Abstract
The aim of this systematic review and meta-analysis is to summarize evidence regarding the relationship between psoriatic arthritis (PsA) and sleep problems. We identified 36 eligible studies (26 cross-sectional, 7 cohort and 3 interventional) in PubMed and EMBASE. The prevalence of self-reported sleep problems in patients with PsA ranged from 30–85%. Meta-analysis of six studies that used the Pittsburgh Sleep Quality Index (PSQI) revealed a prevalence of poor sleep quality for patients with PsA of 72.9% [95%CI 63–81.8; I2:78%], statistically higher than in healthy controls (HC) (26.9% [11.7–45.4; I2: 81%]) but not significantly different than patients with psoriasis (PsO) (59.8% [46.9–72.1; I2: 51%]). Sleep disturbance was ranked in the top 4 health-related quality of life domains affected by PsA. One study suggested a bidirectional relationship between PsA and obstructive sleep apnea. Predictors of sleep problems included anxiety, pain, erythrocyte sedimentation rate, depression, fatigue, physical function, and tender/swollen joint count. TNF-inhibitors, guselkumab, and filgotinib (a JAK-inhibitor) were associated with improved sleep outcomes. Objective sleep measures (i.e., actigraphy and polysomnography) have not been used in PsA studies, and evidence on the validity of patient-reported sleep measures in PsA is lacking. In summary, poor sleep quality is prevalent in patients with PsA. Future studies should validate self-reported sleep measures in PsA, explore how sleep quality relates to PsA disease activity and symptoms using both objective and subjective sleep measures, assess the efficacy of strategies to manage sleep problems, and the effects of such management on symptoms and disease signs in patients with PsA.
Keywords: Psoriatic Arthritis, Psoriasis, Sleep, Sleep disorders, Quality of life
Introduction
Psoriatic arthritis (PsA) is a complex inflammatory disease that manifests as peripheral arthritis, dactylitis, enthesitis, and/or spondylitis.1 PsA is diagnosed in approximately 30% of patients with skin psoriasis (PsO)2 and is associated with other systemic inflammatory diseases, including inflammatory bowel disease, cardiovascular disease, and type II diabetes.3
PsA has also been associated with sleep problems, including short sleep duration, poor sleep quality, and sleep disorders. Sleep is vital for proper physiologic and psychologic functioning.4–6 Sleep problems are associated with chronic physical and mental health problems including cardiovascular disease, type II diabetes, obesity, and depression. Multiple studies have also shown diminished quality of life in patients who suffer from sleep problems.7,8 Sleep problems, therefore, may present a potential additional source of risk - and of intervention - for patients with PsA who are already at risk for such comorbidities.
A growing body of literature has investigated the relationship between psoriatic disease and sleep. The aim of this systematic review is to synthesize available evidence regarding the (i) prevalence and incidence of sleep problems in patients with PsA; (ii) prevalence and incidence of PsA in patients with sleep problems; (iii) factors associated with sleep problems in patients with PsA; (iv) use of validated versus unvalidated sleep outcome measures in patients with PsA; (v) beliefs about sleep in patients with PsA; and (vi) effect of PsA therapies on sleep problems in patients with PsA.
Methodology
This review was conducted in accordance with the PRISMA guideline for systematic reviews.9
Inclusion criteria
We included studies that were full-text original articles in English featuring older adolescents and adults (≥16 years) in which any of the following conditions were met: (i) sleep problems were evaluated as an outcome in patients with PsA, (ii) the incidence or prevalence of PsA was evaluated in patients with sleep disorders; (iii) the quality (i.e., measurement properties) of sleep measures was evaluated in PsA; and (iv) beliefs about sleep were elicited by patients with PsA. Sub-analysis of sleep problems for the PsA population was required for studies including other populations (e.g., psoriasis, ankylosing spondylitis).
Exclusion criteria
We excluded any non-English language studies, review articles, research letters, opinion letters, notes, abstracts, and animal studies.
Literature Search
PubMed and Embase databases were searched on January 5, 2023. The PICOS criteria, Emtree, and MeSH were used to create a thorough search strategy (Supplementary Table 2). Search results were uploaded to Covidence and reviewed first by title and abstract followed by full-text review. Studies were chosen for inclusion by three independent reviewers: CG, MW, and LPC.
Data Extraction
Data extraction was performed by each independent reviewer with subsequent discussion about any disagreements. Data were organized per study aim (i) prevalence and incidence of sleep problems in patients with PsA; (ii) prevalence and incidence of PsA in patients with sleep problems; (iii) factors associated with sleep problems in patients with PsA; (iv) use of validated versus unvalidated sleep outcome measures in patients with PsA; (v) beliefs about sleep in patients with PsA; and (vi) effect of PsA therapies on sleep problems in patients with PsA.
For the purpose of this work, we agreed that an outcome measure would be considered as “validated” if any of the following measurement properties was assessed in a validation study for any population: content validity, construct validity, structural validity, reliability, and responsiveness. If none of these measurement properties was assessed in any population, the instrument was deemed as “unvalidated”.
Quality Evaluation
Two independent reviewers (LPC, MW) evaluated the quality of the data using the Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields developed by Kmet et al.10 This quality assessment tool was selected given the heterogeneity of study designs included in the review. Studies were classified as of high quality (>8 out of 10), good quality (6–8 out of 10), moderate quality (4–6 out of 10), and poor quality (<4).11
Statistical analysis
Proportional meta-analyses were implemented to evaluate the pooled prevalence of poor sleep quality as determined by the Pittsburgh Sleep Quality Index (PSQI)12. Heterogeneity between studies was evaluated with I2 statistics and considered present if I2 was greater than 50% (p<0.1).13 The random-effect model was applied given that the I2 statistics indicated high heterogeneity. Additional meta-analyses with single means and standard deviations were incorporated to examine pooled PSQI parameters (i.e., subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction). Individual meta-analysis was performed on patients with PsA, patients with PsO, and healthy controls (HC) if at least two studies were available.14 The assessment of publication bias was not considered relevant during the proportional meta-analyses,15 and not relevant during the meta-analyses with single means due to low number of included studies. The statistical analysis was conducted using statistical software R with additional packages meta.
Results
In all, 810 references were retrieved; duplicates were removed resulting in 567 unique references for screening (Figure 1). After screening the title and/or abstract, 152 articles remained for full-text assessment. There were 116 full-text studies excluded. A total of 36 articles were included (Table 1).
Figure 1.
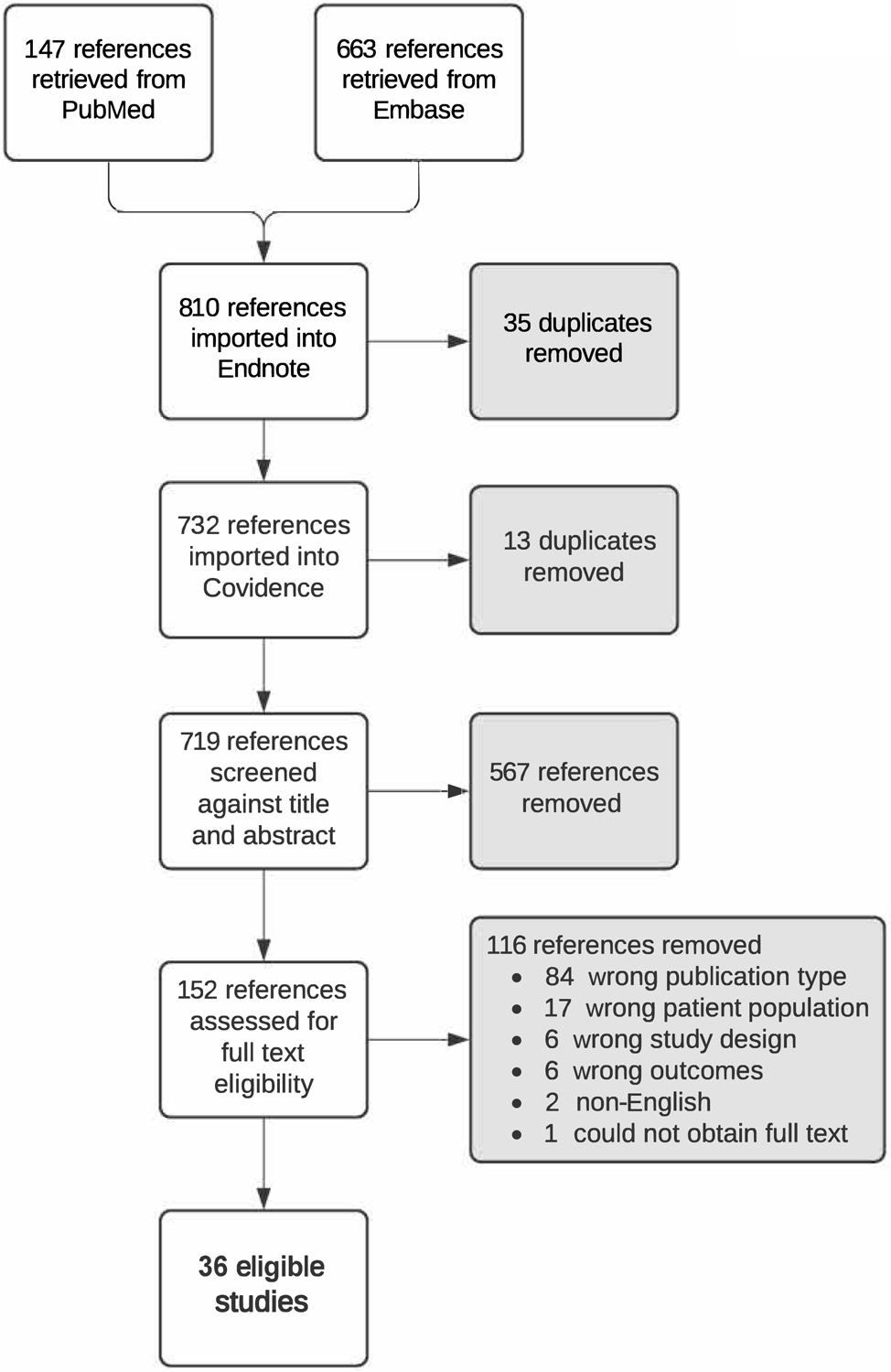
PRISMA Flow Diagram.
Table 1.
Characteristics of included studies.
| Author, year | Study Aim | Study Design | Sample Size, n | Sex, n [M/F] | Age, years [Mean (SD)] | Sleep measure | Summary of main sleep-related results |
|---|---|---|---|---|---|---|---|
| Studies measuring sleep with an unvalidated sleep PROM | |||||||
| Callis Duffin 200917 | Determine what clinical features of PsO predict sleep interference | Cross-Sectional |
|
198/222 (PsA and PsO patients) | 28.9 (16.4) (PsA and PsO patients) | Sleep interference question |
|
| Haugeberg, Hoff 202023 | Explore the occurrence of sleep disturbances, fatigue, and anxiety/depression in PsA | Cross-Sectional |
|
68/69 | 52.3 (10) | Sleep difficulty question (NRS) |
|
| Haugeberg, Michelsen 202025 | Explore the impact of the various disease manifestations and disease consequences on HRQoL in PsA patients treated with biologics | Cross-Sectional |
|
66/65 | 51.9 (10.1) | Sleep disturbance question (NRS) |
|
| Haugeberg 202124 | Explore associations between sleep disturbance, fatigue, pain, anxiety, depression, general health status, and satisfaction with life before and after a diagnosis of PsA | Cross-Sectional |
|
|
|
Sleep difficulty question (NRS) |
|
| Smith 201934 | Understand the nature of sleep disturbance in patients with PsA | Cross-Sectional |
|
960/2153 (whole sample) | NR | Sleep difficulty question |
|
| Wendler 202248 | Assess long-term effectiveness, impact on quality of life, and safety of ustekinumab treatment for PsA | Cohort |
|
NR | NR | Sleep quality (100-mm VAS) - question and anchors not described | Absolute change from baseline to week 4: 3.3; week 28: 4.9; week 52: 6.2; week 76: 1.4; week 100: 6.5; week 124: 7.1; week 160: 9.5. |
| Studies measuring sleep with a sleep question embedded in a psoriatic disease PROM | |||||||
| Azarfar 202116 | Determine the prevalence and correlation of anxiety, depression, sleep disturbance, fibromyalgia, and obesity in rheumatic patients | Cross-Sectional |
|
NR | NR | RAPID3 (“good night sleep” question) |
|
| Desthieux 201719 | Assess patient-physician discordance in PsA and patient-reported domains of health associated with discordance | Cross-Sectional |
|
223/237 | 50.6 (12.9) | PsAID (“sleep disturbance” question) |
|
| Palominos 202031 | Analyze determinants of impaired sleep in patients with PsA | Cross-Sectional |
|
194/202 | 51.9 (12.6) | PsAID (“sleep disturbance” question) |
|
| Puyraimond-Zemmour 201732 | Explore the link between a PASS and patient-perceived impact in RA and PsA | Cross-Sectional |
|
|
|
PsAID (“sleep disturbance” question) |
|
| Pincus 199946 | Develop components of a MHAQ through the addition of new items in the patient friendly HAQ format | Cohort |
|
NR | 50.2 | MDHAQ (“good night sleep” question) |
|
| Orbai 202049 | Examine the effects of filgotinib on HRQoL in active PsA | RCT |
|
NR | NR | PsAID (“sleep disturbance” question) |
|
| Studies measuring sleep with a sleep PROM validated in other populations | |||||||
| Cano-García 202118 | Describe sleep disorders and positive psychological factors in patients with AxSp and PsA | Cross-Sectional |
|
|
|
OSQ |
|
| Duruoz, Baklacioglu 201820 | Evaluate the static and dynamic balances in PsA and investigate their relationship with clinical and functional parameters | Cross-Sectional |
|
|
|
PSQI |
|
| Gezer 201721 | Determine the effects of PsA on sleep quality, quality of life and psychological state | Cross-Sectional |
|
|
|
PSQI |
|
| Krajewska-Wlodarczyk 201828 | Assess and measure occurrence of sleep disorders in patients with PsA and PsO | Cross-Sectional |
|
|
|
PSQI |
|
| Orbai 202250 | Assess the effects of treatment on general health outcomes in patients with PsA | RCT |
|
186/195 | 48.4 (11.4) | PROMIS Sleep Disturbance |
|
| Sandikci 201933 | Determine the prevalence of RLS in PsA patients and investigate a relationship between sleep and quality of life impairments of RLS in PSA patients | Cross-Sectional |
|
|
|
RLS Study Group Criteria / PSQI / ESS/ ISI |
|
| Skougaard 202238 | Determine the prevalence of sleep disturbances in PsA, PsO, and HC and explore associations between PSQI and PROMs/treatment | Cross-sectional |
|
|
|
PSQI |
|
| Ulutatar 202035 | Examine the presence of neuropathic pain in PsA and its relationship with functional parameters | Cross-Sectional |
|
18/32 | Median (IQR)=50.5 (17) | PSQI |
|
| Ulutatar 202136 | Evaluate the relationship of fibromyalgia with enthesopathy, sleep, fatigue, and quality of life in PsA | Cross-Sectional |
|
19/31 | 50 (10.3) | PSQI |
|
| Wong 201737 | Determine the prevalence and quality of sleep-in patients with PsA and those with PsO to compare findings to controls | Cross-Sectional |
|
|
|
PSQI |
|
| Tektonidou 202047 | Evaluate the effect of adalimumab on work productivity measures, overall activity impairment, and sleep quality in patients with active moderate to severe RA, PsA, or AS | Cohort |
|
|
Median (IQR)=
|
MOS-SS |
|
| Strober 201251 | Assess the extent of sleep impairment, the effect of adalimumab on sleep, patient-reported outcomes, and correlations between changes in these outcomes and sleep quality | Non-RCT |
|
NR | 47.6 (13.7) (whole sample) | MOS-SS |
|
| Studies evaluating sleep beliefs in PsA | |||||||
| Hu 201026 | Piloted a novel application of WTP as a Patient Reported Outcome to measure the relative impact of PsA in 8 domains of HRQoLmk | Cross-Sectional |
|
56/44 | 30% <45 years, 70% ≥ 45 years | Domain evaluated: “Ability to Sleep” |
|
| Kotulska 201827 | Evaluate various aspects of satisfaction and dissatisfaction of Polish patients treated with biologics | Cross-Sectional |
|
|
18–29: 183 (15.2) 30–39: 255 (21) 40–49: 239 (19.7) 50–59: 332 (27.4) (whole sample) |
Question: effect of biologics on sleep quality |
|
| Ogdie 202030 | Characterize and develop a conceptual model to represent the patient experience of PsA | Cross-Sectional |
|
6/13 |
|
Factor impacting PsA: Sleep disturbance |
|
| Ogdie 202239 | Evaluate patients’ experiences regarding the burden of PsA symptoms and disease impacts, and patients’ preferences for treatment | Cross-Sectional |
|
66/266 | 53.9 (11.4) |
|
|
| Nowell 202145 | Understand which patient-reported outcomes patients with rheumatic and musculoskeletal disease are considered most important to track for disease management | Cohort |
|
16/124 (whole sample) | 55.1 (9.3) (whole sample) | Sleep measure ranked: PROMIS Sleep Disturbance |
|
| Tsang 202240 | Assess the correlation between cannabis use and psoriatic disease severity, health-related quality of life, pain, psychosocial outcomes, and cytokine levels in patients with PsO and PsA. | Cross-Sectional |
|
80/71 (whole sample) | 55.75 (13.4) (whole sample) | Perceived benefit of cannabis on sleep | 63% patients believed that use of cannabis helped them sleep better |
| Claims-based studies using ICD codes | |||||||
| Cohen 201542 | Evaluate association between OSA and snoring and incidence of PsO and PsA | Cohort |
|
All females |
|
OSA ICD code | Women with OSA had a significantly increased risk of PsO, but not PsA RR of PsA among women with OSA |
| Egeberg 201643 | Investigate the potential bidirectional association between PsO and OSA | Cohort |
|
|
|
OSA ICD code | PsO and PsA were associated with increased risk of OSA, and OSA was associated with increased risk of PsO and PsA |
| Studies evaluating measurement properties or interpretability of patient-reported sleep measures in PsA | |||||||
| Duruoz, Erdem 201841 | Investigate the validity and reliability of the Turkish version of JSS in PsA | Cross-Sectional |
|
12/44 | 42.71 (11.79) | JSS |
|
| Guler 202022 | Validate the Turkish version of the PsAID | Cross-Sectional |
|
20/60 | 50.2 (9.9) | PsAID (“sleep disturbance” question) |
|
| Morante 202129 | Address the clinimetric properties of the instrument ASAS-HI in patients with PsA | Cross-Sectional |
|
52/38 | Median (IQR): 53 (45–65) | ASAS-HI (‘I sleep badly at night’ question) |
|
| Kwok 201044 | Determine the MID for the HAQ-DI, pain, fatigue, sleep, and global VAS in patients with PsA using a patient-reported overall health status anchor | Cohort |
|
83/117 | 51.05 (14.08) | Sleep (VAS) |
|
AS, ankylosing spondylitis; ASAS-HI, Assessment of SpondyloArthritis international Society-Health Index; AxSp, axial spondyloarthritis; BDI, Beck Depression Inventory; BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; DAPSA, Disease Activity in Psoriatic Arthritis; DLQI, Dermatology Life Quality Index; EQ5D, European Quality of Life-5 Dimensions; ESR, erythrocyte sedimentation rate; ESS, Epworth sleepiness scale; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; FIQ, Fibromyalgia Impact Questionnaire; FM, fibromyalgia; FSS, fatigue severity scale; HAQ-DI, health assessment questionnaire disability index; HC, healthy controls; ICD, international classification of diseases; IQR, interquartile range; ISI, insomnia severity index; JIA, juvenile idiopathic arthritis; JSS, Jenkins Sleep Scale; MAF, Multidimensional Assessment of Fatigue; MDHAQ, Multidimensional Health Assessment Questionnaire; MHAQ, modified health assessment questionnaire; MID, minimal important difference; MOS-SS, Medical Outcomes Study Sleep Scale; NP, neuropathic pain; NR, not reported; NRS, numeric rating scale; OA, osteoarthritis; OP, osteoporosis; OR, odds ratio; OSA, obstructive sleep apnea; OSQ, Oviedo Sleep Questionnaire; PASI, psoriasis area and severity index; PASS, patient acceptable symptom state; PDQ, pain DETECT questionnaire; PGA, patient global assessment; PhGA, physician global assessment; PROM, patient-reported outcome measure; PROMIS, patient-reported outcomes measurement information system; PsA, psoriatic arthritis; PsO, psoriasis; PsAID, Psoriatic Arthritis Impact of Disease; PSQI, Pittsburgh Sleep Quality Index; QoL, quality of life; RA, rheumatoid arthritis; RAPID3, Routine Assessment of Patient Index Data 3; RCT, randomized controlled trial; RLS, restless legs syndrome; RR, relative risk; SF-36 MCS, Short Form-36 Mental Health Component Score; SF-36 PCS, Short-Form 36 Physical Component Score; SLE, systemic lupus erythematosus; SPI, sleep problem index; TJC, tender joint count; TNF, tumor necrosis factor; TSJC, tender and swollen joint count; WPAI, Work Productivity and Activity Impairment; WTP, willingness-to-pay; VAS, visual analogue scale
Study Design and Quality Evaluation
We identified (i) 33 observational studies, including 26 cross-sectional studies16–41, 7 cohort studies42–48, and (ii) 3 interventional studies, including 2 RCT49,50 and 1 non-RCT51. Supplementary Table 3 includes the quality evaluation for studies. Most studies were of high quality (i.e., low risk of bias). Common limitations of these studies included absence of sample size calculations and limited methods to adjust for confounders. Two qualitative studies26,45 had moderate risk of bias due to limitations in sampling strategy, verification of study results, and data collection methods.
Prevalence and incidence of sleep problems in patients with PsA
Sleep problems were defined in different ways across studies, using a variety of measures. Based on these multiple definitions, the prevalence of sleep problems in patients with PsA ranged from 30 to 85% (Table 1 and Supplementary Table 4). Meta-analysis of the prevalence of poor sleep quality across 6 studies20,21,28,36–38 that used the PSQI12 revealed a prevalence of poor sleep quality for patients with PsA of 72.9% [95%CI 63–81.8; I2:78%], statistically higher than in healthy controls (HC) (26.9% [11.7–45.4; I2: 81%])28,37,38 but not significantly different than patients with psoriasis (PsO) (59.8% [46.9–72.1; I2: 51%])21,28,37,38 Figure 2 and Supplementary Table 5).
Figure 2.
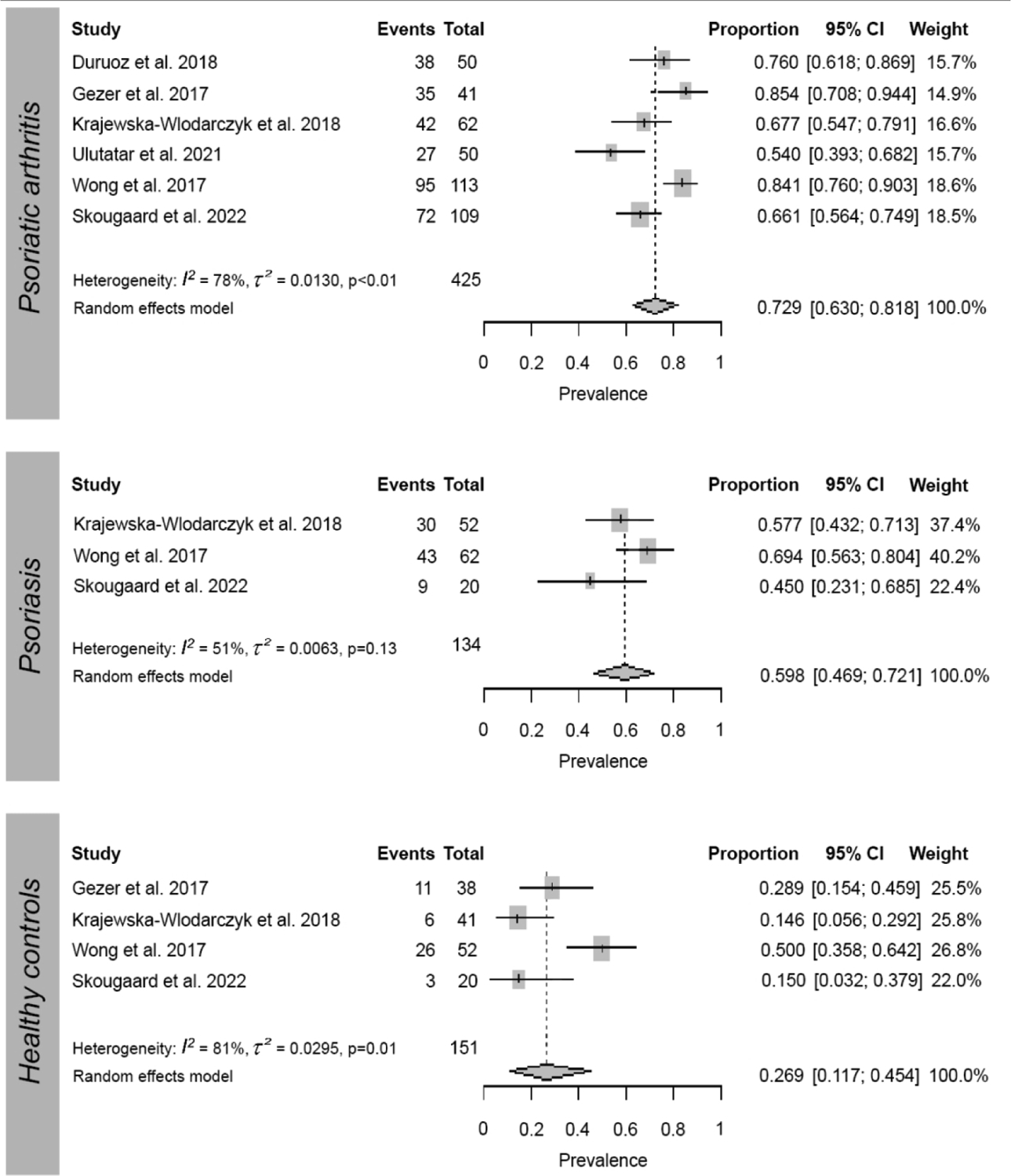
Meta-analysis of the prevalence of ‘poor sleep quality’ as measured by the Pittsburgh Sleep Quality Index (PSQI) in patients with psoriatic arthritis, patients with psoriasis, and healthy controls.
Meta-analysis of the prevalence of ‘poor sleep quality’ as measured by the Pittsburgh Sleep Quality Index (PSQI) in patients with psoriatic arthritis, patients with psoriasis, and healthy controls.
Regarding discrete sleep disorders, as defined by the International Classification of Sleep Disorders, one study reported that the prevalence of restless legs syndrome (RLS) in patients with PsA was 64%; significantly higher compared to patients with PsO (20%) and HC (14%).33 Insomnia was more frequent in patients with axial spondylarthritis (AxSp) than patients with PsA (PsA insomnia score [Median (IQR): 26.0 (18.0–30.7)] vs. AxSp [Median (IQR): 22.0 (15.2–28.7)], p=0.014); prevalence estimates, however, were not reported18 (Table 1 and Supplementary Table 4).
Using data from the Danish National Patient Register, Egeberg et al. reported an incidence rate ratio (IRR) of 1.75 (95% CI 1.35–2.26) for sleep apnea (ICD-10 G473) in patients with PsA, after adjusting for age, sex, smoking, alcohol, comorbidities, and socioeconomic status43 (Table 1 and Supplementary Table 6).
Prevalence and incidence of PsA in patients with sleep apnea
Egeberg et al. identified a high incidence of PsA in patients with sleep apnea (IRR of PsA in sleep apnea treated with continuous positive airway pressure (CPAP): 1.94 (95% CI 1.34–2.79 and without CPAP: 5.59 (95% CI 3.74–8.37), showing a significant bidirectional association in adjusted models.43 Cohen et al. further reported that patients with obstructive sleep apnea (OSA) had an increased risk of developing PsA (Table 1 and Supplementary Table 6).42 No studies determining the prevalence and incidence of PsA in other sleep disorders (e.g., insomnia, RLS) were found.
Factors associated with sleep problems in patients with PsA
Overall, we found moderate-to-strong (0.3 ≤ r < 0.7) correlations between sleep problems and pain, fatigue, physical function, emotional distress, tender joint counts, enthesitis, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), patient age, and number of tender joints (Supplementary Table 7). In studies in which the sample consisted of patients with psoriasis and PsA, PsA was identified as an independent predictor of sleep problems.17,34,51 Other factors independently associated with sleep problems in this population were itch, physical pain or soreness, female sex, obesity, OSA, moderate and severe psoriasis severity, smoking, poor dermatologic quality of life, and work productivity impairment (Supplementary Table 8).
In studies in which the sample consisted of patients with PsA (with or without psoriasis),18,21,23,28,31,37,38 pain was the most frequently identified factor independently associated with sleep problems, followed by actively inflamed or tender joints. Other factors independently associated with sleep problems included depression, anxiety, emotional recovery, physical function, itch, ESR, CRP, age, and duration of psoriasis (Supplementary Table 8).
Sleep problems were identified as a predictor of fatigue52 [i.e., a sustained, overwhelming sense of exhaustion associated with decreased capacity for physical and mental work that is different from sleepiness (i.e., propensity to sleep53]. Sleep problems were also identified as a predictor of poor health-related quality of life [as measured by the 15-Dimensional Questionnaire], and active PsA [as measured by the Disease Activity in Psoriatic Arthritis (DAPSA) score]29 (Supplementary Table 8).
Use of validated sleep PROMs in patients with PsA
Twenty-four studies (67%) utilized a patient-reported outcome measure (PROM) to assess sleep (Table 1). Six of these studies used a sleep question that had not been previously validated in any population. Six studies used a sleep question embedded in a validated psoriatic disease PROM (i.e., PsAID, RAPID3, and MDHAQ). Twelve studies used a PROM that was previously validated to measure sleep in other populations but not in PsA (i.e., Ottawa Sleep Scale, PSQI, PROMIS Sleep disturbance, Epworth Sleepiness Scale, Insomnia Severity Index, and the Medical Outcomes Sleep Scale).
We identified four studies evaluating one or more measurement properties of sleep measures in patients with PsA.22,29,41,44 The evaluated sleep measures included the Jenkins Sleep Scale (JSS), the PsAID item “sleep disturbance”, the Assessment of SpondyloArthritis International Society Health Index (ASAS-HI) item “I sleep badly at night’, and the sleep Visual Analogue Scale (VAS, 0–100 mm; 0 mm = no/minimal symptoms, 100mm = worse symptoms) (Table 1).
Sleep problems as measured by the PSQI
Eight studies assessed sleep problems in patients with PsA with the PSQI.20,21,28,33,35–38 PSQI scores ranged from 5.0–10.0. Patients with PsA consistently presented with worse sleep quality than HC and patients with PsO. Patients with PsA that had neuropathic pain or fibromyalgia had worse sleep quality than patients without neuropathic pain or fibromyalgia.35,36
Meta-analysis of the mean scores for the sleep dimensions assessed by the PSQI (i.e., PSQI parameters) across 3 studies showed that mean sleep quality, latency, disturbances, and day dysfunction were significantly higher in patients with PsA compared to HC (Supplementary Table 10 and Supplementary Figures 1–4).21,28,37 Evaluating the overlapping confidence interval, differences between patients with PsA and PsO were considered not significant. Use of sleep medication was comparable across groups. PSQI parameters are each scored on a 0–3 scale, where a higher score reflects worse sleep. Sleep efficiency was the sleep dimension most affected (Mean (SD) 2 [1.1; 2.9]), followed by sleep latency (1.6 [1.3; 1.9]), sleep disturbance (1.6 [1.4; 1.8]), and day dysfunction (1.5 [1.1; 2]) (Supplementary Table 10).
Sleep problems as measured by the PsAID questionnaire
Four studies evaluated sleep with the ‘sleep disturbance’ item from the PsAID questionnaire.19,31,32,49 The score ranged from 0–7, with a mean (SD) ranging from 1.9–3.4 (2.4–3.3). In Palominos et al., a score of 4 or higher was considered as having ‘sleep disturbance’ mirroring the PsAID cut-off value used to identify patients at a patient acceptable symptom state (PASS).31 Other studies did not clarify what cut-off value was used to define ‘sleep disturbance’. Puyraimond et al. described that a value of 2 for the ‘sleep disturbance’ item corresponded to PASS level.32 This threshold had a sensitivity of 0.77 and specificity of 0.5 (Table 1).
Studies evaluating beliefs about sleep problems in patients with PsA
Six studies explored patients’ beliefs about sleep problems in patients with PsA (Table 1).26,27,30,45 Hu et al. described that 60% of participants believed that sleep was affected by PsA and ranked sleep disturbances in the top 4 domains affected by PsA. Additionally, they reported that 59% of participants were willing to pay for a cure in this domain [median (25th quartile, 75th quartile): $10,000 ($5000, $50,000)].26 In Nowell et al., patients with PsA ranked the PROMIS sleep disturbance domain as the 4th most important PROM to track disease management.45 In one study, 78 (67%) participants believed that biologics had a ‘very beneficial’ or ‘beneficial’ effect on sleep quality,27 and in another study 29 (63%) patients believed that the use of cannabis helped them sleep better.40 More that 80% of participants further identified sleep disturbance as an important impact in patients with PsA, along with physical disability, effects on daily activities, and feelings of frustration.30 In a study involving 332 participants, improving sleep quality was identified as the third most important disease impact that required improvement, ranking higher than the ability to do physical activities, participate in work, social or leisure activities, and emotional well-being.
Effect of PsA therapies on sleep problems in patients with PsA
Three studies, including 1 non-RCT51, 1 cohort47, and 1 cross-sectional study28, showed TNF-inhibitors improved sleep problems in patients with PsA as measured by the PSQI and the MOS-SS. One cohort study showed improvement in sleep quality (VAS) with ustekinumab but changes were not statistically significant, whereas a RCT reported improved sleep with guselkumab50. Another RCT showed significant improvement in the PsAID ‘sleep disturbance’ item following treatment with filgotinib, a JAK-inhibitor49. In a cohort study including initiators of TNF- and IL17 inhibitors, the proportion of patients who had poor sleep quality at baseline dropped from 71.9% to 47.4% (p= 0.01) by month 4. However, mixed-effect models found that initiating either of those therapies did not influence changes in PSQI scores38. (Table 2).
Table 2.
Effect of therapies on sleep problems.
| Author, year | Study Aim | Study Design | Sample Size, n | Sex, n [M/F] | Age, years [Mean (SD)] | Sleep measure | Summary of main sleep-related results |
|---|---|---|---|---|---|---|---|
| Studies measuring sleep with a sleep question embedded in a psoriatic disease measure | |||||||
| Orbai 202049 | Examine the effects of filgotinib on HRQoL in active PsA | RCT |
|
NR | NR | PsAID (“sleep disturbanc e” question) |
|
| Studies measuring sleep with a sleep PROM validated in other populations | |||||||
| Krajewska-Wlodarczyk 201828 | Assess and measure occurrence of sleep disorders in patients with PsA and PsO | Cross-Sectional |
|
|
|
PSQI |
|
| Orbai 202250 | Assess the effects of guselkumab on general health outcomes in patients with PsA | RCT |
|
186/195 | 48.4 (11.4) | PROMIS Sleep Disturbanc e |
|
| Tektonidou 202047 | Evaluate the effect of adalimumab on work productivity measures, overall activity impairment, and sleep quality in patients with active moderate to severe RA, PsA, or AS | Cohort |
|
|
Median (IQR)=
|
MOS-SS |
|
| Skougaard 202238 | Determine the prevalence of sleep disturbances in PsA, PsO, and HC and explore associations between PSQI and PROMs/treatment | Cohort |
|
|
|
PSQI |
|
| Strober 201251 | Assess the extent of sleep impairment, the effect of adalimumab on sleep, patient-reported outcomes, and correlations between changes in these outcomes and sleep quality | Non-RCT |
|
NR | 47.6 (13.7) (whole sample) | MOS-SS |
|
| Wendler 202248 | Assess the long-term effectiveness, impact on quality of life, and safety of ustekinumab treatment for PsA | Cohort |
|
NR | NR | Sleep quality (100-mm VAS)-question and anchors not described |
|
AS, ankylosing spondylitis; HC, healthy controls; HRQoL, health-related quality of life; IQR, interquartile range; MOS-SS, Medical Outcomes Study Sleep Scale; NR, not reported; PGA, patient global assessment; PhGA, physician global assessment; PsA, psoriatic arthritis; PsAID, Psoriatic Arthritis Impact of Disease; PsO, psoriasis; PSQI, Pittsburgh Sleep Quality Index; RA, rheumatoid arthritis; RCT, randomized control trial; SPI, sleep problem index; TNF, tumor necrosis factor
Discussion
In this systematic review, we found that 30 to 85% of patients with PsA reported sleep problems. The difference in the rates of sleep problems across studies may respond partly to the different sleep problems evaluated and the instruments used to measure them. The sleep problems evaluated included ‘sleep disturbance’, ‘sleep interference’, ‘sleep difficulty’, ‘sleep impairment’, ‘sleep disorders’, and ‘poor sleep quality’. Even among studies measuring the same sleep problem, different measurement instruments were used. For example, ‘sleep disturbance’ was measured with an unvalidated Numeric Rating Scale (NRS) question, with the ‘sleep disturbance’ question from the PsAID and with the ‘sleep disturbance’ sub-score from the PSQI. The lack of a common terminology and common measurement instrument for a given sleep problem across studies hinders comparison of results.
Despite the variability in sleep problems evaluated and sleep measures used among studies, we identified 6 studies reporting on the prevalence of ‘poor sleep quality’ as measured by the PSQI.20,21,28,35,37,38 A meta-analysis based on these studies concluded that 73% of patients with PsA had poor sleep quality. Pooled data from 421,28,37,38 of these studies further showed that the prevalence of poor sleep quality in patients with PsA was significantly higher compared to HC (27%). Compared to patients with PsO, pooled data from 328,37,38 of these studies revealed that differences were not significant. Additionally, our meta-analysis of mean scores of PSQI parameters found that patients with PsA suffered from worse subjective sleep quality, latency, disturbances, and day dysfunction than HC, but not compared to patients with PsO. Results of these meta-analyses, however, are limited by the large heterogeneity identified and the small number of studies included.54 Differences in patient demographics and disease severity, the presence of comorbidities including sleep disorders, and medication status may account for the variability of results among studies.
In this review, only self-reported sleep measures were used; objective sleep measures (i.e., actigraphy or polysomnography) and sleep diaries were not used in PsA. Henry et al. described similar findings in their review for patients with PsO.55 Although they identified a few studies that utilized polysomnography in patients with PsO, actigraphy and sleep diaries were not applied. Both subjective and objective sleep measures are required to identify and assess sleep problems,56 especially for targeting interventions. Future studies in psoriatic disease should include both type of sleep assessments.
The JSS was the only sleep PROM whose measurement properties were formally assessed in a validation study. However, we did not find studies reporting results for the JSS in patients with PsA.41 In this validation study, investigators explored the internal consistency and construct validity of the JSS, but its content validity in PsA has not been explored. The PSQI is the most widely used sleep PROM in clinical and non-clinical populations. It was developed for patients with depression and later validated in several other disorders.57 Yet, the PSQI has not been validated in patients with PsA. Furthermore, even in other populations, its dimensionality (i.e., factor structure) warrants further investigation.58 Given that patients with psoriatic disease were not involved in the development of these instruments and that such instruments do not quantify symptoms that may cause sleep problems in these patients such as pruritus, skin pain, joint pain, and stiffness, evaluation of the content validity and other measurement properties of sleep PROMs in PsA is warranted. To address the potential lack of comprehensiveness of these measures in psoriatic disease, a set of psoriatic disease-specific sleep measures - the “PsO Sleepy Q” and the “PsA-Sleepy-Q” - are currently being validated.59
This review identified several predictors of sleep problems in patients with PsA including anxiety, pain, ESR, emotional recovery, depression, fatigue, physical function, and tender or swollen joint count (TSJC). Yet, the directionality of the association between sleep and these predictors has not been established. Longitudinal studies including both PROMs and actigraphy are needed to explore whether the symptoms and/or signs of PsA (e.g., joint pain or TSJC) hold a bidirectional relationship with sleep problems as suggested in qualitative studies.59,60
Finally, we found that TNF-inhibitors, guselkumab, and filgotinib were associated with improved sleep outcomes. However, these assessments presented certain limitations. First, only 249,50 of 628,47,49,51 studies consisted of an RCT. Second, sleep was not the primary outcome in any of these 4 studies. Therefore, power calculations were not based on sleep outcomes. Third, sleep PROMs used had not been validated in PsA and objective sleep measures were not used as previously noted. Properly powered clinical trials exploring the efficacy of (i) psoriatic disease interventions and (ii) sleep-directed interventions (e.g., cognitive behavioral therapy for insomnia) to treat sleep outcomes in PsA are required.
Limitations:
Our search was restricted to two databases, PubMed and Embase, and inclusion criteria were restricted to data from individuals 16 years and older, studies evaluating sleep in patients with PsA (or at least sub-analysis for the PsA population was required), and studies in English. Therefore, it is possible that studies outside of these parameters with information relevant to our study aims were not included.
Conclusion
Sleep problems are common in patients with PsA, and patients consider sleep problems as having an important impact on PsA. The prevalence of poor sleep quality was significantly higher in patients with PsA compared to HC, but not compared to those with PsO. Our results rely on a small number of heterogenous studies. Sleep apnea holds a bidirectional relationship with PsA in adjusted models, but data about the association between PsA and other sleep disorders is lacking. The PSQI and the PsAID item ‘sleep disturbance’ were the most widely used sleep PROMs across studies, though evidence supporting their validity is needed. Longitudinal studies combining daily measurement of PsA signs and symptoms, and subjective (i.e., validated sleep PROMs and sleep diaries) and objective sleep measures (e.g., actigraphy) are needed to further understand how sleep quality relates to PsA disease activity and symptoms. Finally, while a few studies support the beneficial effect of biologics and JAK inhibitors for PsA on sleep problems, further RCT evaluating the efficacy of diverse therapies to manage and improve sleep in PsA are needed.
Supplementary Material
Disclosures:
Elizabeth B. Klerman has consulted for the American Association of Sleep Medicine Foundation, National Sleep Foundation, Circadian Therapeutics and Yale University Press. Joseph F. Merola, MD, MMSc: JFM has served as a consultant and/or investigator for Dermavant, Eli Lilly, UCB, Sun Pfizer and Leo Pharma. Alexis Ogdie, MD has consulted for Abbvie, Amgen, BMS, Celgene, CorEvitas, Gilead, GSK, HappifyHealth, Janssen, Novartis, Pfizer and UCB and received grants from Abbvie, Amgen, Novartis and Pfizer. All other authors have no relevant disclosures.
Source of Support:
EBK: Leducq Trans-Atlantic Network of Excellence On Circadian Effects in Stroke; NIH R01HD107064, R01NS114526-02S1, R21DA052861, U54-AG062322, U01NS114001; US DoD W81XWH201076. LPC: National Psoriasis Foundation 815789.
Contributor Information
Carly Grant, Department of Dermatology, Harvard Medical School, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Michael Woodbury, Department of Dermatology, Harvard Medical School, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Marie Skougaard, Department of Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark.
Jens K. Boldsen, Department of Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark.
Alexis Ogdie, Department of Medicine/Division of Rheumatology, Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Elizabeth B. Klerman, Department of Neurology, Massachusetts General Hospital; Boston, MA, USA and Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA.
Joseph F. Merola, Department of Dermatology and Medicine, Division of Rheumatology, Harvard Medical School, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Lourdes Perez-Chada, Department of Dermatology, Harvard Medical School, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
References
- 1.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic Arthritis. N Engl J Med. May 25 2017;376(21):2095–6. doi: 10.1056/NEJMc1704342 [DOI] [PubMed] [Google Scholar]
- 2.Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. Nov 2013;69(5):729–735. doi: 10.1016/j.jaad.2013.07.023 [DOI] [PubMed] [Google Scholar]
- 3.Perez-Chada LM, Merola JF. Comorbidities associated with psoriatic arthritis: Review and update. Clin Immunol. May 2020;214:108397. doi: 10.1016/j.clim.2020.108397 [DOI] [PubMed] [Google Scholar]
- 4.Zhou L, Tang Z, Zuo Z, Zhou K. Neural Mechanism Underlying the Sleep Deprivation-Induced Abnormal Bistable Perception. Cereb Cortex. Jan 22 2022;32(3):583–592. doi: 10.1093/cercor/bhab235 [DOI] [PubMed] [Google Scholar]
- 5.Zhu G, Catt M, Cassidy S, et al. Objective sleep assessment in >80,000 UK mid-life adults: Associations with sociodemographic characteristics, physical activity and caffeine. PLoS One. 2019;14(12):e0226220. doi: 10.1371/journal.pone.0226220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zielinski MR, McKenna JT, McCarley RW. Functions and Mechanisms of Sleep. AIMS Neurosci. 2016;3(1):67–104. doi: 10.3934/Neuroscience.2016.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S, Kim JH, Chung JH. The association between sleep quality and quality of life: a population-based study. Sleep Med. Aug 2021;84:121–126. doi: 10.1016/j.sleep.2021.05.022 [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Park EC, Yoo KB, Park S. The Association between Short or Long Sleep Times and Quality of Life (QOL): Results of the Korea National Health and Nutrition Examination Survey (KNHANES IV-V). J Clin Sleep Med. Jun 15 2015;11(6):625–34. doi: 10.5664/jcsm.4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. Oct 2009;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 10.Kmet LM, Lee RC, Cook LS. Standard quality assessment criteria for evaluating primary research papers from a variety of fields. Edmonton: Alberta Heritage Foundation for Medical Research (AHFMR). AHFMR - HTA Initiative #13. 2004 [Google Scholar]
- 11.Friedel M, Aujoulat I, Dubois AC, Degryse JM. Instruments to Measure Outcomes in Pediatric Palliative Care: A Systematic Review. Pediatrics. Jan 2019;143(1)doi: 10.1542/peds.2018-2379 [DOI] [PubMed] [Google Scholar]
- 12.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. May 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. Sep 6 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan R. Heterogeneity and subgroup analyses in Cochrane Consumers and Communication Group reviews: planning the analysis at protocol stage. Cochrane Consumers and Communication Review Group; Dec 2016. [Google Scholar]
- 15.Barker TH, Migliavaca CB, Stein C, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. Sep 20 2021;21(1):189. doi: 10.1186/s12874-021-01381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azarfar A, Ahmed A, Bég S. Prevalence of Anxiety, Depression, Sleep Disturbance, Fibromyalgia, Obesity, and Gastroesophageal Disease in Patients with Rheumatic Diseases. Curr Rheumatol Rev. 2021;17(2):252–257. doi: 10.2174/1573397116666201211124815 [DOI] [PubMed] [Google Scholar]
- 17.Callis Duffin K, Wong B, Horn EJ, Krueger GG. Psoriatic arthritis is a strong predictor of sleep interference in patients with psoriasis. Article. Journal of the American Academy of Dermatology. 2009;60(4):604–608. doi: 10.1016/j.jaad.2008.10.059 [DOI] [PubMed] [Google Scholar]
- 18.Cano-García L, Mena-Vázquez N, Manrique Arija S, et al. Psychological factors associated with sleep disorders in patients with axial spondyloarthritis or psoriatic arthritis: A multicenter cross-sectional observational study. Article. Journal of clinical nursing. 2021;30(1–2):266–275. doi: 10.1111/jocn.15546 [DOI] [PubMed] [Google Scholar]
- 19.Desthieux C, Granger B, Balanescu AR, et al. Determinants of Patient-Physician Discordance in Global Assessment in Psoriatic Arthritis: A Multicenter European Study. Arthritis Care Res (Hoboken). Oct 2017;69(10):1606–1611. doi: 10.1002/acr.23172 [DOI] [PubMed] [Google Scholar]
- 20.Duruoz MT, Baklacioglu HS, Sanal Toprak C, Gencer Atalay K, Atagunduz MP. The evaluation of the static and dynamic balance disorders in patients with psoriatic arthritis. Rheumatol Int. Nov 2018;38(11):2063–2068. doi: 10.1007/s00296-018-4137-7 [DOI] [PubMed] [Google Scholar]
- 21.Gezer O, Batmaz İ, Sariyildiz MA, et al. Sleep quality in patients with psoriatic arthritis. Article. International Journal of Rheumatic Diseases. 2017;20(9):1212–1218. doi: 10.1111/1756-185X.12505 [DOI] [PubMed] [Google Scholar]
- 22.Güler T, Bora Karslı P, Ataman Ş, Bodur H. Psoriatic arthritis impact of disease questionnaire: validity, reliability and its clinical potential. Article. Rheumatology International. 2020;40(6):959–967. doi: 10.1007/s00296-020-04575-8 [DOI] [PubMed] [Google Scholar]
- 23.Haugeberg G, Hoff M, Kavanaugh A, Michelsen B. Psoriatic arthritis: exploring the occurrence of sleep disturbances, fatigue, and depression and their correlates. Arthritis Res Ther. Aug 26 2020;22(1):198. doi: 10.1186/s13075-020-02294-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haugeberg G, Lund Nilsen TI, Kavanaugh A, Thomsen RS, Gulati AM, Hoff M. Physical and Psychosocial Burden of Psoriatic Arthritis: Longitudinal Data From a Population-Based Study in Norway. Arthritis Care Res (Hoboken). Jan 2021;73(1):138–145. doi: 10.1002/acr.24412 [DOI] [PubMed] [Google Scholar]
- 25.Haugeberg G, Michelsen B, Kavanaugh A. Impact of skin, musculoskeletal and psychosocial aspects on quality of life in psoriatic arthritis patients: A cross-sectional study of outpatient clinic patients in the biologic treatment era. Article. RMD Open. 2020;6(1)doi: 10.1136/rmdopen-2020-001223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu SW, Holt EW, Husni ME, Qureshi AA. Willingness-to-Pay stated preferences for 8 health-related quality-of-life domains in psoriatic arthritis: A Pilot Study. Review. Seminars in Arthritis and Rheumatism. 2010;39(5):384–397. doi: 10.1016/j.semarthrit.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 27.Kotulska A, Kucharz EJ, Wiland P, et al. Satisfaction and discontent of Polish patients with biological therapy of rheumatic diseases: Results of a multi-center questionnaire study. Article. Reumatologia. 2018;56(3):140–148. doi: 10.5114/reum.2018.76901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krajewska-Włodarczyk M, Owczarczyk-Saczonek A, Placek W. Sleep disorders in patients with psoriatic arthritis and psoriasis. Article. Reumatologia. 2018;56(5):301–306. doi: 10.5114/reum.2018.79501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morante I, Aurrecoechea E, Villa I, Santos M, Riancho L, Queiro R. Construct validity of the ASAS health index in psoriatic arthritis: a cross-sectional analysis. Rheumatology (Oxford). Mar 2 2021;60(3):1465–1473. doi: 10.1093/rheumatology/keaa626 [DOI] [PubMed] [Google Scholar]
- 30.Ogdie A, Michaud K, Nowak M, et al. Patient’s experience of psoriatic arthritis: A conceptual model based on qualitative interviews. Article. RMD Open. 2020;6(3)doi: 10.1136/rmdopen-2020-001321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palominos PE, Coates L, Kohem CL, et al. Determinants of sleep impairment in psoriatic arthritis: An observational study with 396 patients from 14 countries. Article. Joint Bone Spine. 2020;87(5):449–454. doi: 10.1016/j.jbspin.2020.03.014 [DOI] [PubMed] [Google Scholar]
- 32.Puyraimond-Zemmour D, Etcheto A, Fautrel B, et al. Associations Between Five Important Domains of Health and the Patient Acceptable Symptom State in Rheumatoid Arthritis and Psoriatic Arthritis: A Cross-Sectional Study of 977 Patients. Arthritis Care Res (Hoboken). Oct 2017;69(10):1504–1509. doi: 10.1002/acr.23176 [DOI] [PubMed] [Google Scholar]
- 33.Sandikci SC, Colak S, Aydoğan Baykara R, et al. Evaluation of restless legs syndrome and sleep disorders in patients with psoriatic arthritis. Article. Zeitschrift fur Rheumatologie. 2019;78(10):987–995. doi: 10.1007/s00393-018-0562-y [DOI] [PubMed] [Google Scholar]
- 34.Smith MP, Ly K, Thibodeaux Q, et al. Factors Influencing Sleep Difficulty and Sleep Quantity in the Citizen Pscientist Psoriatic Cohort. Article. Dermatology and Therapy. 2019;9(3):511–523. doi: 10.1007/s13555-019-0306-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulutatar Ç, Ulutatar F, DuruÖz MT. Neuropathic Pain: Unexplored and Significant Relationship With Psoriatic Arthritis and Functional Parameters. Arch Rheumatol. Sep 2020;35(3):409–415. doi: 10.46497/ArchRheumatol.2020.7215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulutatar F, Unal-Ulutatar C, Tuncay Duruoz M. Fibromyalgia in patients with psoriatic arthritis: Relationship with enthesopathy, sleep, fatigue and quality of life. Article. International Journal of Rheumatic Diseases. 2021;24(2):183–188. doi: 10.1111/1756-185X.13963 [DOI] [PubMed] [Google Scholar]
- 37.Wong ITY, Chandran V, Li S, Gladman DD. Sleep disturbance in psoriatic disease: Prevalence and associated factors. Article. Journal of Rheumatology. 2017;44(9):1369–1374. doi: 10.3899/jrheum.161330 [DOI] [PubMed] [Google Scholar]
- 38.Skougaard M, Stisen ZR, Jørgensen TS, et al. Increased prevalence of sleep disturbance in psoriatic arthritis is associated with inflammatory and non-inflammatory measures. Scand J Rheumatol. Mar 18 2022:1–9. doi: 10.1080/03009742.2022.2044116 [DOI] [PubMed] [Google Scholar]
- 39.Ogdie A, Myers K, Mansfield C, et al. Experiences and Treatment Preferences in Patients With Psoriatic Arthritis: A Cross-Sectional Study in the ArthritisPower Registry. Rheumatol Ther. Apr 2022;9(2):735–751. doi: 10.1007/s40744-022-00436-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsang J, Silverberg O, Machhar R, et al. Exploring cannabis use and perspectives among psoriatic disease patients. Clin Rheumatol. May 2022;41(5):1431–1437. doi: 10.1007/s10067-022-06066-6 [DOI] [PubMed] [Google Scholar]
- 41.Duruöz MT, Erdem D, Gencer K, Ulutatar F, Baklacıoğlu H. Validity and reliability of the Turkish version of the Jenkins Sleep Scale in psoriatic arthritis. Rheumatol Int. Feb 2018;38(2):261–265. doi: 10.1007/s00296-017-3911-2 [DOI] [PubMed] [Google Scholar]
- 42.Cohen JM, Jackson CL, Li TY, Wu S, Qureshi AA. Sleep disordered breathing and the risk of psoriasis among US women. Article. Archives of Dermatological Research. 2015;307(5):433–438. doi: 10.1007/s00403-015-1536-4 [DOI] [PubMed] [Google Scholar]
- 43.Egeberg A, Khalid U, Gislason GH, Mallbris L, Skov L, Hansen PR. Psoriasis and sleep apnea: A Danish nationwide cohort study. Article. Journal of Clinical Sleep Medicine. 2016;12(5):663–671. doi: 10.5664/jcsm.5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwok T, Pope JE. Minimally important difference for patient-reported outcomes in psoriatic arthritis: Health Assessment Questionnaire and pain, fatigue, and global visual analog scales. J Rheumatol. May 2010;37(5):1024–8. doi: 10.3899/jrheum.090832 [DOI] [PubMed] [Google Scholar]
- 45.Nowell WB, Gavigan K, Kannowski CL, et al. Which patient-reported outcomes do rheumatology patients find important to track digitally? A real-world longitudinal study in ArthritisPower. Article. Arthritis Research and Therapy. 2021;23(1)doi: 10.1186/s13075-021-02430-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pincus T, Swearingen C, Wolfe F. Toward a multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient-friendly health assessment questionnaire format. Arthritis Rheum. Oct 1999;42(10):2220–30. doi: [DOI] [PubMed] [Google Scholar]
- 47.Tektonidou MG, Katsifis G, Georgountzos A, et al. Real-world evidence of the impact of adalimumab on work productivity and sleep measures in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Ther Adv Musculoskelet Dis. 2020;12:1759720×20949088. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wendler J, Damann N, Röcken M, et al. Ustekinumab Is Rapid-Acting and Is an Effective Long-Term Treatment for Patients with Active Psoriatic Arthritis: Real-World Evidence from the Non-interventional SUSTAIN Study. Rheumatol Ther. Oct 2022;9(5):1435–1450. doi: 10.1007/s40744-022-00484-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orbai AM, Ogdie A, Gossec L, et al. Effect of filgotinib on health-related quality of life in active psoriatic arthritis: a randomized phase 2 trial (EQUATOR). Rheumatology (Oxford). Jul 1 2020;59(7):1495–1504. doi: 10.1093/rheumatology/kez408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orbai AM, Coates LC, Deodhar A, et al. Meaningful Improvement in General Health Outcomes with Guselkumab Treatment for Psoriatic Arthritis: Patient-Reported Outcomes Measurement Information System-29 Results from a Phase 3 Study. Patient. Nov 2022;15(6):657–668. doi: 10.1007/s40271-022-00588-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strober BE, Sobell JM, Duffin KC, et al. Sleep quality and other patient-reported outcomes improve after patients with psoriasis with suboptimal response to other systemic therapies are switched to adalimumab: Results from PROGRESS, an open-label Phase IIIB trial. Article. British Journal of Dermatology. 2012;167(6):1374–1381. doi: 10.1111/bjd.12000 [DOI] [PubMed] [Google Scholar]
- 52.Haugeberg G, Hoff M, Michelsen B, Kavanaugh A. Exploring occurrence and correlates of sleep disturbances and fatigue in psoriatic arthritis patients. Conference Abstract. Annals of the Rheumatic Diseases. 2020;79(SUPPL 1):1689. doi: 10.1136/annrheumdis-2020-eular.1105 [DOI] [Google Scholar]
- 53.Billones R, Liwang JK, Butler K, Graves L, Saligan LN. Dissecting the fatigue experience: A scoping review of fatigue definitions, dimensions, and measures in non-oncologic medical conditions. Brain Behav Immun Health. Aug 2021;15:100266. doi: 10.1016/j.bbih.2021.100266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj. Jul 22 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 55.Henry AL, Kyle SD, Bhandari S, Chisholm A, Griffiths CE, Bundy C. Measurement, Classification and Evaluation of Sleep Disturbance in Psoriasis: A Systematic Review. PLoS One. 2016;11(6):e0157843. doi: 10.1371/journal.pone.0157843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. Sep 2001;2(5):389–96. doi: 10.1016/s1389-9457(00)00098-8 [DOI] [PubMed] [Google Scholar]
- 57.Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev. Feb 2016;25:52–73. doi: 10.1016/j.smrv.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 58.Manzar MD, BaHammam AS, Hameed UA, et al. Dimensionality of the Pittsburgh Sleep Quality Index: a systematic review. Health and quality of life outcomes. 2018;16(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ford AR, Mascia E, Boehncke WH, Ritchlin CT. GRAPPA Trainees Symposium 2018: A Report from the GRAPPA 2018 Annual Meeting. J Rheumatol Suppl. Jun 2019;95:4–10. doi: 10.3899/jrheum.190123 [DOI] [PubMed] [Google Scholar]
- 60.Henry AL, Bundy C, Kyle SD, Griffiths CEM, Chisholm A. Understanding the experience of sleep disturbance in psoriasis: a qualitative exploration using the Common-Sense Model of Self-Regulation. Br J Dermatol. Jun 2019;180(6):1397–1404. doi: 10.1111/bjd.17685 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


