental Health: A Framework for Early Intervention and Detection. MIT Press: 2020. We wish to dedicate this paper to the memory of Prof. George Patton who passed during the preparation of this manuscript. George was a pioneer in youth mental health and an eminent psychiatrist and psychiatric epidemiologist whose career focussed on improving the health of young people in Australia and across the globe.
Author contributions
PJU, CGD, UMM, JS and JT drafted the manuscript. NBA, SA, TBA, AC, EYHC, CUC, KQD, HLF, SF, IBH, MSK, KK, FSL, CHL, BL, PDM, AML, MD, DÖ, GCP, TP, UR, AS, MS, GS; VHS, ES. SKV, TWW, LWY, ARY and SJW approved the work.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
The authors declare no competing interests.
Footnotes
We chose “youth” over other concepts, such as adolescence, as youth encompasses a broader definition of developmental phenomena and age-ranges.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Insel TR, Fenton WS. Psychiatric epidemiology: it’s not just about counting anymore. Arch Gen Psychiatry. 2005;62:590–2. doi: 10.1001/archpsyc.62.6.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborators, GBDMD. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–50. doi: 10.1016/S2215-0366(21)00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borsboom D, Cramer AOJ, Kalis A. Brain disorders? Not really: Why network structures block reductionism in psychopathology research. Behav Brain Sci. 2018;42:e2. doi: 10.1017/S0140525X17002266. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho AF, Solmi M, Sanches M, Machado MO, Stubbs B, Ajnakina O, et al. Evidence-based umbrella review of 162 peripheral biomarkers for major mental disorders. Transl Psychiatry. 2020;10:152. doi: 10.1038/s41398-020-0835-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leichsenring F, Steinert C, Rabung S, Ioannidis J. The efficacy of psychotherapies and pharmacotherapies for mental disorders in adults: an umbrella review and meta-analytic evaluation of recent meta-analyses. World Psychiatry. 2022;21:133–45. doi: 10.1002/wps.20941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howes OD, Thase ME, Pillinger T. Treatment resistance in psychiatry: state of the art and new directions. Mol Psychiatry. 2022;27:58–72. doi: 10.1038/s41380-021-01200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhlhaas PJ, Wood, SJ, Youth Mental Health: A Paradigm for Prevention and Early Intervention. Strüngmann Forum Reports. Vol. 28. 2020: MIT Press.
- 8.Fusar-Poli P, Correll CU, Arango C, Berk M, Patel V, Ioannidis J. Preventive psychiatry: a blueprint for improving the mental health of young people. World Psychiatry. 2021;20:200–21. doi: 10.1002/wps.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2022;27:281–95. doi: 10.1038/s41380-021-01161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 11.McGorry PD, Killackey E, Yung A. Early intervention in psychosis: concepts, evidence and future directions. World Psychiatry. 2008;7:148–56. doi: 10.1002/j.2051-5545.2008.tb00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin A, Wood SJ, Nelson B, Beavan A, McGorry P, Yung AR. Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis. Am J Psychiatry. 2015;172:249–58. doi: 10.1176/appi.ajp.2014.13030418. [DOI] [PubMed] [Google Scholar]
- 13.Chanen AM, Betts JK, Jackson H, Cotton SM, Gleeson J, Davey CG, et al. A comparison of adolescent versus young adult outpatients with first-presentation borderline personality disorder: findings from the MOBY randomized controlled trial. Can J Psychiatry. 2022;67:26–38. doi: 10.1177/0706743721992677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalindjian N, Hirot F, Stona AC, Huas C, Godart N. Early detection of eating disorders: a scoping review. Eat Weight Disord. 2022;27:21–68. doi: 10.1007/s40519-021-01164-x. [DOI] [PubMed] [Google Scholar]
- 15.Vieta E, Salagre E, Grande I, Carvalho AF, Fernandes BS, Berk M, et al. Early Intervention in Bipolar Disorder. Am J Psychiatry. 2018;175:411–26. doi: 10.1176/appi.ajp.2017.17090972. [DOI] [PubMed] [Google Scholar]
- 16.Radez J, Reardon T, Creswell C, Lawrence PJ, Evdoka-Burton G, Waite P. Why do children and adolescents (not) seek and access professional help for their mental health problems? A systematic review of quantitative and qualitative studies. Eur Child Adolesc Psychiatry. 2021;30:183–211. doi: 10.1007/s00787-019-01469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson, EJ, et al. Changes in the adult consequences of adolescent mental ill-health: findings from the 1958 and 1970 British birth cohorts. Psychol Med, 2021: 1-10. [DOI] [PubMed]
- 18.Hafstad GS, Augusti EM. A lost generation? COVID-19 and adolescent mental health. Lancet Psychiatry. 2021;8:640–1. doi: 10.1016/S2215-0366(21)00179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caspi A, Houts RM, Ambler A, Danese A, Elliott ML, Hariri A, et al. Longitudinal assessment of mental health disorders and comorbidities across 4 decades among participants in the dunedin birth cohort study. JAMA Netw Open. 2020;3:e203221. doi: 10.1001/jamanetworkopen.2020.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–25. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 21.Reh RK, Dias BG, Nelson CA, Kaufer D, Werker JF, Kolb B, et al. Critical period regulation across multiple timescales. Proc Natl Acad Sci USA. 2020;117:23242–51. doi: 10.1073/pnas.1820836117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hooks BM, Chen C. Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron. 2007;56:312–26. doi: 10.1016/j.neuron.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Fuhrmann D, Knoll LJ, Blakemore SJ. Adolescence as a sensitive period of brain development. Trends Cogn Sci. 2015;19:558–66. doi: 10.1016/j.tics.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Essau CA, Lewinsohn PM, Olaya B, Seeley JR. Anxiety disorders in adolescents and psychosocial outcomes at age 30. J Affect Disord. 2014;163:125–32. doi: 10.1016/j.jad.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerhard DM, Meyer HC, Lee FS. An adolescent sensitive period for threat responding: impacts of stress and sex. Biol Psychiatry. 2021;89:651–8. doi: 10.1016/j.biopsych.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, et al. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci USA. 2012;109:16318–23. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J, et al. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun. 2016;7:11475. doi: 10.1038/ncomms11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nederhof E, van Oort FV, Bouma EM, Laceulle OM, Oldehinkel AJ, Ormel J. Predicting mental disorders from hypothalamic-pituitary-adrenal axis functioning: a 3-year follow-up in the TRAILS study. Psychol Med. 2015;45:2403–12. doi: 10.1017/S0033291715000392. [DOI] [PubMed] [Google Scholar]
- 29.Romeo RD. The metamorphosis of adolescent hormonal stress reactivity: a focus on animal models. Front Neuroendocrinol. 2018;49:43–51. doi: 10.1016/j.yfrne.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes FV, Zhu X, Grace AA. The pathophysiological impact of stress on the dopamine system is dependent on the state of the critical period of vulnerability. Mol Psychiatry. 2020;25:3278–91. doi: 10.1038/s41380-019-0514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunnar MR, DePasquale CE, Reid BM, Donzella B, Miller BS. Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proc Natl Acad Sci USA. 2019;116:23984–8. doi: 10.1073/pnas.1909699116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DePasquale CE, Herzberg MP, Gunnar MR. The pubertal stress recalibration hypothesis: potential neural and behavioral consequences. Child Dev Perspect. 2021;15:249–56. doi: 10.1111/cdep.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sisk LM, Gee DG. Stress and adolescence: vulnerability and opportunity during a sensitive window of development. Curr Opin Psychol. 2022;44:286–92. doi: 10.1016/j.copsyc.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen B, Luna B. Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci Biobehav Rev. 2018;94:179–95. doi: 10.1016/j.neubiorev.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chini M, Hanganu-Opatz IL. Prefrontal cortex development in health and disease: lessons from rodents and humans. Trends Neurosci. 2021;44:227–40. doi: 10.1016/j.tins.2020.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Caballero A, Flores-Barrera E, Cass DK, Tseng KY. Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence. Brain Struct Funct. 2014;219:395–406. doi: 10.1007/s00429-013-0508-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen B, Cui Z, Adebimpe A, Pines A, Alexander-Bloch A, Bertolero M, et al. A developmental reduction of the excitation:inhibition ratio in association cortex during adolescence. Sci Adv. 2022;8:eabj8750. doi: 10.1126/sciadv.abj8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park BY, Paquola C, Bethlehem R, Benkarim O, Neuroscience in Psychiatry Network (NSPN) C, Mišić B, et al. Adolescent development of multiscale structural wiring and functional interactions in the human connectome. Proc Natl Acad Sci USA. 2022;119:e2116673119. doi: 10.1073/pnas.2116673119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jalbrzikowski M, Larsen B, Hallquist MN, Foran W, Calabro F, Luna B. Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol Psychiatry. 2017;82:511–21. doi: 10.1016/j.biopsych.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calabro FJ, Murty VP, Jalbrzikowski M, Tervo-Clemmens B, Luna B. Development of hippocampal-prefrontal cortex interactions through adolescence. Cereb Cortex. 2020;30:1548–58. doi: 10.1093/cercor/bhz186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nabel EM, Garkun Y, Koike H, Sadahiro M, Liang A, Norman KJ, et al. Adolescent frontal top-down neurons receive heightened local drive to establish adult attentional behavior in mice. Nat Commun. 2020;11:3983. doi: 10.1038/s41467-020-17787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benoit LJ, Holt ES, Posani L, Fusi S, Harris AZ, Canetta S, et al. Adolescent thalamic inhibition leads to long-lasting impairments in prefrontal cortex function. Nat Neurosci. 2022;25:714–25. doi: 10.1038/s41593-022-01072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casey BJ, Heller AS, Gee DG, Cohen AO. Development of the emotional brain. Neurosci Lett. 2019;693:29–34. doi: 10.1016/j.neulet.2017.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silvers JA. Adolescence as a pivotal period for emotion regulation development. Curr Opin Psychol. 2022;44:258–63. doi: 10.1016/j.copsyc.2021.09.023. [DOI] [PubMed] [Google Scholar]
- 45.Sheppes G, Suri G, Gross JJ. Emotion regulation and psychopathology. Annu Rev Clin Psychol. 2015;11:379–405. doi: 10.1146/annurev-clinpsy-032814-112739. [DOI] [PubMed] [Google Scholar]
- 46.Chapman AL. Borderline personality disorder and emotion dysregulation. Dev Psychopathol. 2019;31:1143–56. doi: 10.1017/S0954579419000658. [DOI] [PubMed] [Google Scholar]
- 47.Duell N, Steinberg L, Icenogle G, Chein J, Chaudhary N, Di Giunta L, et al. Age patterns in risk taking across the world. J Youth Adolesc. 2018;47:1052–72. doi: 10.1007/s10964-017-0752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jordan CJ, Andersen SL. Sensitive periods of substance abuse: early risk for the transition to dependence. Dev Cogn Neurosci. 2017;25:29–44. doi: 10.1016/j.dcn.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luciana M, Collins PF. Is adolescence a sensitive period for the development of incentive-reward motivation? Curr Top Behav Neurosci. 2022;53:79–99. doi: 10.1007/7854_2021_275. [DOI] [PubMed] [Google Scholar]
- 50.Verrico CD, Gu H, Peterson ML, Sampson AR, Lewis DA. Repeated Delta9-tetrahydrocannabinol exposure in adolescent monkeys: persistent effects selective for spatial working memory. Am J Psychiatry. 2014;171:416–25. doi: 10.1176/appi.ajp.2013.13030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrews JL, Ahmed SP, Blakemore SJ. Navigating the social environment in adolescence: the role of social brain development. Biol Psychiatry. 2021;89:109–18. doi: 10.1016/j.biopsych.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 52.Crone EA, Fuligni AJ. Self and others in adolescence. Annu Rev Psychol. 2020;71:447–69. doi: 10.1146/annurev-psych-010419-050937. [DOI] [PubMed] [Google Scholar]
- 53.Blakemore SJ, Mills KL. Is adolescence a sensitive period for sociocultural processing? Annu Rev Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- 54.Smith AR, Steinberg L, Strang N, Chein J. Age differences in the impact of peers on adolescents’ and adults’ neural response to reward. Dev Cogn Neurosci. 2015;11:75–82. doi: 10.1016/j.dcn.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gruenenfelder-Steiger AE, Harris MA, Fend HA. Subjective and objective peer approval evaluations and self-esteem development: a test of reciprocal, prospective, and long-term effects. Dev Psychol. 2016;52:1563–77. doi: 10.1037/dev0000147. [DOI] [PubMed] [Google Scholar]
- 56.Bicks LK, Yamamuro K, Flanigan ME, Kim JM, Kato D, Lucas EK, et al. Prefrontal parvalbumin interneurons require juvenile social experience to establish adult social behavior. Nat Commun. 2020;11:1003. doi: 10.1038/s41467-020-14740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foulkes L, Blakemore SJ. Individual differences in adolescent mental health during COVID-19: The importance of peer relationship quality. Neuron. 2021;109:3203–5. doi: 10.1016/j.neuron.2021.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paus T. Population neuroscience. Handb Clin Neurol. 2016;138:17–37. doi: 10.1016/B978-0-12-802973-2.00002-1. [DOI] [PubMed] [Google Scholar]
- 59.Paus T. Population neuroscience: why and how. Hum Brain Mapp. 2010;31:891–903. doi: 10.1002/hbm.21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paus, T, Population Neuroscience. 2013, Berlin/Heidelberg: Springer.
- 61.Masten AS, Cicchetti D. Developmental cascades. Dev Psychopathol. 2010;22:491–5. doi: 10.1017/S0954579410000222. [DOI] [PubMed] [Google Scholar]
- 62.Shah JL, Jones N, van Os J, McGorry PD, Gülöksüz S. Early intervention service systems for youth mental health: integrating pluripotentiality, clinical staging, and transdiagnostic lessons from early psychosis. Lancet Psychiatry. 2022;9:413–22. doi: 10.1016/S2215-0366(21)00467-3. [DOI] [PubMed] [Google Scholar]
- 63.Lahey BB, Lee SS, Sibley MH, Applegate B, Molina B, Pelham WE. Predictors of adolescent outcomes among 4-6-year-old children with attention-deficit/hyperactivity disorder. J Abnorm Psychol. 2016;125:168–81. doi: 10.1037/abn0000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arslan IB, Lucassen N, van Lier P, de Haan AD, Prinzie P. Early childhood internalizing problems, externalizing problems and their co-occurrence and (mal)adaptive functioning in emerging adulthood: a 16-year follow-up study. Soc Psychiatry Psychiatr Epidemiol. 2021;56:193–206. doi: 10.1007/s00127-020-01959-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orben A, Przybylski AK, Blakemore SJ, Kievit RA. Windows of developmental sensitivity to social media. Nat Commun. 2022;13:1649. doi: 10.1038/s41467-022-29296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gee DG. When do sensitive periods emerge later in development? Trends Cogn Sci. 2022;26:97–98. doi: 10.1016/j.tics.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Guloksuz S, van Os J, Rutten BPF. The exposome paradigm and the complexities of environmental research in psychiatry. JAMA Psychiatry. 2018;75:985–6. doi: 10.1001/jamapsychiatry.2018.1211. [DOI] [PubMed] [Google Scholar]
- 68.Choi KW, Wilson M, Ge T, Kandola A, Patel CJ, Lee SH, et al. Integrative analysis of genomic and exposomic influences on youth mental health. J Child Psychol Psychiatry. 2022;63:1196–205. doi: 10.1111/jcpp.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 70.Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, et al. The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J Abnorm Psychol. 2017;126:454–77. doi: 10.1037/abn0000258. [DOI] [PubMed] [Google Scholar]
- 71.Durbin CE, Wilson S, MacDonald AW. Integrating development into the Research Domain Criteria (RDoC) framework: Introduction to the special section. J Psychopathol Clin Sci. 2022;131:535–41. doi: 10.1037/abn0000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biol Psychiatry. 2014;76:350–3. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 73.Fusar-Poli P, Salazar de Pablo G, Correll CU, Meyer-Lindenberg A, Millan MJ, Borgwardt S, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77:755–65. doi: 10.1001/jamapsychiatry.2019.4779. [DOI] [PubMed] [Google Scholar]
- 74.Van Meter AR, Burke C, Youngstrom EA, Faedda GL, Correll CU. The bipolar prodrome: meta-analysis of symptom prevalence prior to initial or recurrent mood episodes. J Am Acad Child Adolesc Psychiatry. 2016;55:543–55. doi: 10.1016/j.jaac.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 75.Stice E, Desjardins CD, Rohde P, Shaw H. Sequencing of symptom emergence in anorexia nervosa, bulimia nervosa, binge eating disorder, and purging disorder and relations of prodromal symptoms to future onset of these disorders. J Abnorm Psychol. 2021;130:377–87. doi: 10.1037/abn0000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benasi G, Fava GA, Guidi J. Prodromal symptoms in depression: a systematic review. Psychother Psychosom. 2021;90:365–72. doi: 10.1159/000517953. [DOI] [PubMed] [Google Scholar]
- 77.Fineberg NA, Dell’Osso B, Albert U, Maina G, Geller D, Carmi L, et al. Early intervention for obsessive compulsive disorder: An expert consensus statement. Eur Neuropsychopharmacol. 2019;29:549–65. doi: 10.1016/j.euroneuro.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 78.Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust NZJ Psychiatry. 2005;39:964–71. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 79.Fusar-Poli P, Cappucciati M, Rutigliano G, Schultze-Lutter F, Bonoldi I, Borgwardt S, et al. At risk or not at risk? A meta-analysis of the prognostic accuracy of psychometric interviews for psychosis prediction. World Psychiatry. 2015;14:322–32. doi: 10.1002/wps.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salazar de Pablo G, Radua J, Pereira J, Bonoldi I, Arienti V, Besana F, et al. Probability of Transition to Psychosis in Individuals at Clinical High Risk: An Updated Meta-analysis. JAMA Psychiatry. 2021;78:970–8. doi: 10.1001/jamapsychiatry.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, et al. An Individualized Risk Calculator for Research in Prodromal Psychosis. Am J Psychiatry. 2016;173:980–8. doi: 10.1176/appi.ajp.2016.15070890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Catalan A, Salazar de Pablo G, Aymerich C, Damiani S, Sordi V, Radua J, et al. Neurocognitive Functioning in Individuals at Clinical High Risk for Psychosis: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2021;78:859–67. doi: 10.1001/jamapsychiatry.2021.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Group, E.C.H.R.F.P.W. Jalbrzikowski M, Hayes RA, Wood SJ, Nordholm D, Zhou JH, et al. Association of structural magnetic resonance imaging measures with psychosis onset in individuals at clinical high risk for developing psychosis: an ENIGMA working group mega-analysis. JAMA Psychiatry. 2021;78:753–66. doi: 10.1001/jamapsychiatry.2021.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Correll CU, Olvet DM, Auther AM, Hauser M, Kishimoto T, Carrión RE, et al. The Bipolar Prodrome Symptom Interview and Scale-Prospective (BPSS-P): description and validation in a psychiatric sample and healthy controls. Bipolar Disord. 2014;16:505–22. doi: 10.1111/bdi.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bechdolf A, Ratheesh A, Cotton SM, Nelson B, Chanen AM, Betts J, et al. The predictive validity of bipolar at-risk (prodromal) criteria in help-seeking adolescents and young adults: a prospective study. Bipolar Disord. 2014;16:493–504. doi: 10.1111/bdi.12205. [DOI] [PubMed] [Google Scholar]
- 86.Waszczuk MA, Zavos HM, Gregory AM, Eley TC. The phenotypic and genetic structure of depression and anxiety disorder symptoms in childhood, adolescence, and young adulthood. JAMA Psychiatry. 2014;71:905–16. doi: 10.1001/jamapsychiatry.2014.655. [DOI] [PubMed] [Google Scholar]
- 87.Hickie IB, Scott EM, Hermens DF, Naismith SL, Guastella AJ, Kaur M, et al. Applying clinical staging to young people who present for mental health care. Early Inter Psychiatry. 2013;7:31–43. doi: 10.1111/j.1751-7893.2012.00366.x. [DOI] [PubMed] [Google Scholar]
- 88.Shah JL, Scott J, McGorry PD, Cross S, Keshavan MS, Nelson B, et al. Transdiagnostic clinical staging in youth mental health: a first international consensus statement. World Psychiatry. 2020;19:233–42. doi: 10.1002/wps.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McGorry PD, Hickie IB, Yung AR, Pantelis C, Jackson HJ. Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions. Aust N. Z J Psychiatry. 2006;40:616–22. doi: 10.1080/j.1440-1614.2006.01860.x. [DOI] [PubMed] [Google Scholar]
- 90.Bethlehem RAI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, et al. Brain charts for the human lifespan. Nature. 2022;604:525–33. doi: 10.1038/s41586-022-04554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rutherford S, Fraza C, Dinga R, Kia SM, Wolfers T, Zabihi M, et al. Charting brain growth and aging at high spatial precision. Elife. 2022;11:e72904. doi: 10.7554/eLife.72904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marquand AF, Kia SM, Zabihi M, Wolfers T, Buitelaar JK, Beckmann CF. Conceptualizing mental disorders as deviations from normative functioning. Mol Psychiatry. 2019;24:1415–24. doi: 10.1038/s41380-019-0441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McGorry PD, Mei C, Chanen A, Hodges C, Alvarez-Jimenez M, Killackey E. Designing and scaling up integrated youth mental health care. World Psychiatry. 2022;21:61–76. doi: 10.1002/wps.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Howes OD, Whitehurst T, Shatalina E, Townsend L, Onwordi EC, Mak T, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20:75–95. doi: 10.1002/wps.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Austin A, Flynn M, Richards K, Hodsoll J, Duarte TA, Robinson P, et al. Duration of untreated eating disorder and relationship to outcomes: A systematic review of the literature. Eur Eat Disord Rev. 2021;29:329–45. doi: 10.1002/erv.2745. [DOI] [PubMed] [Google Scholar]
- 96.Drancourt N, Etain B, Lajnef M, Henry C, Raust A, Cochet B, et al. Duration of untreated bipolar disorder: missed opportunities on the long road to optimal treatment. Acta Psychiatr Scand. 2013;127:136–44. doi: 10.1111/j.1600-0447.2012.01917.x. [DOI] [PubMed] [Google Scholar]
- 97.Correll CU, Galling B, Pawar A, Krivko A, Bonetto C, Ruggeri M, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75:555–65. doi: 10.1001/jamapsychiatry.2018.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mei C, van der Gaag M, Nelson B, Smit F, Yuen HP, Berger M, et al. Preventive interventions for individuals at ultra high risk for psychosis: An updated and extended meta-analysis. Clin Psychol Rev. 2021;86:102005. doi: 10.1016/j.cpr.2021.102005. [DOI] [PubMed] [Google Scholar]
- 99.Hansen HG, Starzer M, Nilsson SF, Hjorthøj C, Albert N, Nordentoft M. Clinical recovery and long-term association of specialized early intervention services vs treatment as usual among individuals with first-episode schizophrenia spectrum disorder: 20-year follow-up of the OPUS trial. JAMA Psychiatry. 2023;80:371–9. doi: 10.1001/jamapsychiatry.2022.5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chanen AM, Thompson KN. Early intervention for personality disorder. Curr Opin Psychol. 2018;21:132–5. doi: 10.1016/j.copsyc.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 101.Stockings E, Hall WD, Lynskey M, Morley KI, Reavley N, Strang J, et al. Prevention, early intervention, harm reduction, and treatment of substance use in young people. Lancet Psychiatry. 2016;3:280–96. doi: 10.1016/S2215-0366(16)00002-X. [DOI] [PubMed] [Google Scholar]
- 102.Austin A, Flynn M, Shearer J, Long M, Allen K, Mountford VA, et al. The first episode rapid early intervention for eating disorders - upscaled study: clinical outcomes. Early Interv Psychiatry. 2022;16:97–105. doi: 10.1111/eip.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Casey BJ, Glatt CE, Lee FS. Treating the developing versus developed brain: translating preclinical mouse and human studies. Neuron. 2015;86:1358–68. doi: 10.1016/j.neuron.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee H, Dvorak D, Kao HY, Duffy ÁM, Scharfman HE, Fenton AA. Early cognitive experience prevents adult deficits in a neurodevelopmental schizophrenia model. Neuron. 2012;75:714–24. doi: 10.1016/j.neuron.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cabungcal JH, Counotte DS, Lewis E, Tejeda HA, Piantadosi P, Pollock C, et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. 2014;83:1073–84. doi: 10.1016/j.neuron.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mukherjee A, Carvalho F, Eliez S, Caroni P. Long-lasting rescue of network and cognitive dysfunction in a genetic schizophrenia model. Cell. 2019;178:1387–1402.e14. doi: 10.1016/j.cell.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 107.Ginsburg GS, Becker-Haimes EM, Keeton C, Kendall PC, Iyengar S, Sakolsky D, et al. Results from the child/adolescent anxiety multimodal extended long-term study (CAMELS): primary anxiety outcomes. J Am Acad Child Adolesc Psychiatry. 2018;57:471–80. doi: 10.1016/j.jaac.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 108.Odriozola P, Gee DG. Learning about safety: conditioned inhibition as a novel approach to fear reduction targeting the developing brain. Am J Psychiatry. 2021;178:136–55. doi: 10.1176/appi.ajp.2020.20020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Susai SR, Sabherwal S, Mongan D, Föcking M, Cotter DR. Omega-3 fatty acid in ultra-high-risk psychosis: a systematic review based on functional outcome. Early Inter Psychiatry. 2022;16:3–16. doi: 10.1111/eip.13133. [DOI] [PubMed] [Google Scholar]
- 110.Bradlow RCJ, Berk M, Kalivas PW, Back SE, Kanaan RA. The potential of N-Acetyl-L-cysteine (NAC) in the treatment of psychiatric disorders. CNS Drugs. 2022;36:451–82. doi: 10.1007/s40263-022-00907-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chesney E, Oliver D, McGuire P. Cannabidiol (CBD) as a novel treatment in the early phases of psychosis. Psychopharmacol (Berl) 2022;239:1179–90. doi: 10.1007/s00213-021-05905-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McGorry PD, Tanti C, Stokes R, Hickie IB, Carnell K, Littlefield LK, et al. Headspace: Australia’s National Youth Mental Health Foundation–where young minds come first. Med J Aust. 2007;187:S68–70. doi: 10.5694/j.1326-5377.2007.tb01342.x. [DOI] [PubMed] [Google Scholar]
- 113.McGorry PD, Goldstone SD, Parker AG, Rickwood DJ, Hickie IB. Cultures for mental health care of young people: an Australian blueprint for reform. Lancet Psychiatry. 2014;1:559–68. doi: 10.1016/S2215-0366(14)00082-0. [DOI] [PubMed] [Google Scholar]
- 114.Rickwood, D, McEachran, J, Saw, A, Telford, N, Trethowan, J, McGorry, P, Sixteen years of innovation in youth mental healthcare in Australia: Outcomes for young people attending headspace centre services. medRxiv 2022. 2022.08.24.22279102. [DOI] [PMC free article] [PubMed]
- 115.Iorfino F, Scott EM, Hickie IB. Social and occupational outcomes for young people who attend early intervention mental health services: a longitudinal study. Med J Aust. 2022;217:218. doi: 10.5694/mja2.51653. [DOI] [PubMed] [Google Scholar]
- 116.Iyer SN, Shah J, Boksa P, Lal S, Joober R, Andersson N, et al. A minimum evaluation protocol and stepped-wedge cluster randomized trial of ACCESS Open Minds, a large Canadian youth mental health services transformation project. BMC Psychiatry. 2019;19:273. doi: 10.1186/s12888-019-2232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abba-Aji A, Hay K, Kelland J, Mummery C, Urichuk L, Gerdes C, et al. Transforming youth mental health services in a large urban centre: ACCESS Open Minds Edmonton. Early Inter Psychiatry. 2019;13:14–19. doi: 10.1111/eip.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boonstra, A, et al., @ease peer-to-peer youth walk-in centres in The Netherlands: A protocol for evaluating longitudinal outcomes, follow-up results and cost-of-illness. Early Interv Psychiatry, 2023. [DOI] [PubMed]
- 119.Shah JL, Moinfar Z, Anderson KK, Gould H, Hutt-Macleod D, Jacobs P, et al. Return on investment from service transformation for young people experiencing mental health problems: Approach to economic evaluations in ACCESS Open Minds (Esprits ouverts), a multi-site pan-Canadian youth mental health project. Front Psychiatry. 2023;14:1030407. doi: 10.3389/fpsyt.2023.1030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Uhlhaas P, Torous J. Digital tools for youth mental health. NPJ Digit Med. 2019;2:104. doi: 10.1038/s41746-019-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lattie EG, Stiles-Shields C, Graham AK. An overview of and recommendations for more accessible digital mental health services. Nat Rev Psychol. 2022;1:87–100. doi: 10.1038/s44159-021-00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Domhardt M, Engler S, Nowak H, Lutsch A, Baumel A, Baumeister H. Mechanisms of Change in Digital Health Interventions for Mental Disorders in Youth: Systematic Review. J Med Internet Res. 2021;23:e29742. doi: 10.2196/29742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Domhardt M, Messner EM, Eder AS, Engler S, Sander LB, Baumeister H, et al. Mobile-based interventions for common mental disorders in youth: a systematic evaluation of pediatric health apps. Child Adolesc Psychiatry Ment Health. 2021;15:49. doi: 10.1186/s13034-021-00401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gleeson J, Lederman R, Koval P, Wadley G, Bendall S, Cotton S, et al. Moderated Online Social Therapy: A Model for Reducing Stress in Carers of Young People Diagnosed with Mental Health Disorders. Front Psychol. 2017;8:485. doi: 10.3389/fpsyg.2017.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alvarez-Jimenez M, Koval P, Schmaal L, Bendall S, O’Sullivan S, Cagliarini D, et al. The Horyzons project: a randomized controlled trial of a novel online social therapy to maintain treatment effects from specialist first-episode psychosis services. World Psychiatry. 2021;20:233–43. doi: 10.1002/wps.20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Engel, L, Alvarez-Jimenez M, Cagliarini D, D’Alfonso S, Faller J, Valentine L, et al., The Cost-Effectiveness of a Novel Online Social Therapy to Maintain Treatment Effects From First-Episode Psychosis Services: Results From the Horyzons Randomized Controlled Trial. Schizophr Bull, 2023. [DOI] [PMC free article] [PubMed]
- 127.Chang S, Gray L, Torous J. Smartphone app engagement and clinical outcomes in a hybrid clinic. Psychiatry Res. 2023;319:115015. doi: 10.1016/j.psychres.2022.115015. [DOI] [PubMed] [Google Scholar]
- 128.Reininghaus U, Paetzold I, Rauschenberg C, Hirjak D, Banaschewski T, Meyer-Lindenberg A, et al. Effects of a novel, transdiagnostic ecological momentary intervention for prevention, and early intervention of severe mental disorder in youth (EMIcompass): findings from an exploratory randomized controlled trial. Schizophr Bull. 2023;49:592–604. doi: 10.1093/schbul/sbac212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fusar-Poli P, Sullivan SA, Shah JL, Uhlhaas PJ. Improving the detection of individuals at clinical risk for psychosis in the community, primary and secondary care: an integrated evidence-based approach. Front Psychiatry. 2019;10:774. doi: 10.3389/fpsyt.2019.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cohen A, Naslund JA, Chang S, Nagendra S, Bhan A, Rozatkar A, et al. Relapse prediction in schizophrenia with smartphone digital phenotyping during COVID-19: a prospective, three-site, two-country, longitudinal study. Schizophrenia (Heidelb) 2023;9:6. doi: 10.1038/s41537-023-00332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Torous J, Bucci S, Bell IH, Kessing LV, Faurholt-Jepsen M, Whelan P, et al. The growing field of digital psychiatry: current evidence and the future of apps, social media, chatbots, and virtual reality. World Psychiatry. 2021;20:318–35. doi: 10.1002/wps.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lloyd K, White J. Democratizing clinical research. Nature. 2011;474:277–8. doi: 10.1038/474277a. [DOI] [PubMed] [Google Scholar]
- 133.Baumann PS, Crespi S, Marion-Veyron R, Solida A, Thonney J, Favrod J, et al. Treatment and early intervention in psychosis program (TIPP-Lausanne): Implementation of an early intervention programme for psychosis in Switzerland. Early Inter Psychiatry. 2013;7:322–8. doi: 10.1111/eip.12037. [DOI] [PubMed] [Google Scholar]
- 134.Anderson KK, Norman R, MacDougall A, Edwards J, Palaniyappan L, Lau C, et al. Effectiveness of early psychosis intervention: comparison of service users and nonusers in population-based health administrative data. Am J Psychiatry. 2018;175:443–52. doi: 10.1176/appi.ajp.2017.17050480. [DOI] [PubMed] [Google Scholar]
- 135.Stockings EA, Degenhardt L, Dobbins T, Lee YY, Erskine HE, Whiteford HA, et al. Preventing depression and anxiety in young people: a review of the joint efficacy of universal, selective and indicated prevention. Psychol Med. 2016;46:11–26. doi: 10.1017/S0033291715001725. [DOI] [PubMed] [Google Scholar]
- 136.Breedvelt JJF, Kandola A, Kousoulis AA, Brouwer ME, Karyotaki E, Bockting C, et al. What are the effects of preventative interventions on major depressive disorder (MDD) in young adults? A systematic review and meta-analysis of randomized controlled trials. J Affect Disord. 2018;239:18–29. doi: 10.1016/j.jad.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 137.Lawrence PJ, Rooke SM, Creswell C. Review: Prevention of anxiety among at-risk children and adolescents - a systematic review and meta-analysis. Child Adolesc Ment Health. 2017;22:118–30. doi: 10.1111/camh.12226. [DOI] [PubMed] [Google Scholar]
- 138.Carney T, Myers B. Effectiveness of early interventions for substance-using adolescents: findings from a systematic review and meta-analysis. Subst Abus Treat Prev Policy. 2012;7:25. doi: 10.1186/1747-597X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Addington J, Stowkowy J, Weiser M. Screening tools for clinical high risk for psychosis. Early Inter Psychiatry. 2015;9:345–56. doi: 10.1111/eip.12193. [DOI] [PubMed] [Google Scholar]
- 140.Powers AR, Addington J, Perkins DO, Bearden CE, Cadenhead KS, Cannon TD, et al. Duration of the psychosis prodrome. Schizophr Res. 2020;216:443–9. doi: 10.1016/j.schres.2019.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Merritt K, McGuire PK, Egerton A, -MRS in Schizophrenia I, Aleman A, Block W, et al. Association of age, antipsychotic medication, and symptom severity in schizophrenia with proton magnetic resonance spectroscopy brain glutamate level: a mega-analysis of individual participant-level data. JAMA Psychiatry. 2021;78:667–81. doi: 10.1001/jamapsychiatry.2021.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Van Meter A, Guinart D, Bashir A, Sareen A, Cornblatt BA, Auther A, et al. Bipolar prodrome symptom scale - abbreviated screen for patients: description and validation. J Affect Disord. 2019;249:357–65. doi: 10.1016/j.jad.2019.02.040. [DOI] [PubMed] [Google Scholar]
- 143.Sekhar DL, Schaefer EW, Waxmonsky JG, Walker-Harding LR, Pattison KL, Molinari A, et al. Screening in high schools to identify, evaluate, and lower depression among adolescents: a randomized clinical trial. JAMA Netw Open. 2021;4:e2131836. doi: 10.1001/jamanetworkopen.2021.31836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Scott J, Graham A, Yung A, Morgan C, Bellivier F, Etain B. A systematic review and meta-analysis of delayed help-seeking, delayed diagnosis and duration of untreated illness in bipolar disorders. Acta Psychiatr Scand. 2022;146:389–405. doi: 10.1111/acps.13490. [DOI] [PubMed] [Google Scholar]
- 145.Ghio L, Gotelli S, Marcenaro M, Amore M, Natta W. Duration of untreated illness and outcomes in unipolar depression: a systematic review and meta-analysis. J Affect Disord. 2014;152-154:45–51. doi: 10.1016/j.jad.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 146.Chanen AM, Jovev M, Djaja D, McDougall E, Yuen HP, Rawlings D, et al. Screening for borderline personality disorder in outpatient youth. J Pers Disord. 2008;22:353–64. doi: 10.1521/pedi.2008.22.4.353. [DOI] [PubMed] [Google Scholar]
- 147.Dierker LC, Albano AM, Clarke GN, Heimberg RG, Kendall PC, Merikangas KR, et al. Screening for anxiety and depression in early adolescence. J Am Acad Child Adolesc Psychiatry. 2001;40:929–36. doi: 10.1097/00004583-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 148.Wang PS, Berglund P, Olfson M, Pincus HA, Wells KB, Kessler RC. Failure and delay in initial treatment contact after first onset of mental disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:603–13. doi: 10.1001/archpsyc.62.6.603. [DOI] [PubMed] [Google Scholar]
- 149.Pilowsky DJ, Wu LT. Screening instruments for substance use and brief interventions targeting adolescents in primary care: a literature review. Addict Behav. 2013;38:2146–53. doi: 10.1016/j.addbeh.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Piqueras JA, Rodríguez-Jiménez T, Ortiz AG, Moreno E, Lázaro L, Godoy A. Validation of the Short Obsessive-Compulsive Disorder Screener (SOCS) in children and adolescents. BJPsych Open. 2015;1:21–26. doi: 10.1192/bjpo.bp.115.000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319:1467–8. doi: 10.1136/bmj.319.7223.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bryant E, Miskovic-Wheatley J, Touyz SW, Crosby RD, Koreshe E, Maguire S. Identification of high risk and early stage eating disorders: first validation of a digital screening tool. J Eat Disord. 2021;9:109. doi: 10.1186/s40337-021-00464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Andrés-Pepiñá S, Plana MT, Flamarique I, Romero S, Borràs R, Julià L, et al. Long-term outcome and psychiatric comorbidity of adolescent-onset anorexia nervosa. Clin Child Psychol Psychiatry. 2020;25:33–44. doi: 10.1177/1359104519827629. [DOI] [PubMed] [Google Scholar]
- 154.Oliver, D, et al. Prognostic accuracy and clinical utility of psychometric instruments for individuals at clinical high-risk of psychosis: a systematic review and meta-analysis. Mol Psychiatry, 2022. [DOI] [PMC free article] [PubMed]
- 155.Erickson MA, Ruffle A, Gold JM. A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biol Psychiatry. 2016;79:980–7. doi: 10.1016/j.biopsych.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Koutsouleris N, Dwyer DB, Degenhardt F, Maj C, Urquijo-Castro MF, Sanfelici R, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78:195–209. doi: 10.1001/jamapsychiatry.2020.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fusar-Poli P, De Micheli A, Rocchetti M, Cappucciati M, Ramella-Cravaro V, Rutigliano G, et al. Semistructured Interview for Bipolar At Risk States (SIBARS) Psychiatry Res. 2018;264:302–9. doi: 10.1016/j.psychres.2018.03.074. [DOI] [PubMed] [Google Scholar]
- 158.Birmaher B, Merranko JA, Goldstein TR, Gill MK, Goldstein BI, Hower H, et al. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adolesc Psychiatry. 2018;57:755–763.e4. doi: 10.1016/j.jaac.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Saraf G, Moazen-Zadeh E, Pinto JV, Ziafat K, Torres IJ, Kesavan M, et al. Early intervention for people at high risk of developing bipolar disorder: a systematic review of clinical trials. Lancet Psychiatry. 2021;8:64–75. doi: 10.1016/S2215-0366(20)30188-7. [DOI] [PubMed] [Google Scholar]
- 160.Werner-Seidler A, Perry Y, Calear AL, Newby JM, Christensen H. School-based depression and anxiety prevention programs for young people: A systematic review and meta-analysis. Clin Psychol Rev. 2017;51:30–47. doi: 10.1016/j.cpr.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 161.Hugh-Jones S, Beckett S, Tumelty E, Mallikarjun P. Indicated prevention interventions for anxiety in children and adolescents: a review and meta-analysis of school-based programs. Eur Child Adolesc Psychiatry. 2021;30:849–60. doi: 10.1007/s00787-020-01564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Moreno-Peral P, Conejo-Cerón S, Rubio-Valera M, Fernández A, Navas-Campaña D, Rodríguez-Morejón A, et al. Effectiveness of psychological and/or educational interventions in the prevention of anxiety: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2017;74:1021–9. doi: 10.1001/jamapsychiatry.2017.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Norberg MM, Kezelman S, Lim-Howe N. Primary prevention of cannabis use: a systematic review of randomized controlled trials. PLoS One. 2013;8:e53187. doi: 10.1371/journal.pone.0053187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jensen CD, Cushing CC, Aylward BS, Craig JT, Sorell DM, Steele RG. Effectiveness of motivational interviewing interventions for adolescent substance use behavior change: a meta-analytic review. J Consult Clin Psychol. 2011;79:433–40. doi: 10.1037/a0023992. [DOI] [PubMed] [Google Scholar]
- 165.Shah JL. Bringing clinical staging to youth mental health: from concept to operationalization (and back again) JAMA Psychiatry. 2019;76:1121–3. doi: 10.1001/jamapsychiatry.2019.2003. [DOI] [PubMed] [Google Scholar]