Abstract
Here, we investigate the transcriptome profiles of two S. Enteritidis and one S. Schwarzengrund isolates that present different persister levels when exposed to ciprofloxacin or ceftazidime. It was possible to note a distinct transcript profile among isolates, time of exposure, and treatment. We could not find a commonly expressed transcript profile that plays a role in persister formation after S. enterica exposure to beta-lactam or fluoroquinolone, as only three DEGs presented the same behavior under the conditions and isolates tested. It appears that the formation of persisters in S. enterica after exposure to ciprofloxacin is linked to the overexpression of genes involved in the SOS response (recA), cell division inhibitor (sulA), iron-sulfur metabolism (hscA and iscS), and type I TA system (tisB). On the other hand, most genes differentially expressed in S. enterica after exposure to ceftazidime appeared to be downregulated and were part of the flagellar assembly apparatus, citrate cycle (TCA cycle), glycolysis/gluconeogenesis, carbon metabolism, bacterial secretion system, quorum sensing, pyruvate metabolism pathway, and biosynthesis of secondary metabolites. The different transcriptome profiles found in S. enterica persisters induced by ciprofloxacin and ceftazidime suggest that these cells modulate their response differently according to each stress.
Subject terms: Biofilms, Cellular microbiology, Antimicrobials
Introduction
Salmonella enterica is one of the most common foodborne pathogens that causes self-limiting gastroenteritis and, in some cases, severe systemic infections. Based on 2014 data, the Centers for Disease Control and Prevention (CDC) estimates that nontyphoidal Salmonella causes approximately 1.35 million infections, resulting in 26,500 hospitalizations and 420 deaths annually in the United States1. Moreover, multi-resistant foodborne bacteria are increasing globally, partly as a result of antibiotic overuse, limiting therapeutic options and resulting in treatment failures2. In addition, a hard-to-treat bacterial infection that does not harbor antibiotic-resistance genes and often relapses even after several rounds of antibiotic treatment has been reported3. The recurrence of these infections may be related to the presence of persister cells4–6.
Persisters constitute a small subpopulation of cells that arises from a genetically identical bacterial culture and adopt a non-genetic and non-heritable bet-hedging strategy phenotype, surviving high doses of antibiotics by entering a transient and reversible slow or non-growth state6,7. This phenotype can be evidenced by a biphasic killing curve, where non-persisters are eliminated after the addition of lethal doses of a bactericidal antibiotic, while a persister subpopulation survives and resumes growth as the stressor is removed without undergoing any resistance-determining genetic change, unlike drug-resistant cells8,9. Persisters can be stochastically formed or triggered by multiple stress-induced factors, such as exposure to antimicrobial agents, nutritional deprivation (stringent response—SR), and changes in oxygen availability, pH, or carbon sources10–13.
It is widely acknowledged that the non-growth state found at least in part of these cells implies the modulation of transcriptional activity, including the shutting down of important pathways, especially those related to cell growth and energy production (ATP)8,14–18. Typically, low energy levels drive the reduction of translation, transcription, and DNA replication, leading to the inactivation of important antimicrobial targets that may contribute to persister formation16,17. Although persister cells have been described as metabolically inactive cells, different studies have demonstrated that they may, in fact, remain metabolically active13,19,20, and a dormancy state is not sufficient to explain the persistence phenomenon. The activation of efflux pumps21, quorum sensing cell signaling22–25, DNA repair (SOS response)26–28 and, toxin-antitoxin (TA) systems13,27,29–35 are believed to be involved in persister formation and/or regulation, and they can act alone or overlapped36.
The current knowledge about persistence is very fragmented, and important issues regarding the molecular mechanisms behind persister formation remain unexplored. Although controversial14,37, it is believed that the type II TA system is the main component involved in persister formation in S. enterica30. The TA systems vapBC, relBE35, and shpAB (Salmonella high persistence)38 have been described in S. Typhimurium persisters after ampicillin exposure. High expression of relBE and shpAB is usually activated by SR due to intracellular accumulation of a second messenger, (p)ppGpp, through the synthases RelA/SpoT, which activate the Lon protease. Lon degrades antitoxins, thereby releasing the associated toxins and inducing S. Typhimurium into a transient non- or slow growth inside vacuolar macrophages13. Recently, Rycroft et al. demonstrated the action of three acetyltransferase toxins (TacT, TacT2, and TacT3) on S. Typhimurium and S. Enteritidis, causing the acetylation of aminoacyl-tRNA molecules, which led to the inhibition of translation and persistence34.
Another important TA system involving the regulation of the SOS response, described in Escherichia coli, is the type I TA system TisB/IstR27,32,33. Fluoroquinolones kill bacteria by acting on DNA replication and causing damage that triggers the SOS response. This effect is mediated by RecA activation, which induces the autoproteolysis of the LexA repressor, stimulating the expression of proteins involved in repair, such as SulA, DinG, UvrABCD, RecABCD, and RuvABC26,39,40. In this case, TisB toxin is activated and forms channels in the cell membrane, causing an imbalance in the proton-motive force and decreasing cellular ATP levels, ultimately promoting the loss of antimicrobial activity16,41. Another important mechanism related to persister formation, particularly in Staphylococcus aureus, is the inactivation of tricarboxylic acid (TCA) cycle genes, supporting the hypothesis that depletion of ATP levels significantly increases the number of persister cells42.
Understanding the physiology of persisters is crucial to prevent their formation and recalcitrance to chronic infections. In this study, we investigated the transcriptome profiles of three isolates of S. enterica (two belonging to serovar S. Enteritidis and one to serovar S. Schwarzengrund), which demonstrated varying levels of persistence when exposed to ciprofloxacin or ceftazidime.
Experimental procedures
Bacterial isolates
Salmonella enterica serovar Enteritidis from porcine feces (4SA), chicken carcass (192), and S. Schwarzengrund (S58) from poultry flesh and bone meal were selected according to previous persistence assays by exposure to ciprofloxacin or ceftazidime43. S. Schwarzengrund (S58) was obtained from the veterinary diagnostic laboratory MercoLab (Cascavel, Brazil). The isolates were stored at -80 °C in Luria–Bertani (LB) medium (Sigma-Aldrich, Saint Louis, USA) supplemented with 20% DMSO.
Genomic DNA isolation and sequencing
Genomic DNA was extracted from 1 mL of overnight S. enterica cultures using Qiagen DNeasy kits (Qiagen, Valencia, CA, USA), following the manufacturer’s instructions. DNA quality was analyzed using NanoDrop One (Thermo Scientific, WI, USA), quantified using a Qubit 3.0 fluorometer (Thermo Fisher Scientific Inc., MA, USA), and stored at − 20 °C until further use. Whole-genome sequencing (WGS) was performed using an Illumina MiSeq using V2 chemistry with 2 × 250 paired-end sequencing (Illumina, Inc., CA, USA). The sequenced reads were assembled using the default parameters of CLC Genomics Workbench 12.0 (Qiagen Bioinformatics). The resulting assembled genome was annotated using Prokka (Prokaryotic Genome Annotation) software version 1.13.7 (Supplementary Table S1). A pairwise FastANI comparison was performed with the three genomes sequenced here and the Salmonella enterica reference genome ASM694v2/ATCC 700720 (https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000006945.2/) (Supplementary data).
Persister assay and RNA extraction
Salmonella enterica cultures at the mid-exponential phase were exposed to a 100-fold MIC (Minimal Inhibitory Concentration) of ciprofloxacin or ceftazidime according to the protocol described by Drescher et al.43, (Fig. 1). Briefly, persister levels were evaluated in planktonic cultures that were grown overnight in LB broth flasks. cultures were diluted at 1:30 and incubated without shaking at 37 °C for 2 h 30 min until the mid-exponential growth phase (approximately 108 CFU/mL). Before antibiotic exposure (0 h) and at two time points (6 and 48 h), 5 mL-aliquots were removed and immediately treated for 5 min with RNAprotect Bacteria Reagent (Qiagen, 76506) before RNA extraction. The cultures were centrifuged at 11,088 × g for 5 min at 4 °C, and the pellet was washed with phosphate-buffered saline solution prior to stain with LIVE/DEAD BacLight (Bacterial Viability Kit; Life Technologies, Carlsbad, CA, USA) to access dead and live cells in the cultures. Persister cells were lysed with lysozyme (0.5 mg/mL) (Thermo Scientific, 89833) and 10% sodium dodecyl sulfate for 10 min at room temperature, followed by 10 min of incubation at 65 °C with phenol:chloroform:isoamyl alcohol (25:24:1) (Sigma-Aldrich, 516726). The cells were immediately placed on ice for 5 min and centrifuged at 21693 g for 10 min at 4 °C. The aqueous phase was removed and transferred to a new tube, to which 400 µL of cold chloroform were added, mixed and centrifuged at 21,693 × g for 10 min at 4 °C. One-tenth of the total volume of 3 M sodium acetate and 1 mL of absolute ethanol was added to the aqueous phase. The samples were centrifuged at 21,693 × g for 10 min at 4 °C, the supernatant was removed, and the pellet was washed with 70% ethanol and dried at room temperature for 5 min. The pellet was eluted in 50 µL of RNase-free water, treated with DNase I according to the manufacturer’s protocol (Invitrogen, 18068015) to remove genomic DNA, and stored at − 80 °C until further use. Total RNA concentration was measured using NanoDrop One (Thermo Scientific), and integrity was verified by observing intact 16S rRNA and 23S rRNA bands by formaldehyde agarose gel electrophoresis.
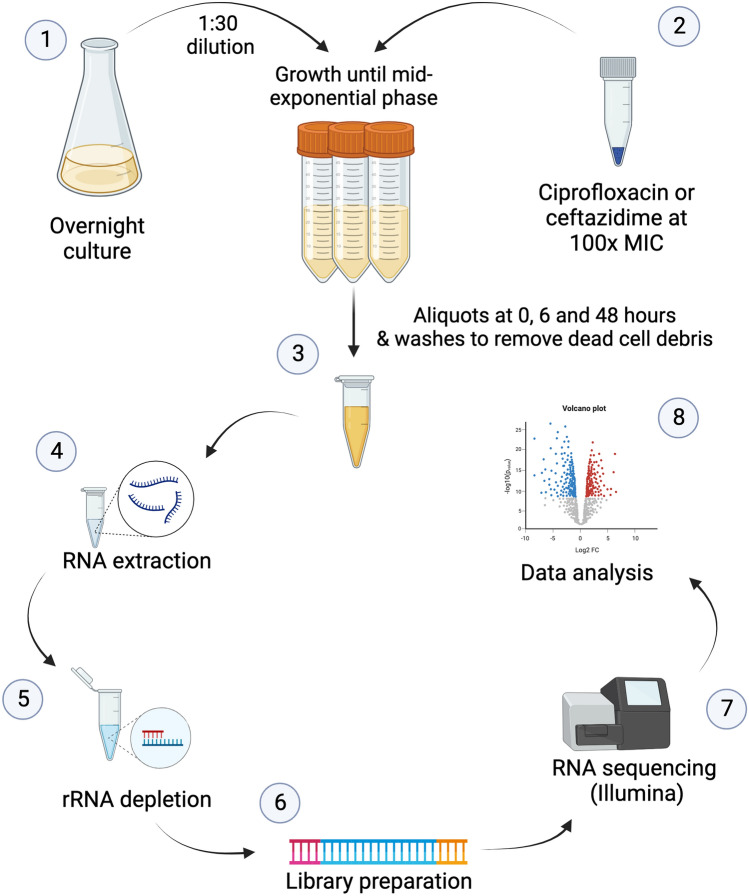
Figure 1.
Experimental procedure for whole-transcriptome analysis of Salmonella enterica persisters induced by exposure to 100-fold MIC of ciprofloxacin or ceftazidime. Three biological replicates were performed for each time point. Overnight cultures were diluted 1:30 in fresh LB and incubated until mid-exponential growth phase (1). Ciprofloxacin or ceftazidime was added to a final concentration 100-fold above the MIC, and the samples were incubated for 48 h (2). 5-mL aliquots were removed at 0, 6, and 48 h after exposure (3) and treated with Bacteria RNAprotect prior to RNA extraction (4). RNA was treated with DNase I, and rRNA was depleted using the RiboZero Magnetic Kit for Gram-negative bacteria (5) before dsDNA synthesis and library preparation (6). RNASeq was performed with Illumina MiSeq platform (7) and the differentially expressed genes were identified with CLC Genomics Workbench 12.0 (8).
RNA-seq library preparation and sequencing
mRNA enrichment was performed using 5 µg of total RNA with the RiboMinus Bacteria Module (Invitrogen, A47335), according to the manufacturer’s specifications. The ribosomal-depleted mRNA was purified with RNeasy Power Clean (Qiagen, 13997-50) and used as a template for generation of the first-strand cDNA using random hexamer-primed anchored-dT primer (dT23VN) (New England BioLabs, S1330S) and ProtoScript II Reverse Transcriptase (New England BioLabs, M0368L), followed by synthesis of the second-strand cDNA (dsDNA) using the NEBNext Ultra II Non-directional RNA second strand synthesis module (New England BioLabs, E6111L), according to the manufacturer’s protocol.
Samples were quantified using a Qubit 3.0 fluorometer (Thermo Fisher Scientific) and adjusted to 0.3 ng/µL. Whole transcriptome sequencing libraries were prepared using the Nextera XT DNA Library Prep Kit for the fragmentation and tagmentation of cDNA. A PCR with 12 cycles of amplification was conducted to enrich the adapter-ligated fragments. A cleanup step using AMPure XP beads was performed to remove short library fragments. Library normalization was carried out according to the Illumina MiSeq protocol for Bead Normalization. Samples were pooled at equal volumes and denatured before dsDNA sequencing was performed on an Illumina MiSeq platform using a V2 kit with 2 × 250 paired-end chemistry (Illumina, Inc.).
The datasets generated and analyzed during the current study are available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) repository, BioProject accession: PRJNA992450.
RNA-seq data analysis
RNA-seq data were analyzed using the standard parameters of CLC Genomics Workbench 12.0 (Qiagen Bioinformatics) to identify the differentially expressed genes between time points (0, 6, and 48 h) after exposure to ciprofloxacin or ceftazidime. The paired-end reads were mapped against their own assembled genome using the following parameters: mismatch cost = 2, insertion cost = 3, deletion cost = 3, length fraction = 0.8, similarity fraction = 0.8, strand specific = both, and maximum number of hits for a read = 10. Library sizes were normalized and expression values were measured in Reads Per Kilobase per million. Differentially expressed genes (DEGs) were identified by comparing sample transcriptomes before and after treatment. Statistically significant differences for DEGs were estimated using a cutoff for false discovery rate (FDR) p-value < 0.01 and an absolute value of log2 fold change > 1 or < − 1. Data analysis was performed using custom codes written in R or Python 3 (Supplementary Table S2).
Results
Global gene expression profiles in Salmonella enterica persister cells vary according to the isolate
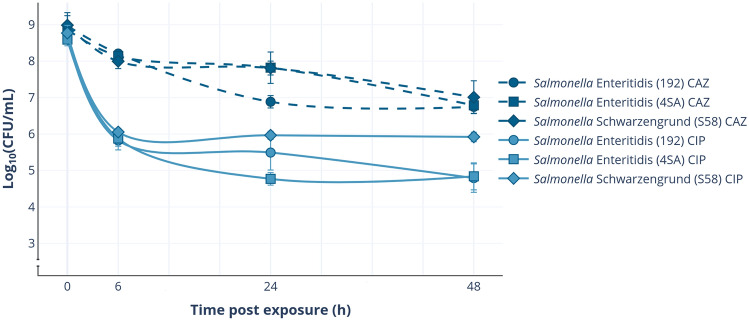
As previously reported43, there is high heterogeneity in persister levels among S. enterica isolates when cultured under the same conditions and exposed to the same antimicrobial, especially with ciprofloxacin. With this in mind, we selected two S. Enteritidis (4SA and 192) and one S. Schwarzengrund (S58) isolates that showed different persister levels after a 48-h exposure to a 100-fold MIC of ciprofloxacin or ceftazidime (ranging from 0.0127 to 0.1415% and 0.5952–1.057%, respectively) (Fig. 2).
Figure 2.
Effect of ciprofloxacin (CIP) or ceftazidime (CAZ) exposure on persister cell levels. Mid-log phase cultures of Salmonella enterica (time 0-h) were exposed to a 100-fold MIC of CIP or CAZ for 48 h. At each time point (6, 24, and 48 h), aliquots were collected to determine the fraction of surviving cells. The plotted values represent the mean of three biological replicates, and the error bars denote the standard deviation (± SD).
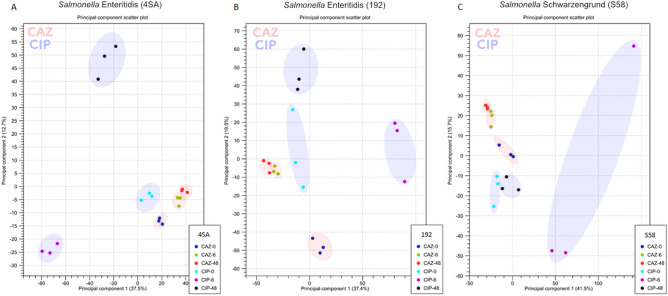
The transcriptome profiles of S. enterica persisters were analyzed after 6 and 48 h of exposure to 100-fold MIC of ciprofloxacin or ceftazidime, and compared with the samples before treatment. At these time points, only a small fraction of surviving cells remained in the sample after washing with phosphate-buffered saline (Fig. S1). The principal component analysis (PCA) plot was able to independently recapitulate the groups based on the similarities in their transcripts (Fig. 3A–C), summarizing the differences in transcription patterns of S. enterica persisters at three time points (0, 6, and 48 h) under different antibiotic treatments (Fig. 3). Remarkably, the expression profiles of persister cells exposed to ceftazidime tended to group more closely between the time points than those exposed to ciprofloxacin.
Figure 3.
PCA plot depicting a comparison of persister transcriptome from (A) Salmonella Enteritidis (4SA), (B) S. Enteritidis (192), and (C) S. Schwarzengrund (S58) after exposure to 100-fold MIC of ceftazidime (CAZ) or ciprofloxacin (CIP) for 6 and 48 h, as well as before the addition of the antibiotic (0 h). The assay was performed in three biological replicates and analyzed using the CLC Genomics Workbench 12.0. The X-axis indicates principal component 1, and the Y-axis indicates principal component 2.
Ceftazidime and ciprofloxacin treatment generate distinct isolate- or exposure-time associated transcription profiles
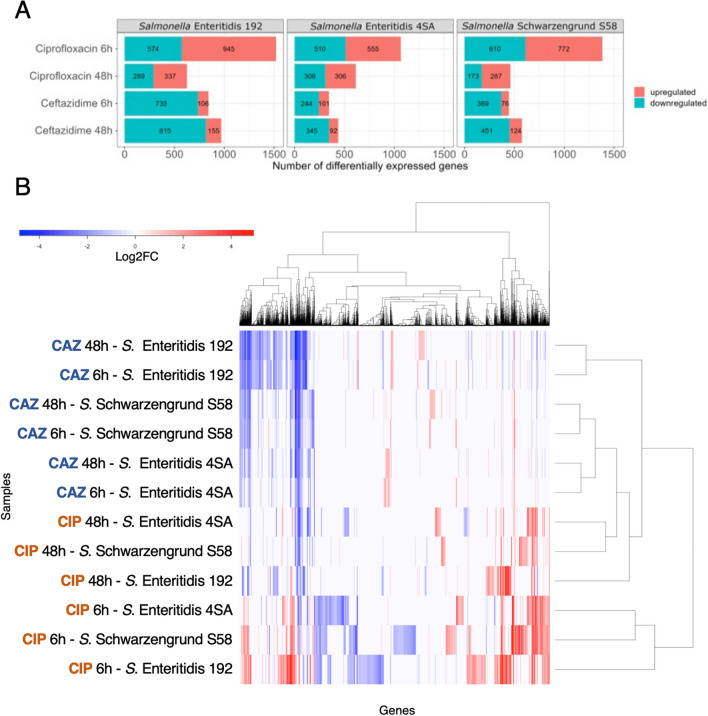
The PCA plots indicated a global change in the transcriptomic profiles of S. enterica cells under antimicrobial treatment. To understand the impact of each treatment and exposure time on persister formation, differential gene expression analysis was performed by filtering out non-significant variations using an FDR-adjusted p-value cut-off of < 0.01 and log2 fold change > 1 or < − 1. A total of 3388 unique genes was differentially expressed (p < 0.01) among the persisters from all S. enterica isolates and time points (Supplementary Table S3). S. enterica persister cells in the ceftazidime-treated group presented a pronounced downregulation of transcripts across all isolates, which was not observed in the ciprofloxacin-treated group (Figs. 4A, S2). When comparing these DEGs from each group, the gene expression profiles from ciprofloxacin-treated persisters interestingly clustered in an isolate-independent manner, primarily grouping according to the time of antimicrobial exposure (Fig. 4B). Meanwhile, transcriptomic changes after ceftazidime exposure appeared to be more correlated within each isolate group.
Figure 4.
Transcriptomic analysis of Salmonella enterica persister cells exposed to ciprofloxacin (CIP) and ceftazidime (CAZ). (A) The Number of Differentially Expressed Genes (DEGs) in persister cells of Salmonella enterica (S. Enteritidis and S. Schwarzengrund) after 6 and 48 h of exposure to 100-fold MIC CIP or CAZ. Statistically significant expressed genes were filtered by a false discovery rate (FDR) p-value < 0.01 and an absolute value of log2 fold change > 1 or < − 1. (B) Heatmap of DEGs. Hierarchical cluster analysis with Euclidean distance correlation and complete linkage. The heatmap displays the DEGs in Salmonella enterica persister cells after exposure to CAZ or CIP, statistically filtered by an FDR p-value ≤ 0.01, and an absolute value of log2 fold change > 1 or < − 1. Different treatments and exposure times (rows) were clustered with the DEGs (columns). Colors indicate expression levels, ranging from highest (red) to lowest (blue). If the transcript was absent or not statistically significant in a given isolate, it is colored in white.
Salmonella enterica persister cells only presented four common transcripts after exposure to ciprofloxacin or ceftazidime
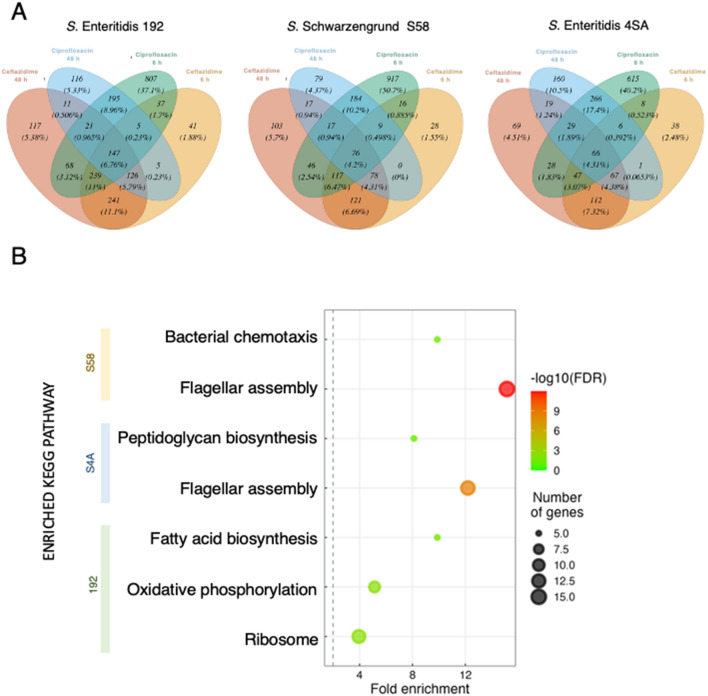
In order to identify a potential set of candidate genes universally associated with persistence, we compared DEGs in the persister cell fraction after each treatment. Across all treatments and time points, we found 66 common DEGs in S. Enteritidis (4SA), 147 in S. Enteritidis (192), and 76 in S. Schwarzengrund (S58) persisters (Fig. 5A). The vast majority of these DEGs were downregulated, as expected, due to the marked downregulation in the ceftazidime-treated persister cells. Pathway enrichment analysis revealed that these genes are mainly involved in flagellar assembly, bacterial chemotaxis, ribosome structure, oxidative phosphorylation, fatty acid metabolism, and peptidoglycan biosynthesis (Fig. 5B).
Figure 5.
RNA-seq data analysis of Salmonella enterica persister cells. (A) Venn Diagram illustrating the overlap of the Differentially Expressed Genes (DEGs) identified in Salmonella Enteritidis (785-4SA), S. Enteritidis (182–192), and S. Schwarzengrund (796-S58) persister cells after exposure to 100-fold MIC of ciprofloxacin (CIP) or ceftazidime (CAZ) for 6 h and 48 h. DEGs were statistically filtered by a false discovery rate (FDR) p-value ≤ 0.01 and an absolute value of log2 fold change > 1 or < − 1. (B) KEGG Pathway Enrichment Analysis performed using the DEGs that were common to all time-antibiotic treatments within each isolate group (S. Schwarzengrund S58, S. Enteritidis S4SA, S. Enteritidis 192). The size of the circles represents the number of genes in each statistically significant pathway, and the color indicates the FDR.
Interestingly, only four DEGs (murG, focA, pspA, and nuoH) were common to all S. enterica isolates after treatment with either ciprofloxacin or ceftazidime. Notably, both murG and nuoH, which encode a peptidoglycan biosynthesis enzyme and a subunit of NADH dehydrogenase I, respectively, were downregulated in all scenarios. In contrast, pspA, a phage shock stress regulator that senses cell membrane stress and has been reported to be important for persistence in E. coli44, was upregulated in all conditions. Also, previously described to influence persistence in E. coli45, focA, which encodes a formate transporter present in the inner membrane, exhibited a discrepant behavior. It showed both down- and up-regulation depending on the isolate and time of exposure to ciprofloxacin. However, under ceftazidime treatment, it showed upregulation in all conditions and isolates. Therefore, any effect derived from focA expression involved in persister development or maintenance may not follow a single mechanism (Supplementary Table S5).
Salmonella persister cells present antibiotic-specific transcription profiles
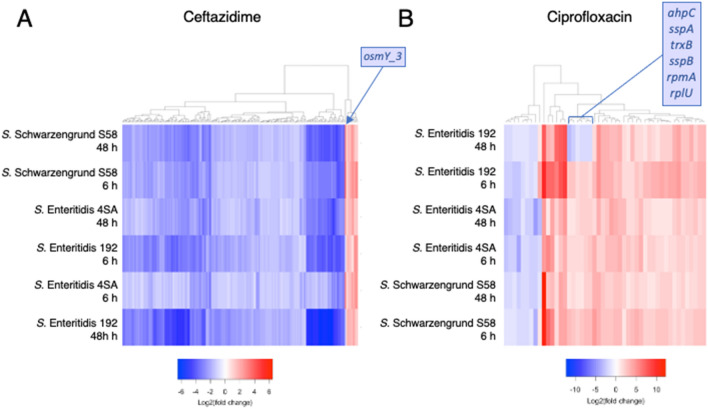
Considering the sharp separation between the persisters surviving ceftazidime and ciprofloxacin treatments, we sought to identify the signature response associated with each antibiotic by separately analyzing the shared DEGs within the two antibiotic groups. We observed a considerable overlap of DEGs when comparing the treatment groups (Fig. 5A). A total of 178 transcripts were found to be differentially regulated and common to all isolates of S. enterica persister cells after exposure to a 100-fold MIC of ceftazidime (Supplementary Table S4, Fig. 6A). The response in these core transcripts was strikingly similar, despite the differences in strains and time points, and they were predominantly downregulated. These DEGs are mainly involved in ribosome production, flagellar assembly, peptidoglycan biosynthesis, ATP synthesis, and secretion. Nearly all DEGs presented a common expression pattern across all isolates, except for one, osmY_3, in S. Enteritidis 192 (Fig. 6A). osmY_3 encodes an osmotically inducible periplasmic protein of unknown function, and it was curiously upregulated exclusively in S. Enteritidis 192, contradicting gene expression in the other two strains. Moreover, a few genes were universally upregulated among all ceftazidime time points and strains: otsB, pspA, dps, pspD, otsA, osmB, osmE, osmX, and spy. The activation of these genes signals an ongoing osmotic stress, given that they are all involved in osmotic responses (osmB, osmE, and osmX), maintenance of cell envelope integrity (pspA and pspD), and trehalose biosynthesis (ostA and ostB), a disaccharide that also plays a role in controlling osmotic stress46.
Figure 6.
Heatmap showing ceftazidime- or ciprofloxacin-shared DEGs, that is, those found in all samples treated with either ceftazidime or ciprofloxacin. The heatmap location of genes that showed divergent expression exclusively in the S. Enteritidis 192 isolate (ie. osmY_3, ahpC, sspA, trxB, sspB, rpmA, and rplU) are indicated.
Likewise, strikingly similar expression profiles were also observed in persister cells that survived ciprofloxacin treatment, although with fewer common DEGs (48) (Fig. 6B). However, the vast majority was upregulated in all samples, including transcripts that play pivotal roles in important processes, such as the SOS response (recA and lexA), TA systems (the toxin gene tisB), cell division inhibitor (sulA), carrier proteins (yebE and yebF), and iron-sulfur cluster assembly (iscA_2, iscR, iscS, iscU, and hscB) (Supplementary Table S5). On the other hand, the nuo operon, responsible for producing part of the respiratory chain complex I components, showed consistent downregulation in all strains, alongside flhE (part of the flagellar regulon) and arnD (involved in lipopolysaccharide modification).
Despite the similarities, a distinctive set of genes (ahpC, trxB, rpmA, rplU, sspA, and sspB) was also exclusively downregulated in the S. Enteritidis 192 isolate at the 48-h time point (Fig. 6B). These genes are involved in redox balance (alkyl hydroperoxidase reductase C, ahpC, and thioredoxin reductase, trxB), ribosomal formation (ribosomal protein L27, rpmA, and ribosomal protein L21, rplU), as well as secretion (sspA and sspB).
Discussion
Despite several genetic approaches, the transcriptome of persister cells remains an understudied topic. Here, we provided insights from a whole transcriptome experiment, revealing possible transcripts that may take part in the establishment or maintenance of the persistence state in multiple strain and antibiotic combinations. Our results indicated heterogeneous responses, especially between the antibiotic treatments. This points to not one, but a diversity of gene expression profiles that vary according to the antibiotic treatment and/or genetic background, potentially contributing to the origin and maintenance of the persistence phenotype. In fact, we could only detect four genes that were differentially expressed in all strains and antibiotic treatments, and only three of them were fully concordant in their expression profiles across samples (pspA was upregulated, and murG and nuoH were downregulated in all treatments). This result reveals a lack of a single pathway responsible for multiple-drug persistence. Indeed, we were able to see similar profiles much more clearly within each class of drug, suggesting that the transcriptional changes that allow persister cell survival are, to a certain extent, antibiotic-specific. Consequently, eradication of persisters is likely better tackled by using a combination of antimicrobial drugs from distinct classes, as proposed in recent studies47–49.
Notwithstanding that only four DEGs were common to all conditions/isolates tested in this study, they bring some interesting clues upon persistence as a heterogeneous phenotype, as well as presenting a somewhat superposed physiological core. Downregulation of murG and nuoH may indicate that cell wall metabolism and the aerobic pulled proton motive force need to be altered50,51. Higher amounts of PspA also point to the need for membrane protection as a core survival strategy, as it was shown to form a protein carpet around damaged membranes, thus preventing proton leakage52,53. This activity seems to be conserved in PspA orthologous proteins and is suggested to be present even in chloroplasts53. The fourth universally common DEG found is focA, which participates in the import/export of formate from the cytoplasm to the periplasmic space, playing essential roles in fermentative metabolism and pH tunning54. Remarkably, or not so, focA expression deviates from the other three common DEGs, as it did not show a common pattern of differential expression when the isolates were treated with ciprofloxacin. This indicates that not a single response and physiological network may be scaffolding persistence over time and/or when different genomes are challenged. It must also be highlighted that all four DEGs code for proteins present in the inner membrane-periplasmic space.
In our transcriptomic analysis, overall, we observed 3388 genes that were differentially expressed (log2ratio > −/+ 1, FDR < 0.01) in the persistence state when compared to pre-treatment. This number is seemingly high, considering that the Salmonella enterica subsp. enterica reference genome typically contains ~ 4500 protein-coding genes. However, in face of the expected drastic differences in physiological states between the non-dividing persister group after antibiotic treatment and the rapidly multiplying pretreatment control group, the observed strong alterations in transcript profiles are plausible. Although we attribute most of these changes to the persistence phenotype, we cannot exclude other factors weighing on this transcriptome remodeling, such as density-dependent gene expression results (e.g., quorum sensing signaling). Moreover, accurately measuring transcript levels in persisters is a complex task because of the high background noise generated by antibiotic-induced cell death and debris. In our study, we attempted to minimize the impact of this noise by washing away most of the cell debris (as observed in Supplementary Fig. 1). We anticipate that this measure, along with the recent report that the half-life of Salmonella transcripts lies in the range of seconds to minutes55, would suffice to selectively capture persister cell transcripts. However, newer transcriptomic technologies (e.g., single-cell RNA sequencing) are required to profile persister cells more precisely in the future. These techniques, despite being highly expensive and technically challenging to perform in bacteria, would not only reduce the background from dead cells but would also provide an understanding of possible genetic interactions at the single-cell level as well as evaluate bona fide individual cell-to-cell variations. Total (“bulk”) RNA-seq presents the limitation of averaging the transcript abundance of the surviving population of cells, losing the heterogeneity information displayed by each individual cell. Indeed, high single-cell phenotypic heterogeneity has been observed in persister cells of E. coli using microfluidic fluorescence microscopy56. Still, we were able to detect consistent transcriptomic profiles in the persister cells that were treated by ceftazidime or ciprofloxacin. For instance, many genes upregulated in the surviving fractions of S. enterica upon ceftazidime exposure, regardless of the isolate, were related to the osmotic response. A potential reason for this response is that these genes were upregulated to combat the stress generated by the inhibition of cell wall synthesis by beta-lactams57, such as ceftazidime, thereby granting persister survival. Alternatively, it has been shown that Salmonella experiences high osmolarity stress inside macrophages58, a condition which curiously induces incredibly high levels (up to 1000-fold increase) of persister cells13. Therefore, it is also possible that the persistence phenotype in Salmonella is associated with the activation of a regulatory “survival” genetic network, which includes coping with osmotic pressure.
Conversely, ciprofloxacin-surviving persister cells showed strong signs of SOS response activation. For instance, recA and lexA genes were both upregulated in all ciprofloxacin-treated groups. These two genes are known to be inducible by ciprofloxacin treatment. We also observed elevated levels of transcripts that code for the TisB toxin (tisB) and the cell division inhibitor sulA, both of which are not only involved in growth arrest, but are also regulated by LexA59. TisB, for instance, has long been associated with increasing persister cell formation33 and accumulation of SulA has been reported as a key element to maintain the cell in a non-dividing state40,60. We also observed an interesting isolate-specific response involving the downregulation of six genes (ahpC, trxB, rpmA, rplU, sspA, and sspB) in S. Enteritidis 192. This isolate was also the only one that diverged in osmY_3 transcript levels upon ceftazidime treatment. This antagonistic response in one of the isolates implies that the genetic backgrounds of different isolates may contribute to different physiological interactions with genes from the core genome, generating heterogeneous and divergent responses, although converging to the same persistent phenotype.
In conclusion, this study provides new information about the heterogeneity of persister cells while also describing common profiles that are present and may sustain cells viable in a harsh environment. Individual genetic/epigenetic compositions, as well as the type and duration of antimicrobial stress, contribute differently to Salmonella persisters metabolism, although common mechanisms of adaptation seem to rely on the membrane and periplasm. Our study shed some light on the transcriptomic state of cells while in the presence of different antimicrobials. However, it also highlights the importance of investigating how diverse metabolic frameworks return to a common baseline phenotype once the stress is removed. Therefore, additional research is needed to uncover the specific transcriptional networks that are modulated, ultimately leading to cell resuscitation. Understanding the nature of persistence, especially its physiological strategies, may enable the development of efficient therapeutics to avoid recurrent infections, hopefully using the already available antimicrobials.
Supplementary Information
Author contributions
S.M. performed the experiments, analyzed the data, and wrote the manuscript. V.C.B.J., C.F., S.O. and J.S. analyzed the data and revised the manuscript. S.O. and J.S. conceived and designed the experiments, analyzed the data and wrote the manuscript.
Funding
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) – [Finance Code 001]. SPM received a scholarship from CAPES, Brazil. SDO is Research Career Awarded of the National Council for Scientific and Technological Development (CNPq).
Data availability
The sequencing datasets generated and analyzed during the current study conducted by Mattiello et al. is available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) repository, BioProject accession: PRJNA992450.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-46142-8.
References
- 1.Collier SA, et al. Estimate of burden and direct healthcare cost of infectious waterborne disease in the United States. Emerg. Infect. Dis. 2021;27:140–149. doi: 10.3201/eid2701.190676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Ciccio PA. Antimicrobial-resistance of food-borne pathogens. Antibiotics (Basel) 2021;10:372. doi: 10.3390/antibiotics10040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant SS, Hung DT. Persistent bacterial infections, antibiotic tolerance, and the oxidative stress response. Virulence. 2013;4:273–283. doi: 10.4161/viru.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fauvart M, De Groote VN, Michiels J. Role of persister cells in chronic infections: Clinical relevance and perspectives on anti-persister therapies. J. Med. Microbiol. 2011;60:699–709. doi: 10.1099/jmm.0.030932-0. [DOI] [PubMed] [Google Scholar]
- 5.Fisher RA, Gollan B, Helaine S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017;15:453–464. doi: 10.1038/nrmicro.2017.42. [DOI] [PubMed] [Google Scholar]
- 6.Lewis K. Persister cells. Annu. Rev. Microbiol. 2010;64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 7.Brauner A, Fridman O, Gefen O, Balaban NQ. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016;14:320–330. doi: 10.1038/nrmicro.2016.34. [DOI] [PubMed] [Google Scholar]
- 8.Lewis K. Persister cells: Molecular mechanisms related to antibiotic tolerance. Handb. Exp. Pharmacol. 2012 doi: 10.1007/978-3-642-28951-4_8. [DOI] [PubMed] [Google Scholar]
- 9.Balaban NQ, et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019;17:441–448. doi: 10.1038/s41579-019-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amato SM, Brynildsen MP. Nutrient transitions are a source of persisters in Escherichia coli biofilms. PLoS One. 2014;9:e93110. doi: 10.1371/journal.pone.0093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amato SM, Orman MA, Brynildsen MP. Metabolic control of persister formation in Escherichia coli. Mol. Cell. 2013;50:475–487. doi: 10.1016/j.molcel.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Donamore BK, Gallo SW, Abreu Ferreira PM, Sanchez Ferreira CA, de Oliveira SD. Levels of persisters influenced by aeration in Acinetobacter calcoaceticus-baumannii. Future Microbiol. 2018;13:209–219. doi: 10.2217/fmb-2017-0153. [DOI] [PubMed] [Google Scholar]
- 13.Helaine S, et al. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science. 2014;343:204–208. doi: 10.1126/science.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conlon BP, et al. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 2016;1:1–7. doi: 10.1038/nmicrobiol.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, et al. The potassium transporter KdpA affects persister formation by regulating ATP levels in Mycobacterium marinum. Emerg. Microbes Infect. 2020;9:129–139. doi: 10.1080/22221751.2019.1710090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan Y, et al. ATP-Dependent Persister Formation in Escherichia coli. mBio. 2017;8:10–1128. doi: 10.1128/mbio.02267-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilmaerts D, Windels EM, Verstraeten N, Michiels J. General mechanisms leading to persister formation and awakening. Trends Genet. 2019;35:401–411. doi: 10.1016/j.tig.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Manuse S, et al. Bacterial persisters are a stochastically formed subpopulation of low-energy cells. PLoS Biol. 2021;19:e3001194. doi: 10.1371/journal.pbio.3001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manina G, Dhar N, McKinney JD. Stress and host immunity amplify Mycobacterium tuberculosis phenotypic heterogeneity and induce nongrowing metabolically active forms. Cell Host Microbe. 2015;17:32–46. doi: 10.1016/j.chom.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Stapels DAC, et al. Salmonella persisters undermine host immune defenses during antibiotic treatment. Science. 2018;362:1156–1160. doi: 10.1126/science.aat7148. [DOI] [PubMed] [Google Scholar]
- 21.Byrd BA, et al. The AcrAB-TolC efflux pump impacts persistence and resistance development in stationary-phase Escherichia coli following delafloxacin treatment. Antimicrob. Agents Chemother. 2021;65:e0028121. doi: 10.1128/aac.00281-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhargava N, Sharma P, Capalash N. Pyocyanin stimulates quorum sensing-mediated tolerance to oxidative stress and increases persister cell populations in Acinetobacter baumannii. Infect. Immun. 2014;82:3417–3425. doi: 10.1128/iai.01600-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung V, Levesque CM. A stress-inducible quorum-sensing peptide mediates the formation of persister cells with noninherited multidrug tolerance. J. Bacteriol. 2012;194:2265–2274. doi: 10.1128/jb.06707-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walawalkar YD, Vaidya Y, Nayak V. Response of Salmonella Typhi to bile-generated oxidative stress: Implication of quorum sensing and persister cell populations. Pathog. Dis. 2016;74:ftw090. doi: 10.1093/femspd/ftw090. [DOI] [PubMed] [Google Scholar]
- 25.Xu T, et al. The Agr quorum sensing system represses persister formation through regulation of phenol soluble modulins in Staphylococcus aureus. Front. Microbiol. 2017;8:2189. doi: 10.3389/fmicb.2017.02189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorr T, Lewis K, Vulic M. SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genet. 2009;5:e1000760. doi: 10.1371/journal.pgen.1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edelmann D, Oberpaul M, Schaberle TF, Berghoff BA. Post-transcriptional deregulation of the tisB/istR-1 toxin-antitoxin system promotes SOS-independent persister formation in Escherichia coli. Environ. Microbiol. Rep. 2021;13:159–168. doi: 10.1111/1758-2229.12919. [DOI] [PubMed] [Google Scholar]
- 28.Podlesek Z, Zgur Bertok D. The DNA damage inducible SOS response is a key player in the generation of bacterial persister cells and population wide tolerance. Front. Microbiol. 2020;11:1785. doi: 10.3389/fmicb.2020.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho J, Carr AN, Whitworth L, Johnson B, Wilson KS. MazEF toxin-antitoxin proteins alter Escherichia coli cell morphology and infrastructure during persister formation and regrowth. Microbiology. 2017;163:308–321. doi: 10.1099/mic.0.000436. [DOI] [PubMed] [Google Scholar]
- 30.Cheverton AM, et al. A Salmonella toxin promotes persister formation through acetylation of tRNA. Mol. Cell. 2016;63:86–96. doi: 10.1016/j.molcel.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gelens L, Hill L, Vandervelde A, Danckaert J, Loris R. A general model for toxin-antitoxin module dynamics can explain persister cell formation in E. coli. PLoS Comput. Biol. 2013;9:e1003190. doi: 10.1371/journal.pcbi.1003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner EG, Unoson C. The toxin-antitoxin system tisB-istR1: Expression, regulation, and biological role in persister phenotypes. RNA Biol. 2012;9:1513–1519. doi: 10.4161/rna.22578. [DOI] [PubMed] [Google Scholar]
- 33.Dorr T, Vulic M, Lewis K. Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLoS Biol. 2010;8:e1000317. doi: 10.1371/journal.pbio.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rycroft JA, et al. Activity of acetyltransferase toxins involved in Salmonella persister formation during macrophage infection. Nat. Commun. 2018;9:1993. doi: 10.1038/s41467-018-04472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva-Herzog E, McDonald EM, Crooks AL, Detweiler CS. Physiologic stresses reveal a Salmonella persister state and TA family toxins modulate tolerance to these stresses. PLoS One. 2015;10:e0141343. doi: 10.1371/journal.pone.0141343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van den Bergh B, Fauvart M, Michiels J. Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol. Rev. 2017;41:219–251. doi: 10.1093/femsre/fux001. [DOI] [PubMed] [Google Scholar]
- 37.Goormaghtigh F, et al. Reassessing the role of type II toxin-antitoxin systems in formation of Escherichia coli Type II persister cells. mBio. 2018;9:10–1128. doi: 10.1128/mbio.00640-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slattery A, Victorsen AH, Brown A, Hillman K, Phillips GJ. Isolation of highly persistent mutants of Salmonella enterica serovar typhimurium reveals a new toxin-antitoxin module. J. Bacteriol. 2013;195:647–657. doi: 10.1128/JB.01397-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilmaerts D, Focant C, Matthay P, Michiels J. Transcription-coupled DNA repair underlies variation in persister awakening and the emergence of resistance. Cell Rep. 2022;38:110427. doi: 10.1016/j.celrep.2022.110427. [DOI] [PubMed] [Google Scholar]
- 40.Mohiuddin SG, Massahi A, Orman MA. lon deletion impairs persister cell resuscitation in Escherichia coli. mBio. 2022;13:e0218721. doi: 10.1128/mbio.02187-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang M, Chan EWC, Wan Y, Wong MH, Chen S. Active maintenance of proton motive force mediates starvation-induced bacterial antibiotic tolerance in Escherichia coli. Commun. Biol. 2021;4:1068. doi: 10.1038/s42003-021-02612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, et al. Inactivation of TCA cycle enhances Staphylococcus aureus persister cell formation in stationary phase. Sci. Rep. 2018;8:10849. doi: 10.1038/s41598-018-29123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drescher SPM, Gallo SW, Ferreira PMA, Ferreira CAS, Oliveira SD. Salmonella enterica persister cells form unstable small colony variants after in vitro exposure to ciprofloxacin. Sci. Rep. 2019;9:7232. doi: 10.1038/s41598-019-43631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vega NM, Allison KR, Khalil AS, Collins JJ. Signaling-mediated bacterial persister formation. Nat. Chem. Biol. 2012;8:431–433. doi: 10.1038/nchembio.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chowdhury N, Kwan BW, Wood TK. Persistence increases in the absence of the alarmone guanosine tetraphosphate by reducing cell growth. Sci. Rep. 2016;6:20519. doi: 10.1038/srep20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howells AM, et al. Role of trehalose biosynthesis in environmental survival and virulence of Salmonella enterica serovar typhimurium. Res. Microbiol. 2002;153:281–287. doi: 10.1016/S0923-2508(02)01321-9. [DOI] [PubMed] [Google Scholar]
- 47.Baek MS, Chung ES, Jung DS, Ko KS. Effect of colistin-based antibiotic combinations on the eradication of persister cells in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2020;75:917–924. doi: 10.1093/jac/dkz552. [DOI] [PubMed] [Google Scholar]
- 48.Chung ES, Ko KS. Eradication of persister cells of Acinetobacter baumannii through combination of colistin and amikacin antibiotics. J. Antimicrob. Chemother. 2019;74:1277–1283. doi: 10.1093/jac/dkz034. [DOI] [PubMed] [Google Scholar]
- 49.Gallo SW, Ferreira CAS, de Oliveira SD. Combination of polymyxin B and meropenem eradicates persister cells from Acinetobacter baumannii strains in exponential growth. J. Med. Microbiol. 2017;66:1257–1260. doi: 10.1099/jmm.0.000542. [DOI] [PubMed] [Google Scholar]
- 50.Sazanov LA. A giant molecular proton pump: structure and mechanism of respiratory complex I. Nat Rev Mol Cell Biol. 2015;16:375–388. doi: 10.1038/nrm3997. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, et al. An alternative and conserved cell wall enzyme that can substitute for the lipid II synthase MurG. mBio. 2021 doi: 10.1128/mbio.03381-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kobayashi R, Suzuki T, Yoshida M. Escherichia coli phage-shock protein A (PspA) binds to membrane phospholipids and repairs proton leakage of the damaged membranes. Mol. Microbiol. 2007;66:100–109. doi: 10.1111/j.1365-2958.2007.05893.x. [DOI] [PubMed] [Google Scholar]
- 53.Junglas B, et al. IM30 IDPs form a membrane-protective carpet upon super-complex disassembly. Commun. Biol. 2020;3:595. doi: 10.1038/s42003-020-01314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kammel M, Sawers RG. The FocA channel functions to maintain intracellular formate homeostasis during Escherichia coli fermentation. Microbiology. 2022;168:001168. doi: 10.1099/mic.0.001168. [DOI] [PubMed] [Google Scholar]
- 55.Jenniches L, et al. Improved RNA stability estimation through Bayesian modeling reveals most bacterial transcripts have sub-minute half-lives. bioRxiv. 2023 doi: 10.1101/2023.06.15.545072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goormaghtigh F, Van Melderen L. Single-cell imaging and characterization of Escherichia coli persister cells to ofloxacin in exponential cultures. Sci. Adv. 2019;5:eaav9462. doi: 10.1126/sciadv.aav9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong F, et al. Understanding beta-lactam-induced lysis at the single-cell level. Front. Microbiol. 2021;12:712007. doi: 10.3389/fmicb.2021.712007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee EJ, Choi J, Groisman EA. Control of a Salmonella virulence operon by proline-charged tRNA(Pro) Proc. Natl. Acad. Sci. U. S. A. 2014;111:3140–3145. doi: 10.1073/pnas.1316209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vogel J, Argaman L, Wagner EG, Altuvia S. The small RNA IstR inhibits synthesis of an SOS-induced toxic peptide. Curr. Biol. 2004;14:2271–2276. doi: 10.1016/j.cub.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Schoemaker JM, Gayda RC, Markovitz A. Regulation of cell division in Escherichia coli: SOS induction and cellular location of the sulA protein, a key to lon-associated filamentation and death. J. Bacteriol. 1984;158:551–561. doi: 10.1128/jb.158.2.551-561.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing datasets generated and analyzed during the current study conducted by Mattiello et al. is available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) repository, BioProject accession: PRJNA992450.