Abstract
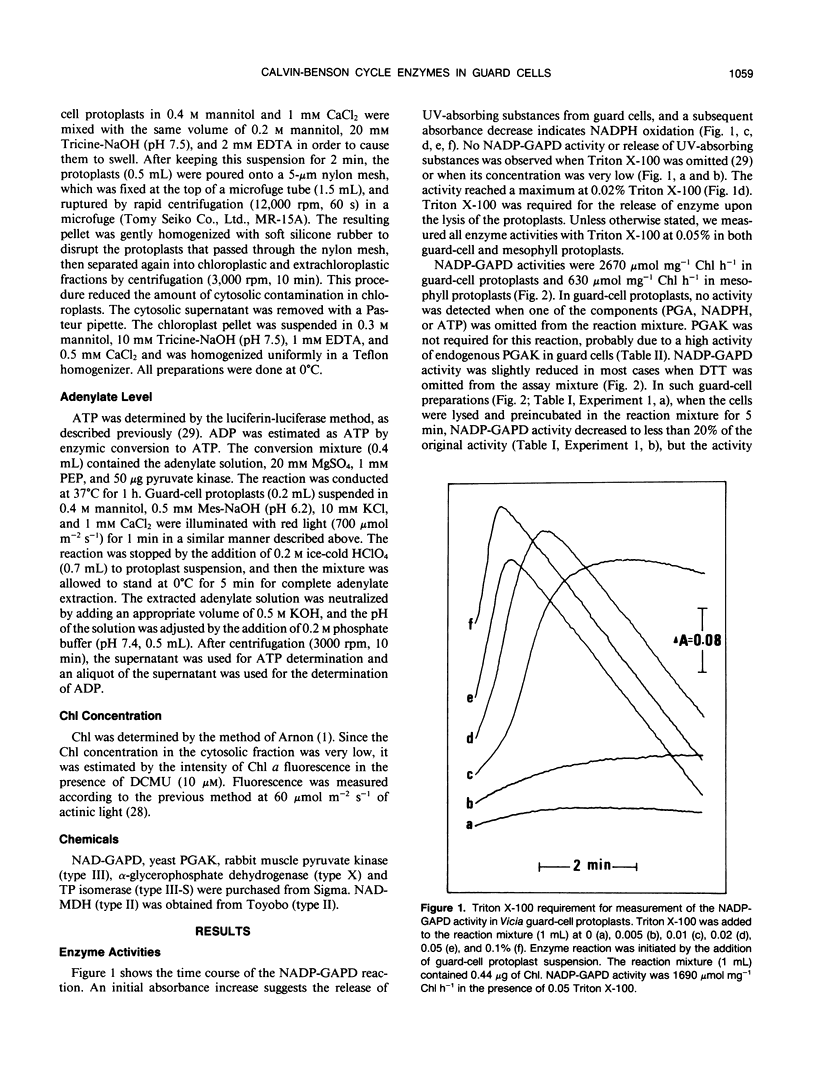
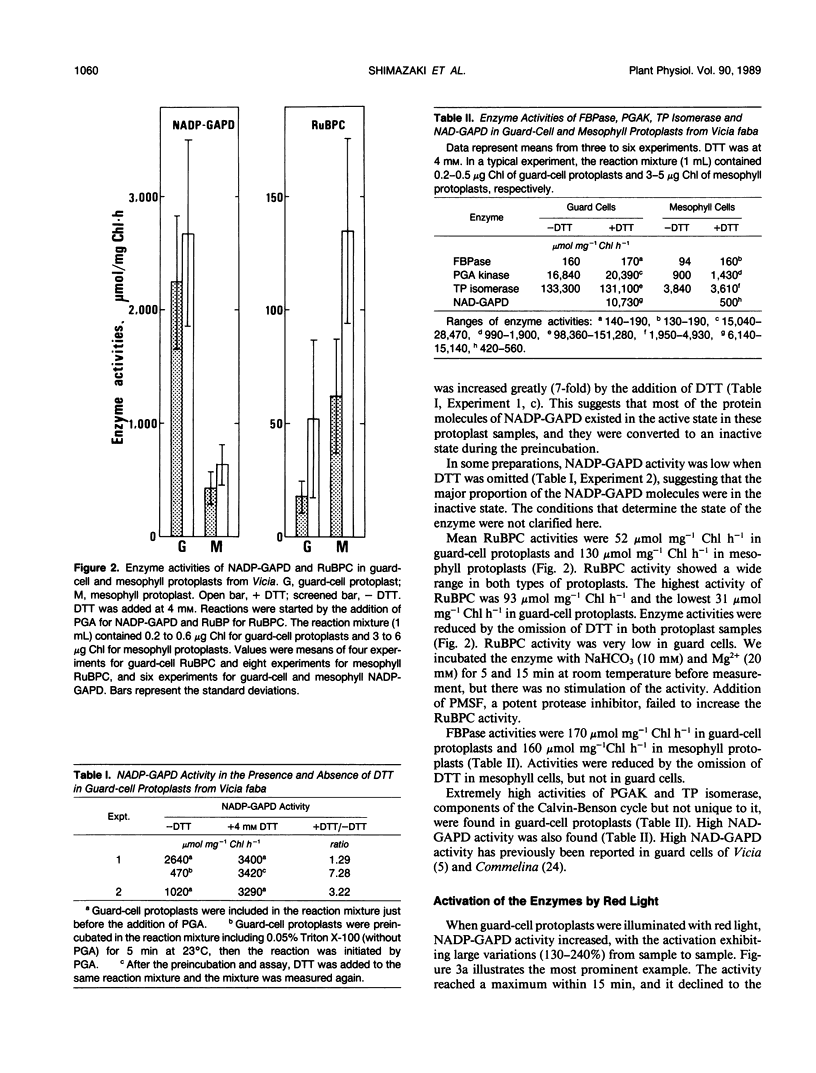
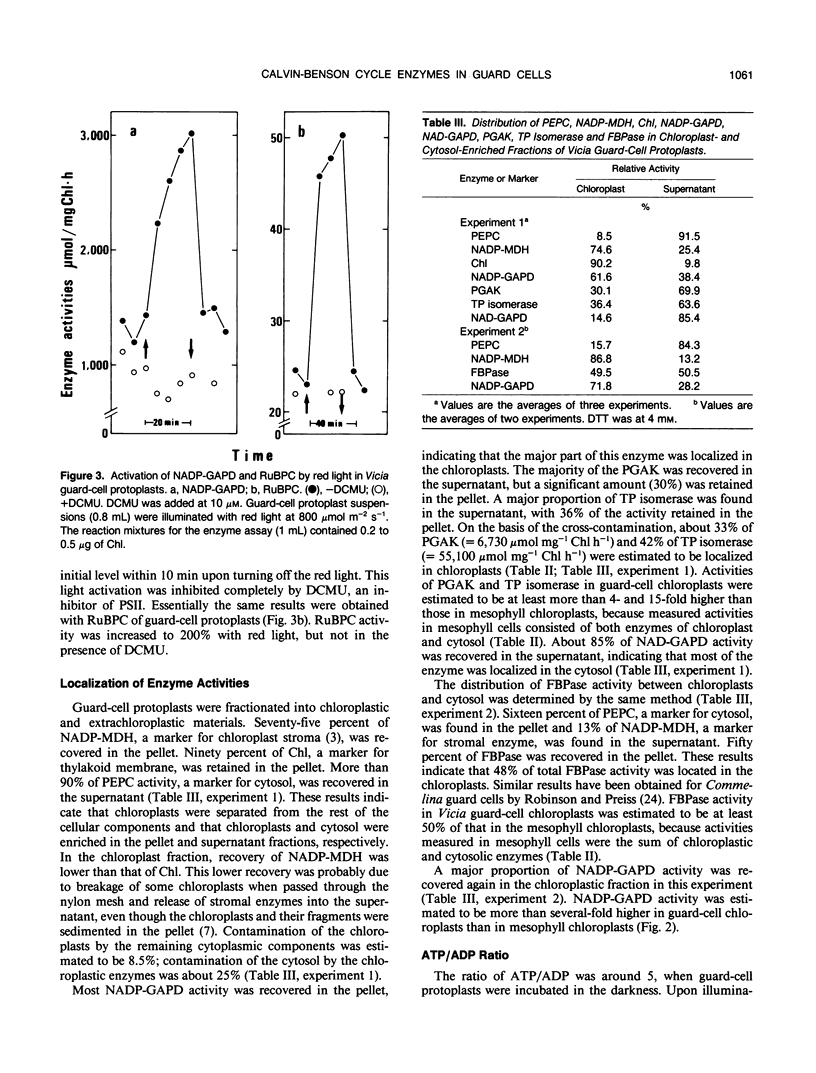
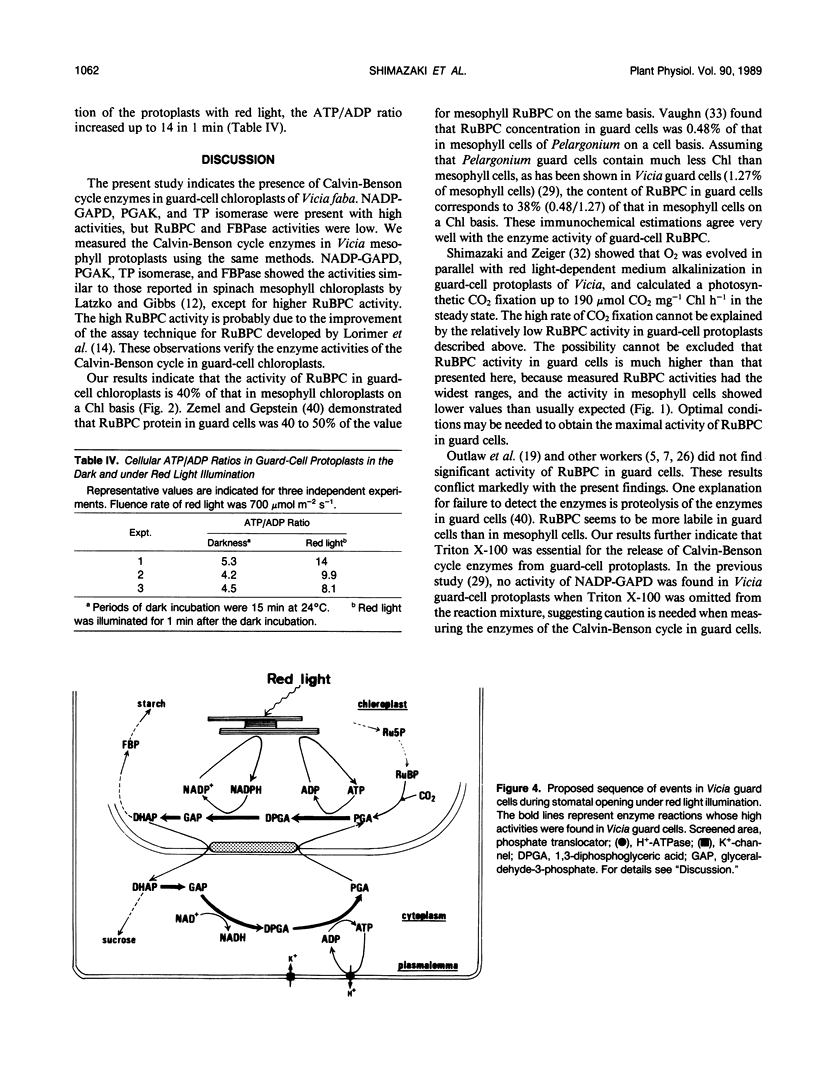
Activities of Calvin-Benson cycle enzymes were found in protoplasts of guard cells from Vicia faba L. The activities of NADP-glyceraldehyde-3-phosphate dehydrogenase (NADP-GAPD) and ribulose-1,5-bisphosphate carboxylase (RuBPC) were 2670 and 52 micromoles per milligrams chlorophyll per hour, respectively. Activities of NADP-GAPD and RuBPC in guard cells were increased by red light illumination, and the light activations were inhibited completely by 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), an inhibitor of photosystem II. Enzymes related to the Calvin-Benson cycle such as 3-phosphoglycerate kinase (PGAK), triose phosphate (TP) isomerase, and fructose-1,6-bisphosphatase (FBPase) were shown to be present in guard-cell chloroplasts. From these results, we conclude that the photosynthetic carbon reduction pathway is present in guard-cell chloroplasts of Vicia faba. We compared these enzyme activities in guard cells with those in mesophyll cells. The activities of NADP-GAPD and PGAK were more than several-fold higher and that of TP isomerase was much higher in guard-cell chloroplasts than in mesophyll chloroplasts. In contrast, activities of RuBPC and FBPase were estimated to be roughly half of those in mesophyll chloroplasts. High activities of PGAK, NAD-GAPD, and TP isomerase were found in fractions enriched in cytosol of guard cells. Illumination of guard-cell protoplasts with red light increased the cellular ATP/ADP ratio from 5 to 14. These results support the interpretation that guard cells utilize a shuttle system (e.g. phosphoglycerate [PGA]/dihydroxyacetone phosphate [DHAP] shuttle) for an indirect transfer of ATP and reducing equivalents from chloroplasts to the cytosol.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULLER R. C., SMILLIE R. M., SISLER E. C., KORNBERG H. L. Carbon metabolism in Chromatium. J Biol Chem. 1961 Jul;236:2140–2149. [PubMed] [Google Scholar]
- Gotow K., Tanaka K., Kondo N., Kobayashi K., Syōno K. Light Activation of NADP-Malate Dehydrogenase in Guard Cell Protoplasts from Vicia faba L. Plant Physiol. 1985 Nov;79(3):829–832. doi: 10.1104/pp.79.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotow K., Taylor S., Zeiger E. Photosynthetic Carbon Fixation in Guard Cell Protoplasts of Vicia faba L. : Evidence from Radiolabel Experiments. Plant Physiol. 1988 Mar;86(3):700–705. doi: 10.1104/pp.86.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp R., Outlaw W. H., Tarczynski M. C. Profile of Basic Carbon Pathways in Guard Cells and Other Leaf Cells of Vicia faba L. Plant Physiol. 1982 Dec;70(6):1582–1585. doi: 10.1104/pp.70.6.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Hsiao T. C., Allaway W. G. Action Spectra for Guard Cell Rb Uptake and Stomatal Opening in Vivia faba. Plant Physiol. 1973 Jan;51(1):82–88. doi: 10.1104/pp.51.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Zimmermann G., Latzko E. Light induced activation of fructose-1, 6-bisphosphatase in isolated intact chloroplasts. Biochem Biophys Res Commun. 1976 May 3;70(1):193–199. doi: 10.1016/0006-291x(76)91127-x. [DOI] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Enzyme activities of the carbon reduction cycle in some photosynthetic organisms. Plant Physiol. 1969 Feb;44(2):295–300. doi: 10.1104/pp.44.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Outlaw W. H., Manchester J., Dicamelli C. A., Randall D. D., Rapp B., Veith G. M. Photosynthetic carbon reduction pathway is absent in chloroplasts of Vicia faba guard cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6371–6375. doi: 10.1073/pnas.76.12.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Mayne B. C., Zenger V. E., Manchester J. Presence of Both Photosystems in Guard Cells of Vicia faba L: IMPLICATIONS FOR ENVIRONMENTAL SIGNAL PROCESSING. Plant Physiol. 1981 Jan;67(1):12–16. doi: 10.1104/pp.67.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Springer S. A., Tarczynski M. C. Histochemical technique : a general method for quantitative enzyme assays of single cell ;extracts' with a time resolution of seconds and a reading precision of femtomoles. Plant Physiol. 1985 Mar;77(3):659–666. doi: 10.1104/pp.77.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N. L., Preiss J. Localization of Carbohydrate Metabolizing Enzymes in Guard Cells of Commelina communis. Plant Physiol. 1987 Oct;85(2):360–364. doi: 10.1104/pp.85.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano E. E., Zeiger E., Hagiwara S. Red light stimulates an electrogenic proton pump in Vicia guard cell protoplasts. Proc Natl Acad Sci U S A. 1988 Jan;85(2):436–440. doi: 10.1073/pnas.85.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K., Zeiger E. Cyclic and Noncyclic Photophosphorylation in Isolated Guard Cell Chloroplasts from Vicia faba L. Plant Physiol. 1985 Jun;78(2):211–214. doi: 10.1104/pp.78.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K., Zeiger E. Red Light-Dependent CO(2) Uptake and Oxygen Evolution in Guard Cell Protoplasts of Vicia faba L.: Evidence for Photosynthetic CO(2) Fixation. Plant Physiol. 1987 May;84(1):7–9. doi: 10.1104/pp.84.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn K. C. Two immunological approaches to the detection of ribulose-1,5-bisphosphate carboxylase in guard cell chloroplasts. Plant Physiol. 1987 May;84(1):188–196. doi: 10.1104/pp.84.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wara-Aswapati O., Kemble R. J., Bradbeer J. W. Activation of Glyceraldehyde-Phosphate Dehydrogenase (NADP) and Phosphoribulokinase in Phaseolus vulgaris Leaf Extracts Involves the Dissociation of Oligomers. Plant Physiol. 1980 Jul;66(1):34–39. doi: 10.1104/pp.66.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosiuk R. A., Buchanan B. B. Studies on the regulation of chloroplast NADP-linked glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1976 Oct 25;251(20):6456–6461. [PubMed] [Google Scholar]
- Zeiger E., Armond P., Melis A. Fluorescence Properties of Guard Cell Chloroplasts: EVIDENCE FOR LINEAR ELECTRON TRANSPORT AND LIGHT-HARVESTING PIGMENTS OF PHOTOSYSTEMS I AND II. Plant Physiol. 1981 Jan;67(1):17–20. doi: 10.1104/pp.67.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel E., Gepstein S. Immunological evidence for the presence of ribulose bisphosphate carboxylase in guard cell chloroplasts. Plant Physiol. 1985 Jul;78(3):586–590. doi: 10.1104/pp.78.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]


