Abstract
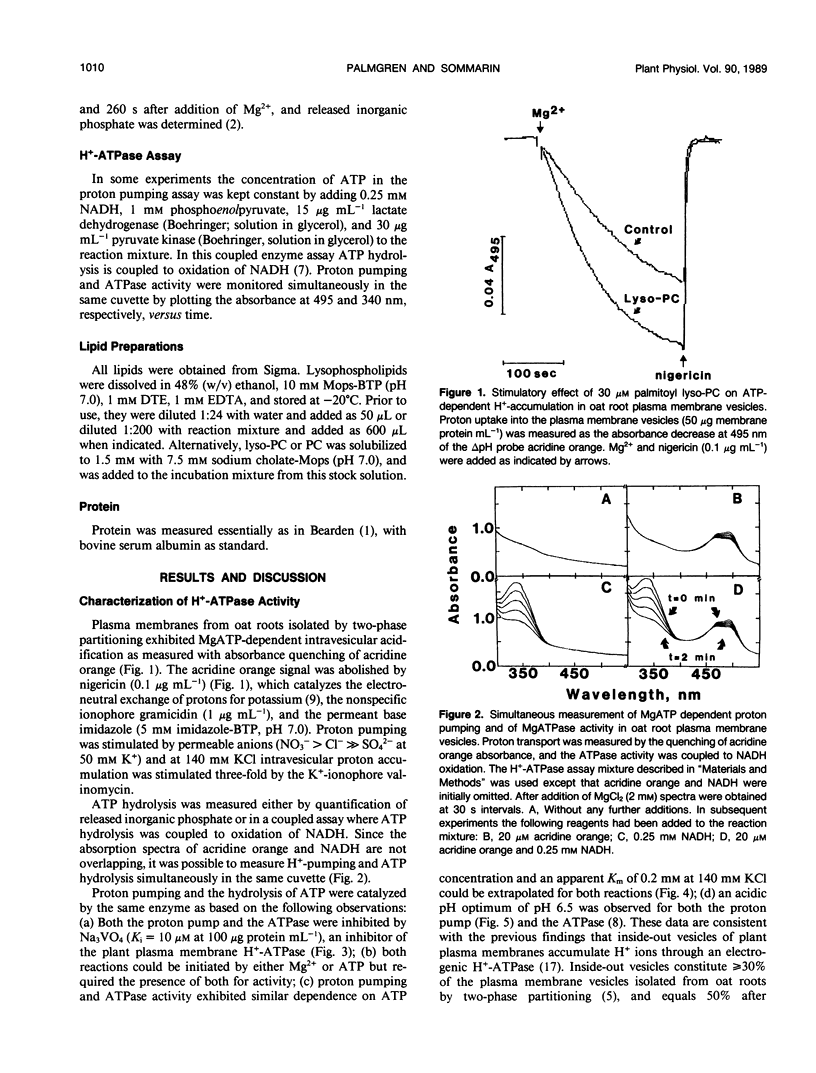
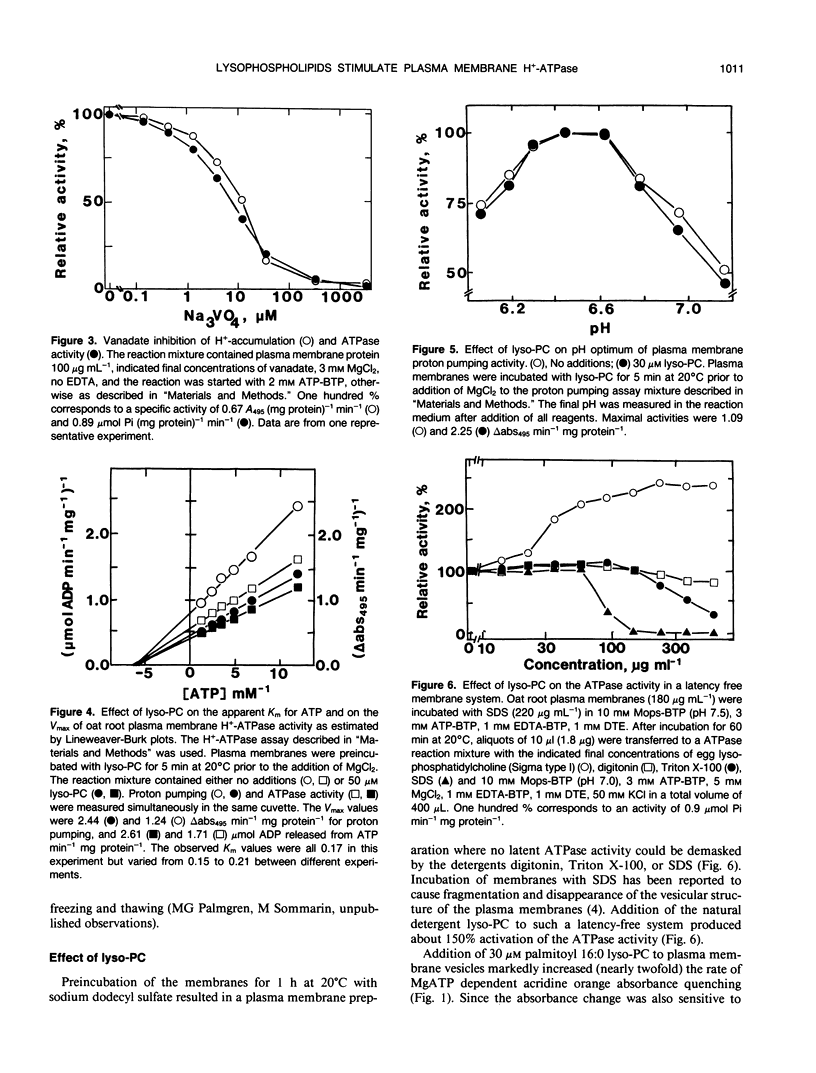
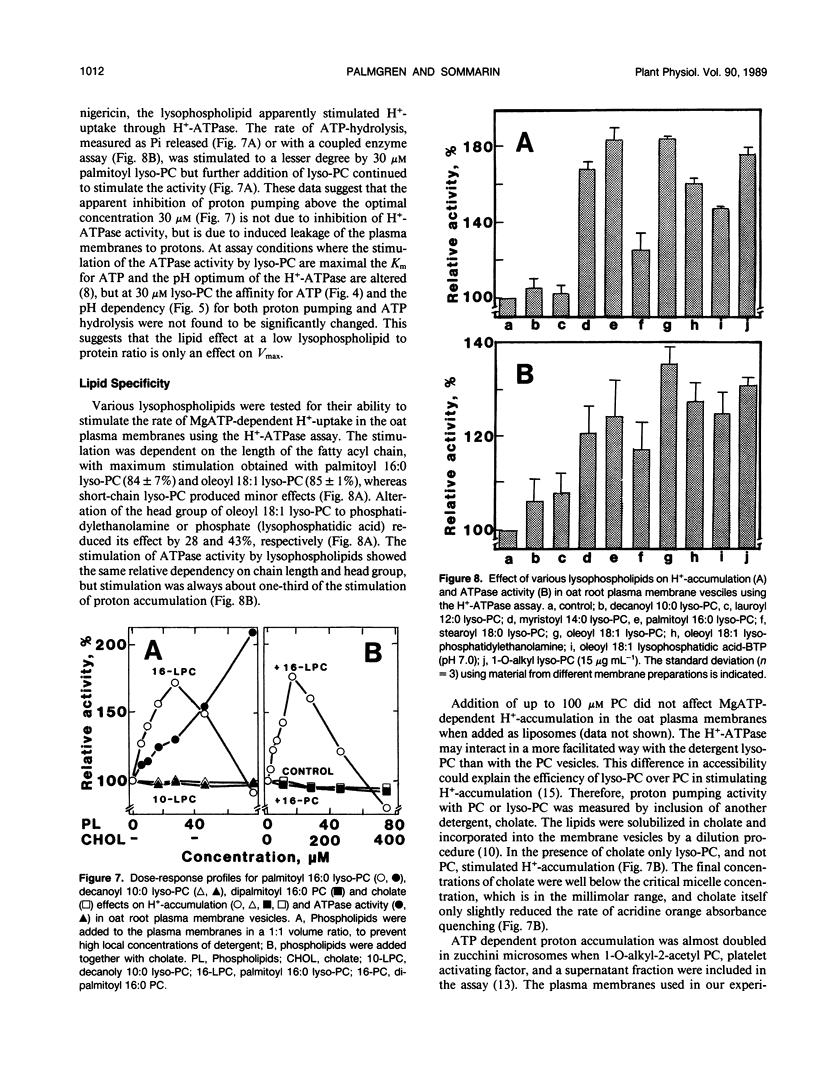
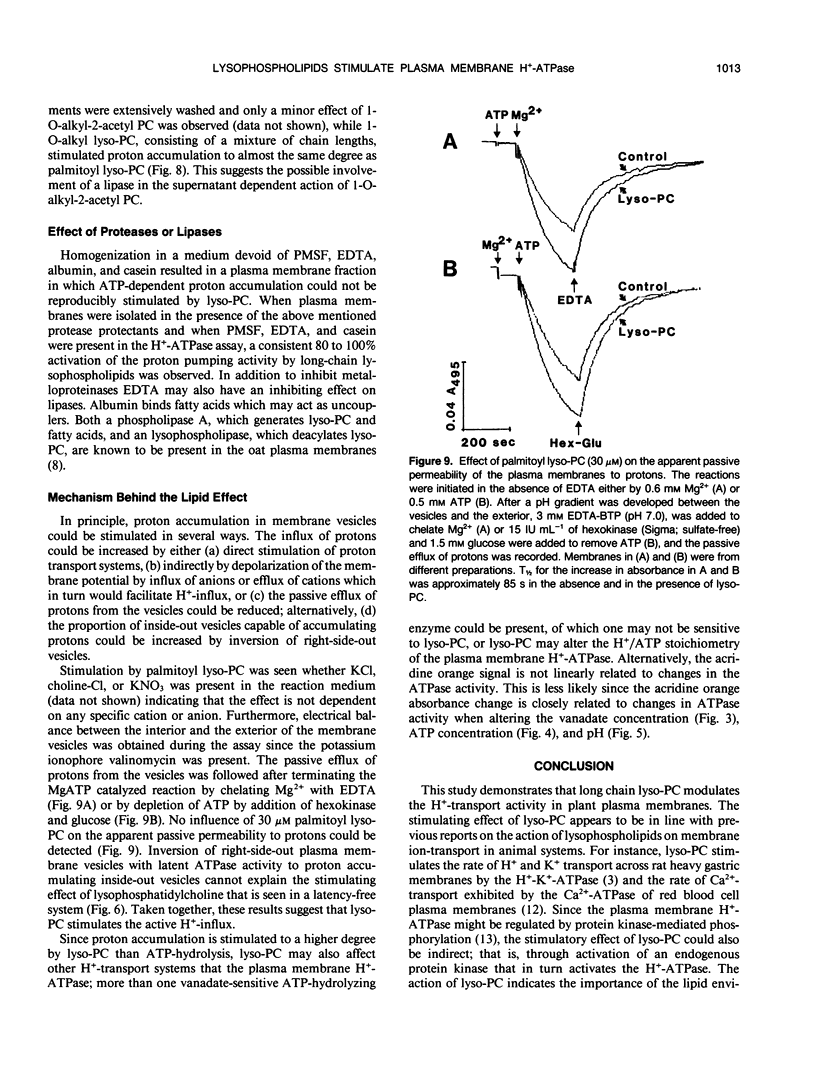
Lysophosphatidylcholine at concentrations of 30 micromolar stimulated the rate of MgATP-dependent H+-accumulation in oat (Avena sativa L. cv Rhiannon) root plasma membrane vesicles about 85% while the passive permeability of H+ was unchanged. Activation was dependent on chain length, degree of saturation, and head group of the lysophospholipid. A H+-ATPase assay was developed that allowed the simultaneous measurement of proton pumping and ATPase activity in the same sample. ATP hydrolysis was also stimulated by lysophospholipids and showed the same lipid specificity, but stimulation was only about 25% at 30 micromolar. At higher concentrations of lysophosphatidylcholine the ATPase activity in a latency-free system could be stimulated about 150%. The enzymic properties of proton pumping and ATP hydrolysis were otherwise identical with respect to vanadate sensitivity, Km for ATP and pH optimum. The stimulatory effect of lysophospholipids suggests that these compounds could be part of the regulatory system for plant plasma membrane H+-ATPase activity in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bearden J. C., Jr Quantitation of submicrogram quantities of protein by an improved protein-dye binding assay. Biochim Biophys Acta. 1978 Apr 26;533(2):525–529. doi: 10.1016/0005-2795(78)90398-7. [DOI] [PubMed] [Google Scholar]
- Brotherus J. R., Jacobsen L., Jørgensen P. L. Soluble and enzymatically stable (Na+ + K+)-ATPase from mammalian kidney consisting predominantly of protomer alpha beta-units. Preparation, assay and reconstitution of active Na+, K+ transport. Biochim Biophys Acta. 1983 Jun 10;731(2):290–303. doi: 10.1016/0005-2736(83)90021-4. [DOI] [PubMed] [Google Scholar]
- Im W. B., Blakeman D. P., Davis J. P. Effect of lysophosphatidylcholine on K+ transport in rat heavy gastric membranes enriched with (H+-K+)-ATPase. Biochem Biophys Res Commun. 1987 Jul 31;146(2):840–848. doi: 10.1016/0006-291x(87)90607-3. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Isolation and characterization of the components of the sodium pump. Q Rev Biophys. 1974 May;7(2):239–274. doi: 10.1017/s0033583500001426. [DOI] [PubMed] [Google Scholar]
- Niggli V., Adunyah E. S., Penniston J. T., Carafoli E. Purified (Ca2+-Mg2+)-ATPase of the erythrocyte membrane. Reconstitution and effect of calmodulin and phospholipids. J Biol Chem. 1981 Jan 10;256(1):395–401. [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Racker E., Chien T. F., Kandrach A. A cholate-dilution procedure for the reconstitution of the Ca++ pump, 32Pi--ATP exchange, and oxidative phosphorylation. FEBS Lett. 1975 Sep 1;57(1):14–18. doi: 10.1016/0014-5793(75)80141-4. [DOI] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Control of plant cell enlargement by hydrogen ions. Curr Top Dev Biol. 1977;11:187–214. doi: 10.1016/s0070-2153(08)60746-2. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Enyedi A., Nyers A., Gárdos G. The function and regulation of the calcium pump in the erythrocyte membrane. Ann N Y Acad Sci. 1982;402:329–348. doi: 10.1111/j.1749-6632.1982.tb25753.x. [DOI] [PubMed] [Google Scholar]
- Serrano R. Structure and function of proton translocating ATPase in plasma membranes of plants and fungi. Biochim Biophys Acta. 1988 Feb 24;947(1):1–28. doi: 10.1016/0304-4157(88)90017-2. [DOI] [PubMed] [Google Scholar]


