Abstract
We applied HNPP (2-hydroxy-3-naphthoic acid-2′-phenylanilide phosphate) to direct in situ PCR for the routine detection of specific bacterial cells at the single-cell level. PCR was performed on glass slides with digoxigenin-labeled dUTP. The digoxigenin-labeled PCR products were detected with alkaline phosphatase-labeled antidigoxigenin antibody and HNPP which was combined with Fast Red TR. A bright red fluorescent signal was produced from conversion to HNP (dephosphorylated form) by alkaline phosphatase. We used the ECOL DNA primer set for amplification of ribosomal DNA of Escherichia coli to identify cells specifically at the single-cell level in a bacterial mixture. High-contrast images were obtained under an epifluorescence microscope with in situ PCR. By image analysis, E. coli cells in polluted river water also were detected.
In situ PCR amplification of a specific sequence can make possible the detection of single-copy genes present in the cell. Hodson et al. (3) have used this technology to detect mRNA and a single-copy gene that was involved in biodegradation of aromatic compounds in bacterial cells. They were able to identify the specific bacterial cells in a model marine community, demonstrating the genetic capabilities and expression of those capabilities. They used FLOUS-dUTP or digoxigenin (DIG)-UTP to label the PCR products. Anti-DIG Fab′ fragments conjugated to fluorescein or rhodamine were used for fluorescence detection, and anti-DIG Fab′ conjugated to alkaline phosphatase with BCIP (5-bromo-4-chloro-3-indolylphosphate toluidinium) and nitroblue tetrazolium was used for detection by bright-field microscopy. When we analyze naturally occurring bacteria, fluorescence dyes such as DAPI (4′, 6-diamidino-2-phenylindole) (14) are generally used to distinguish bacterial cells from nonbacterial particles. Fluorescence detection also is useful when in situ PCR methods are applied to analyze natural bacteria. Previously, we reported that the HNPP (2-hydroxy-3-naphthoic acid-2′-phenylanilide phosphate)-Fast Red TR system was available to detect fluorescent in situ hybridization signals (17). The fluorescent signal was more intense than fluorescein and lasted for more than 2 h, thus allowing precise observation and counting.
For this report, we adapted the HNPP-Fast Red TR system (5, 17) for high-sensitivity detection of PCR products and developed digital image analysis combined with HNNP-TR–DAPI double staining in order to identify specific bacterial cells in a model bacterial community and in river water.
Bacterial strains used in this study are shown in Table 1. All strains were cultured at 30°C in aerobic Luria-Bertani medium (1% tryptone, 0.5% yeast extract, 0.5% NaCl [pH 7.0]). Cells were harvested by centrifugation, washed twice with phosphate-buffered saline (PBS), and suspended in freshly filtered 4% paraformaldehyde in PBS for 14 to 16 h. After fixation, cells were washed twice in PBS and suspended in 50% ethanol with double-distilled water. Fixed cells were stored at −20°C.
TABLE 1.
Bacterial strains used in this study
| Strain | Species | Sourced |
|---|---|---|
| 17908 | Aeromonas juniia | ATCC |
| 12084 | Aeromonas caviaea | GIFU |
| 7966 | Aeromonas hydrophilaa | ATCC |
| 12305 | Alcaligenes eutrophusb | IAM |
| 19018 | Alcaligenes faecalisa | ATCC |
| 17061 | Cytophaga johnsonaea | ATCC |
| K-12 W3110 | Escherichia colia | RIMD |
| 12872 | Bacillus megateriumc | ATCC |
| 13253 | Flavobacterium meningosepticuma | ATCC |
| 254160 | Burkholderia cepaciab | ATCC |
| 657 | Pseudomonas diminutab | GIFU |
| 1168 | Pseudomonas maltophiliab | KM |
| 12633 | Pseudomonas putidaa | ATCC |
| 25920 | Vibrio campbelliia | ATCC |
| 17802 | Vibrio parahaemolyticusa | ATCC |
| 27562 | Vibrio vulnificusa | ATCC |
Strain used in both PCR and direct in situ PCR experiments.
Strain used only in direct in situ PCR experiments.
Strain used only in PCR experiments.
TCC, American Type Culture Collection, Rockville, Md.; GIFU, Department of Microbiology, Gifu University School of Medicine, Gifu, Japan; IAM, IAM Culture Collection, Institute of Applied Microbiology, The University of Tokyo, Tokyo, Japan; RIMD, Culture Collection, Research Institute for Microbial Diseases, Osaka University, Osaka, Japan; KM, Kansoi Medical University, Osaka, Japan.
Two sets of oligonucleotide primer pairs, EUB1 (5′-GGCTTAACACATGCAAGTCG-3′) and EUB2 (5′-GGACTACCAGGGTATCTAATCCTG-3′) and ECOL1 (5′-GAACGGTAACAGGAAGAAGC-3′) ECOL2 (5′-AGAATGTGCCTTCGGGAACC-3′), were designed for PCRs in this study. To design primer sets for specific bacterial groups, small-subunit rRNA sequences were obtained from GenBank (Release 95.0) and the Ribosomal RNA Database Project (11). Multiple-sequence alignment was carried out on a Sun SPARC station 10 (Sun Microsystems, Inc.) with the CLUSTAL W program (16). A 16S ribosomal DNA (rDNA) primer sequence for either eubacteria or Escherichia coli was designed theoretically. Specificity was examined by the FASTA (13) and BLAST (2) programs to compare the rDNA primers with the complete sequence data registered in GenBank. The EUB primers amplified a 761-bp fragment from the rDNA of eubacteria. The ECOL primers were designed from the rDNA sequences of E. coli and are complementary to positions 64 to 83 and positions 1018 to 1037 according to E. coli 16S rRNA numbering.
The specificity of the EUB and ECOL primer sets was tested in PCR performed with total DNA extracts from 12 known strains (Table 1) that had been reported to exist in the natural aquatic environment. Total DNA was extracted from pure cultures of each strain by a standard procedure (4). Agarose gel electrophoresis after PCR showed that a specific DNA fragment from all 12 bacterial strains was amplified by the EUB primers. In contrast, the ECOL primers amplified only E. coli DNA. No band was obtained for any of the other bacteria tested (data not shown).
In situ PCR was carried out according to the method of Hodson et al. (3) with minor modifications. Fixed cells were washed several times with PBS, and a 30-μl aliquot (about 106 to 107 cells) was spotted onto amino alkylsilane-coated slides (Perkin-Elmer Cetus, Norwalk, Conn.) and allowed to air dry. After dehydration in an ethanol series (50, 80, and 100% ethanol for 3 min each), samples were incubated with lysozyme solution (0.5 mg of lysozyme per ml, 100 mM Tris-HCl [pH 8.2], 50 mM EDTA) for 30 min at 15°C, rinsed with double-distilled water, and dehydrated as described above. Permeabilization was furthered by treatment with proteinase K at a final concentration of 0.1 μg/ml for 10 min at room temperature. After permeabilization, RNA was removed from cells by DNase-free RNase at a final concentration of 10 μg/ml for 2 h at room temperature. Finally, samples were rinsed with a double-distilled water and ethanol series. They were sealed with 50 μl of the PCR buffer (10 mM Tris-HCl [pH 9.0], 50 mM KCl, 1% Triton X-100) containing 2.5 mM MgCl2, 0.2 mM (each) deoxynucleoside triphosphate, 0.035 mM DIG-dUTP (Boehringer Mannheim), 0.4 mM (each) primers, and 2.5 U of Taq DNA polymerase. PCR cycles consisted of denaturation at 94°C for 30 s, primer annealing (55°C for the EUB primer set or 60°C for the ECOL primer set, for 30 s), and extension at 72°C for 60 s. Amplification was repeated for 30 cycles with a thermal cycler (GenAmp In Situ PCR System 1000; Perkin-Elmer Cetus). After thermal cycling, the sample was allowed to air dry. Following dehydration, 30 μl of 0.10 μg of proteinase K per ml was spotted onto samples for 3 min at room temperature and then washed with double-distilled water.
In situ-generated amplified samples that had incorporated DIG-labeled nucleotides were directly visualized with alkaline phosphatase-conjugated anti-DIG Fab′ fragments (Boehringer Mannheim) according to methods described by Yamaguchi et al. (17) with modifications. Briefly, 30 μl of blocking buffer (20 μg of bovine serum albumin [Wako Chemicals Corp.] per ml in PBS) was spotted onto the samples for 30 min at room temperature. Then, 15 μl of blocking buffer was replaced by 15 μl of alkaline phosphatase-conjugated anti-DIG Fab′ fragments diluted 1:30 in blocking buffer (final concentration, 375 mU/sample) containing 1.0% Tween 20 and subsequently incubated at room temperature for 45 min. After being washed three times each in buffer 1 (100 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.05% Tween 20) and buffer 2 (100 mM Tris-HCl [pH 8.0], 100 mM NaCl, 10 mM MgCl2), the sample was incubated in the HNPP-Fast Red TR (Boehringer Mannheim) solution (100 μg of HNPP per ml, 250 μg of Fast Red TR per ml in buffer 2) for 60 min at room temperature. Finally, samples were counterstained with 1 ppm of DAPI for 10 min after being washed in filter-sterilized water and were mounted in Macllavaine buffer (53.2 mM citric acid, 93.6 mM Na2HPO4 [pH 4.5]) for examination under an epifluorescence microscope (BH-2; Olympus Corp.) with either UV or B excitation.
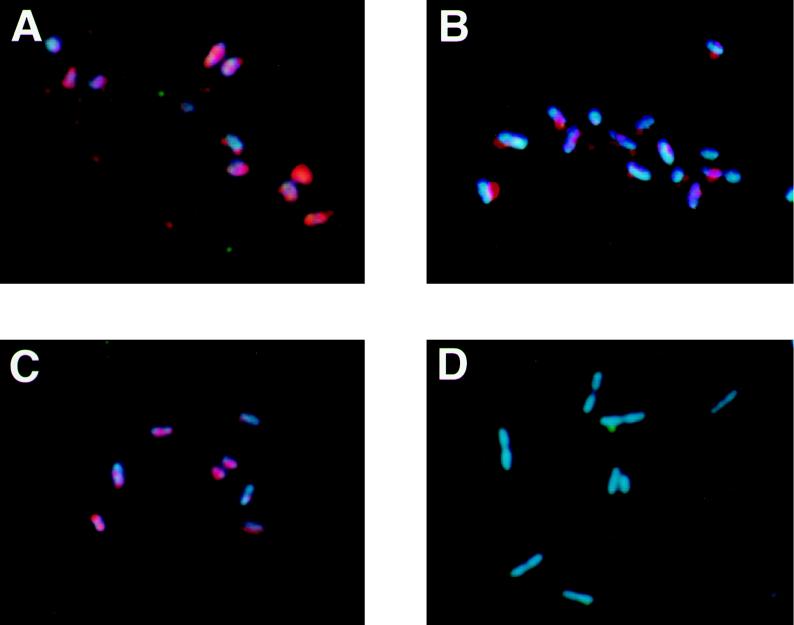
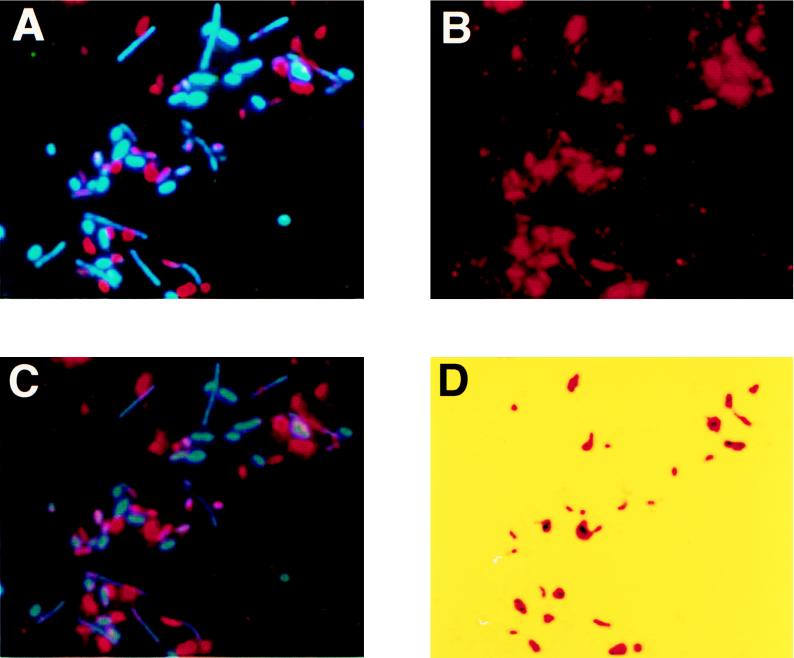
The photo images under UV excitation show the results of direct in situ PCR on glass slides with a mixture of Alcaligenes faecalis and E. coli with the EUB or ECOL primer sets (Fig. 1). Under B excitation, HNP-TR was excited and only the target bacterial cells showed red fluorescence. The HNP-TR signal, however, was very intense and made it possible to detect positive bacterial cells under UV excitation as well. Under UV excitation, both DAPI and HNP-TR were excited, so target cells showed a bluish red fluorescence. Figure 1A and B show E. coli and A. faecalis, respectively, with the EUB primer. The EUB primer hybridized with the rDNA of most cells and amplified the target sequence. Therefore, after PCR cells of both species were excited to bluish red fluorescence. The ECOL primer, however, hybridized only with E. coli. Target cells of E. coli showed bluish red fluorescence, whereas A. faecalis showed only DAPI signals. We performed direct in situ PCR on 15 bacterial species to examine the specificity of this method. Signals were observed only with E. coli. No significant signals were observed with the other 14 bacterial species (Table 1). Furthermore, we examined a mixture consisting of cells of five species: E. coli, Alcaligenes eutrophus, Cytophaga johnsonae, Vibrio vulnificus, and Pseudomonas diminuta. In this model community, we used the direct in situ PCR method with the ECOL primer. E. coli was the only positive bacterial species, while cells of the other four species were negative. Under UV excitation, all cells exhibited blue fluorescence with DAPI (Fig. 2A), and under B excitation, the positive cells demonstrated intense red fluorescence with HNP-Fast Red TR (Fig. 2B).
FIG. 1.
UV excitation images of direct in situ PCR with the EUB or the ECOL primer set. (A) E. coli with EUB primer set. (B) A. faecalis with EUB primer set. (C) E. coli with ECOL primer set. (D) A. faecalis with ECOL primer set.
FIG. 2.
Detection and image analysis of E. coli in the model community. The ECOL primer was used in direct in situ PCR. (A) UV excitation image; (B) B excitation image; (C) composite image of UV and B images; (D) result of image analysis. Only target cells were identified. For an explanation, see the text. The final image analysis (D) was colored with pseudocolor.
The direct in situ PCR method is simple. Detection was rapid with no need for subsequent in situ hybridization (9, 10). In the past, the direct in situ PCR approach has yielded less reliable results than indirect in situ PCR (6–8). In our study, however, highly reliable results for specific amplification were obtained by using glass slide PCR and the HNPP-Fast Red TR system. Serial washes with buffer 1, which contained Tween 20, and buffer 2 after the antibody reaction also contributed to better results by lowering background fluorescence and increasing specificity.
We could detect the target cells with high-intensity fluorescence signals. However, unavoidable fluorescence existed on the B excitation image (Fig. 2B), and it was difficult to separate positive signals from this background fluorescence. Moreover, it is necessary to distinguish bacterial cells from nonbacterial particles when in situ PCR is applied to analyze natural bacteria. We therefore introduced image analysis for in situ PCR to simplify recognition of target cells. Images were obtained under UV and B excitation with an Olympus PM-6 camera and Kodak ISO400 film. Full-color images (8 bits and 512 by 512 pixels) were acquired from the photo images with a digital color image scanner (ScanJet 4C; Hewlett-Packard Co.) and transferred to the Photoshop program (Adobe version 3.0) with a Power Macintosh 9500 (Apple Computer, Inc.). Positive cells, negative cells, and background fluorescence were distinguished by the following method of image analysis. A composite of UV and B images of the same field was examined. The characteristic space was created by the red, green, and blue signal components and the mapped pixels of the images on it. Positive, negative, and background signals were evaluated by the algorithm K-NN (1, 12). Attention was directed at identifying only signals in a positive cluster; thus, the target cells were demarcated with this algorithm. Composite UV and B excitation fluorescence images showed bluish green and red components in the signals from positive cells stained with DAPI and HNP-Fast Red TR, while the negative cells showed only a bluish green color (Fig. 2C). Clustering of positive signals occurred with the K-NN algorithm, and positive cells could be selected automatically by the minimum distance pattern matching method (e.g., thresholding to a 2-bit image). By applying this method to the community mixture, a high-contrast image of composite UV and B excitation was obtained. Only E. coli cells were excited to a purple-red color while cells of the other four species were excited to a blue color. Thus, we were able to sort out the positive and negative cells from the background images. Next, only positive cells (Fig. 2D) were identified by clustering and pattern matching methods. This analysis made it simple to determine the relative number of positive cells in the community mixture. The ratio of positive signals detected by image analysis to total direct counts (DAPI-stained cells) varied in proportion to the ratio of E. coli cells to total cells in the mixture, confirming that positive signals originated from E. coli (data not shown).
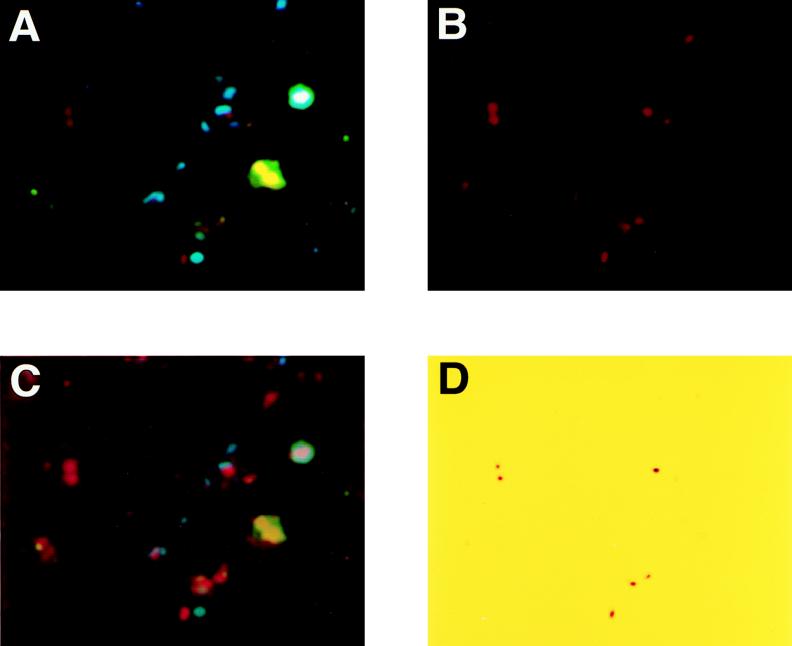
We applied this method to a sample of natural river water. Water was collected from Kitahashi, a tributary of the Neya River in the northern part of Osaka, Japan (15). Kitahashi is located in a commercial area, Osaka Business Park, and is highly polluted. Domestic water flows into this river upstream. In situ PCR was performed as described above. However, a decrease in cell number was observed after the permeabilization treatment. Therefore, the permeabilization conditions for cells from river water were modified as follows. Samples were incubated with the same lysozyme solution for 15 min at 4°C and proteinase K for 3 min at room temperature. Figure 3 shows the photo images of direct in situ PCR for natural river water from Kitahashi with the ECOL primer set. We could count all bacterial cells stained by DAPI under UV excitation (Fig. 3A). B excitation images were not as clear (Fig. 3B). UV and B images were composed on the same field (Fig. 3C). Image analysis removed fluorescence signals, making target cells of E. coli clear and countable (Fig. 3D).
FIG. 3.
Direct in situ PCR with the ECOL primer set in natural river water sample and image analysis. (A) UV excitation image; (B) B excitation image; (C) composite image of UV and B images; (D) result of image analysis.
In this study, we applied the HNPP-Fast Red TR system for the detection of PCR products amplified in bacterial cells. The fluorescent signal obtained in in situ PCR with HNP-Fast Red TR was approximately eightfold more intense than that obtained with fluorescein isothiocyanate (17) and yielded high-contrast images. This high degree of sensitivity allowed us to decrease the number of PCR cycles to 30 from 45, as initially described by Hodson et al. (3), and to reduce by 10-fold the amount of DIG-dUTP required. Due to its sensitivity and ability to identify specific bacteria, the technique of direct in situ PCR described here could become the method of choice in the routine evaluation of bacterial cell numbers and functions in various natural environments.
REFERENCES
- 1.Agui T, Nagao T. Computer image processing and recognition. Tokyo, Japan: Shokodo; 1995. [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Hodson R E, Dustman W A, Garg R P, Moran M A. In situ PCR for visualization of microscale distribution of specific genes and gene products in prokaryotic communities. Appl Environ Microbiol. 1995;61:4074–4082. doi: 10.1128/aem.61.11.4074-4082.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janssen, K. (ed.). Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 5.Kagiyama N, Yoshida K, Hamabata T, Juni N, Awasaki T, Fujita S, Momiyama M, Yoshida M C, Hori S H. A novel fluorescent method for in situ hybridization. Acta Histochem Cytochem. 1993;26:441–445. [Google Scholar]
- 6.Komminoth P, Heitz P U, Long A A. In situ polymerase chain reaction: general methodology and recent advances. Verh Dtsch Ges Pathol. 1994;78:146–152. [PubMed] [Google Scholar]
- 7.Komminoth P, Adams V, Long A A, Roth J, Saremaslani P, Flury R, Schmid M, Heitz P U. Evaluation of methods for hepatitis C virus detection in archival liver biopsies. Comparison of histology, immunohistochemistry, in-situ hybridization, reverse transcriptase polymerase chain reaction (RT-PCR) and in-situ RT-PCR. Pathol Res Pract. 1994;190:1017–1025. doi: 10.1016/s0344-0338(11)80896-4. [DOI] [PubMed] [Google Scholar]
- 8.Long A, Komminoth P, Wolfe H J. Comparison of indirect and direct in situ polymerase chain reaction in cell preparations and tissue sections. Detection of viral DNA, gene rearrangements and chromosomal translocations. Histochemistry. 1993;99:151–162. doi: 10.1007/BF00571876. [DOI] [PubMed] [Google Scholar]
- 9.Nuovo G J. PCR in situ hybridization. In: Choo K H A, editor. In situ hybridization protocols. New York, N.Y: Raven Press; 1992. pp. 223–241. [Google Scholar]
- 10.Nuovo G J, Gallery F, MacConnell P, Becker J, Bloch W. An improved technique for the in situ detection of DNA after polymerase chain reaction amplification. Am J Pathol. 1991;139:1239–1244. [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen G J, Larsen N, Woese C R. The ribosomal RNA database project. Nucleic Acids Res. 1991;19:2017–2021. doi: 10.1093/nar/19.suppl.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pall G H, Hall D J. Proceedings of the IEEE International Communication Conference. 1966. ISODATA; an interactive method of multivariate data analysis and pattern classification; pp. 116–117. [Google Scholar]
- 13.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter K, Feig Y S. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr. 1980;25:943–948. [Google Scholar]
- 15.Tani K, Chen J M, Yamaguchi N, Nasu M. Estimation of bacterial biovolume and biomass by scanning electron microscopic image analysis. Microbes Environ. 1996;11:11–17. [Google Scholar]
- 16.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi N, Inaoka S, Tani K, Kenzaka T, Nasu M. Detection of specific bacterial cells with 2-hydroxy-3-naphthoic acid-2-phenylanilide phosphate and Fast Red TR in situ hybridization. Appl Environ Microbiol. 1996;62:275–278. doi: 10.1128/aem.62.1.275-278.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]